Abstract
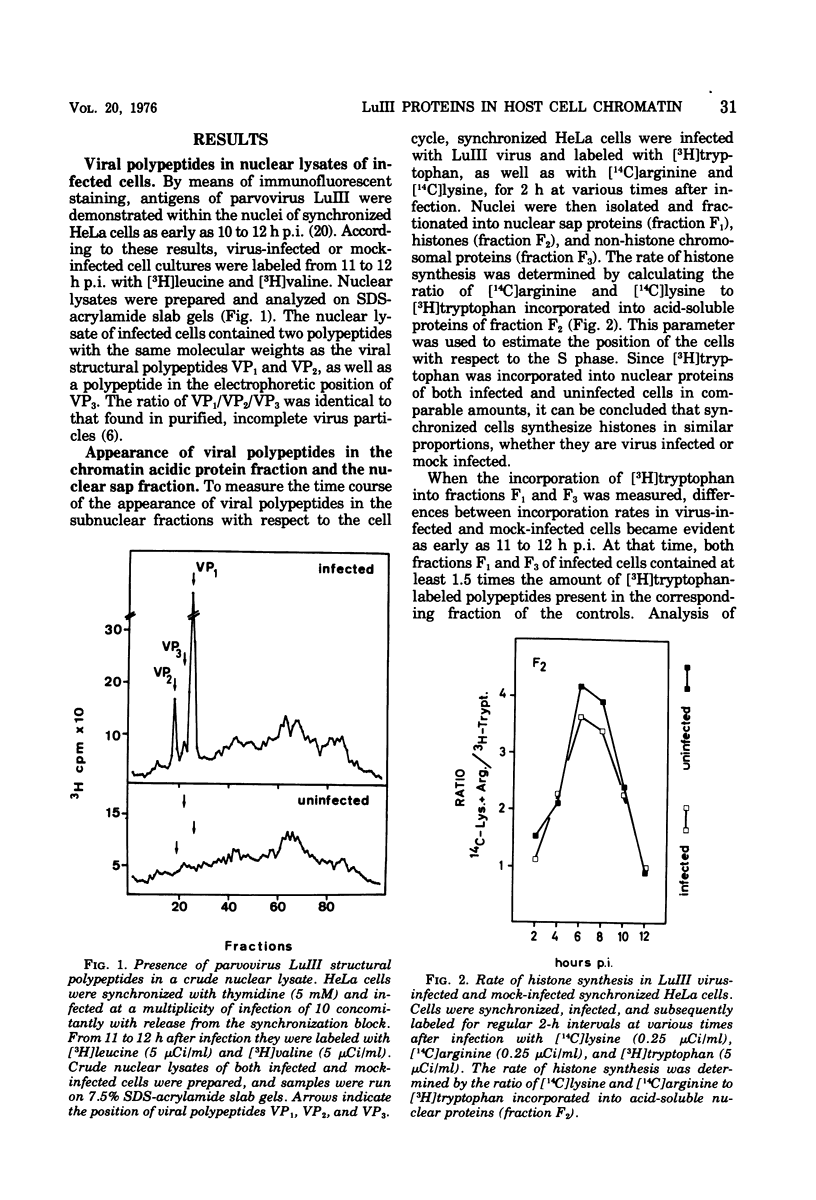
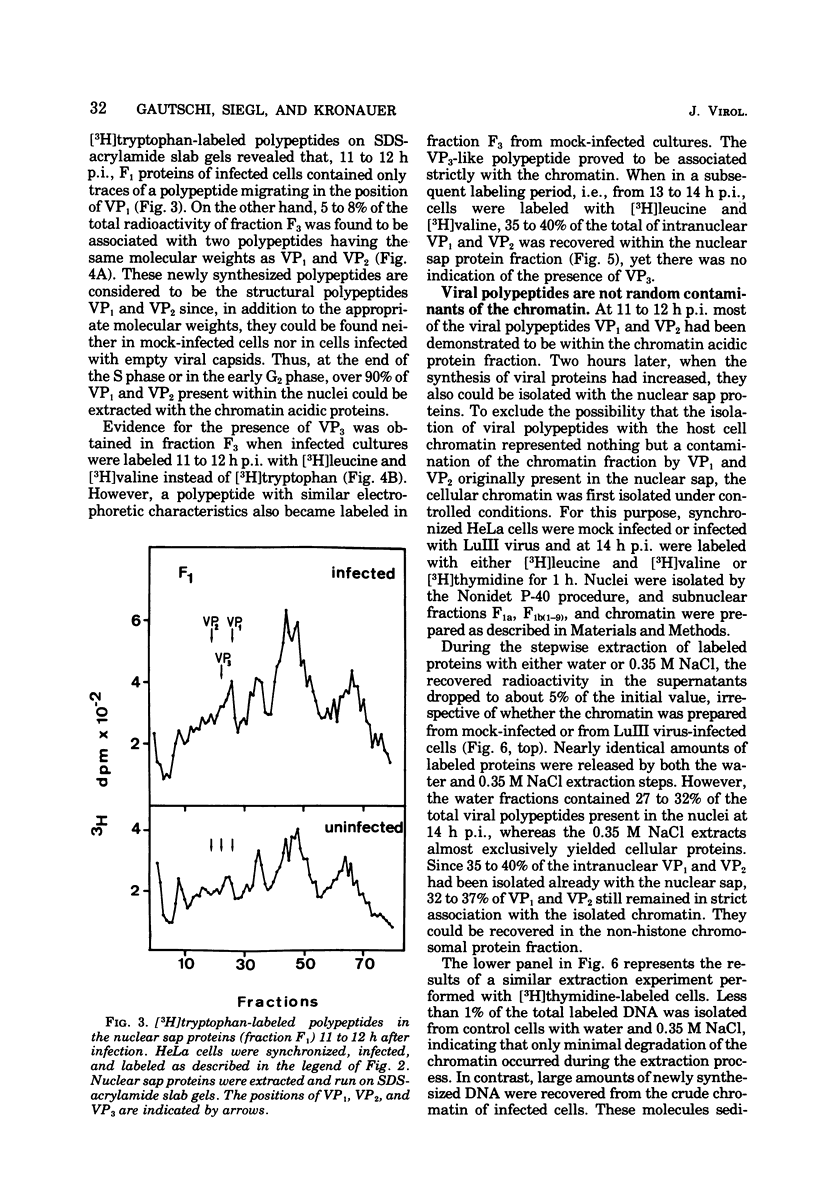
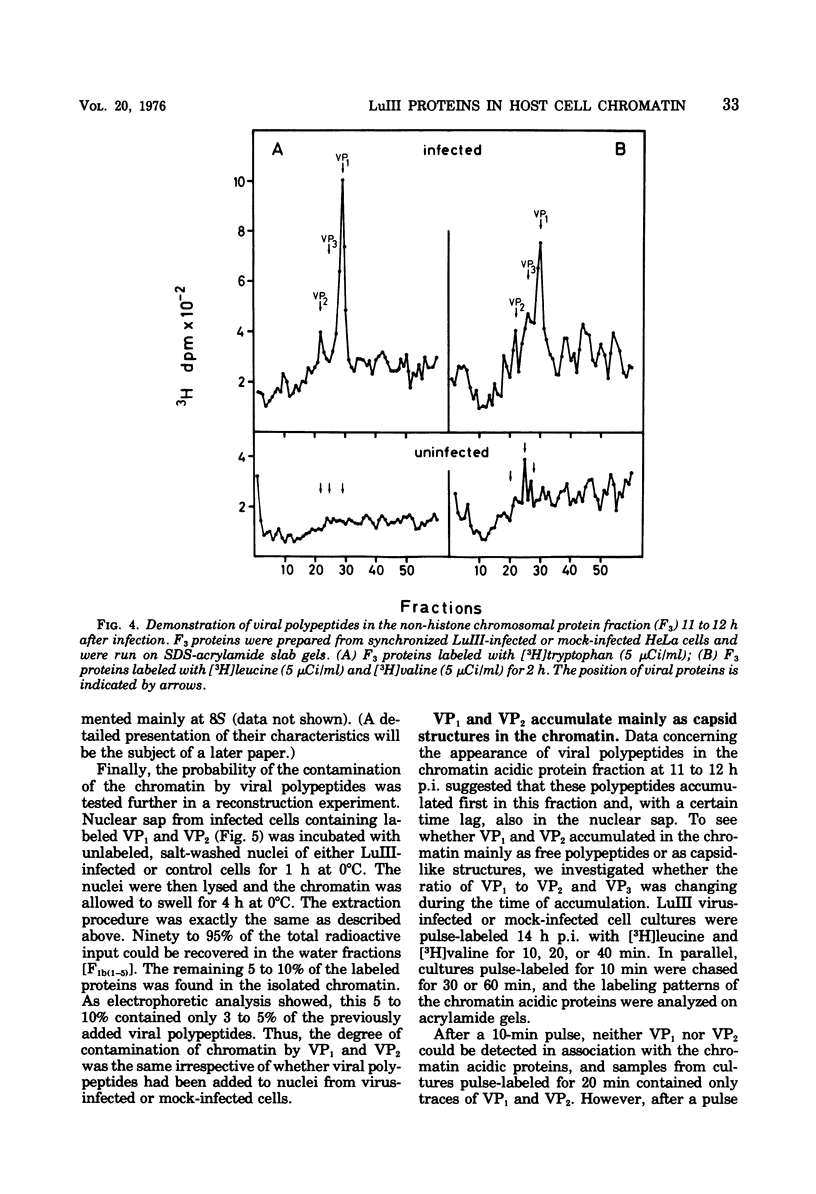
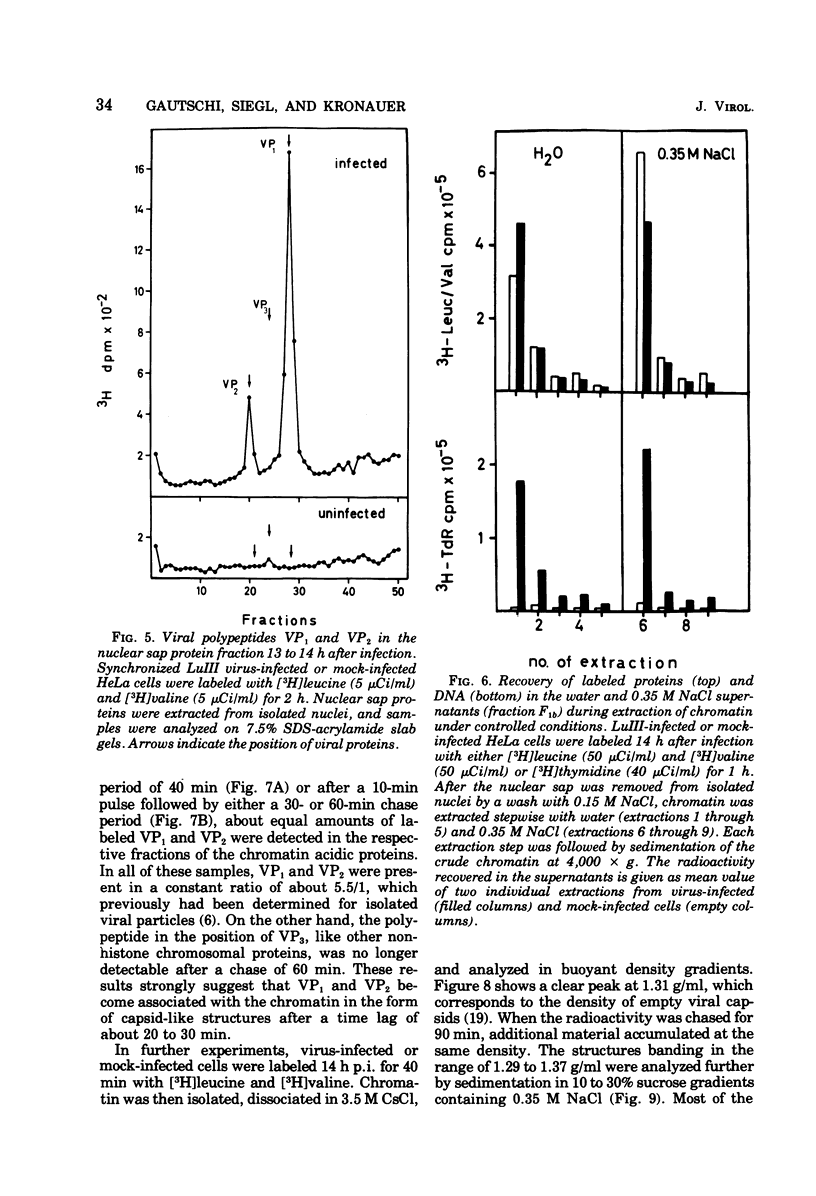
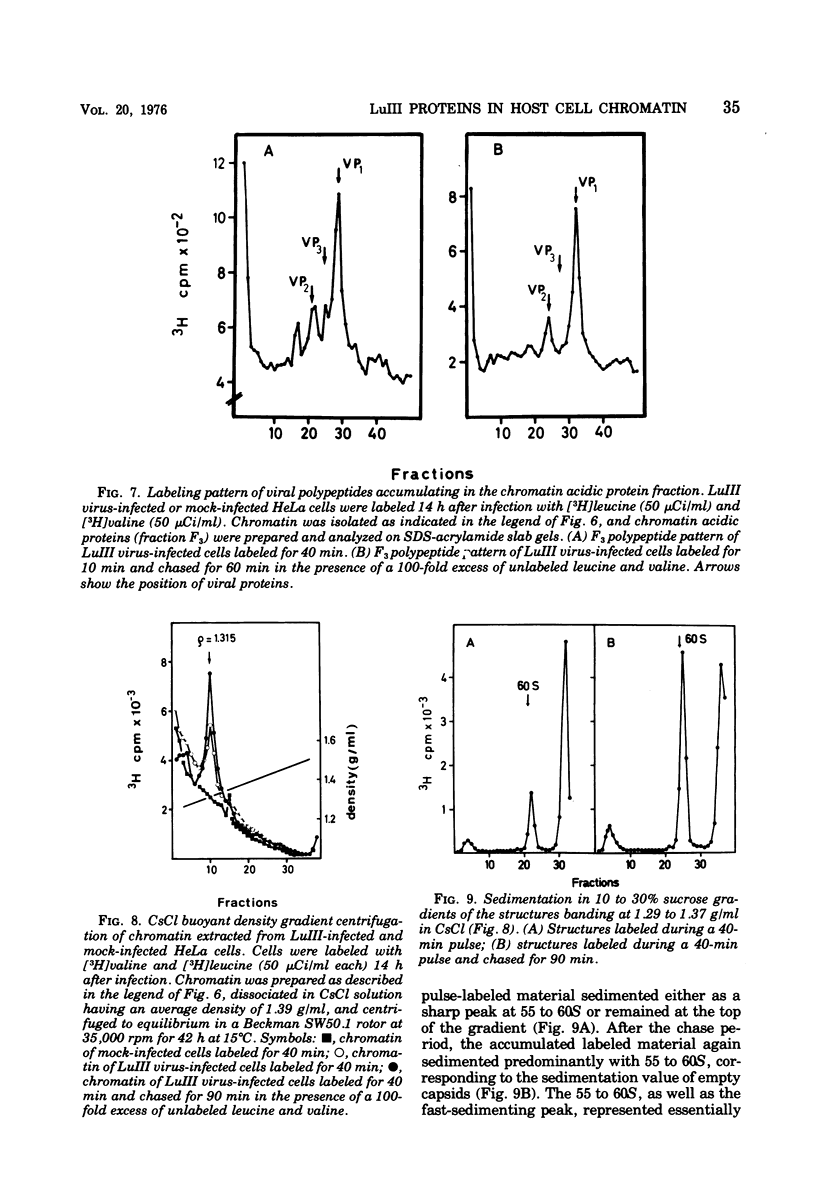
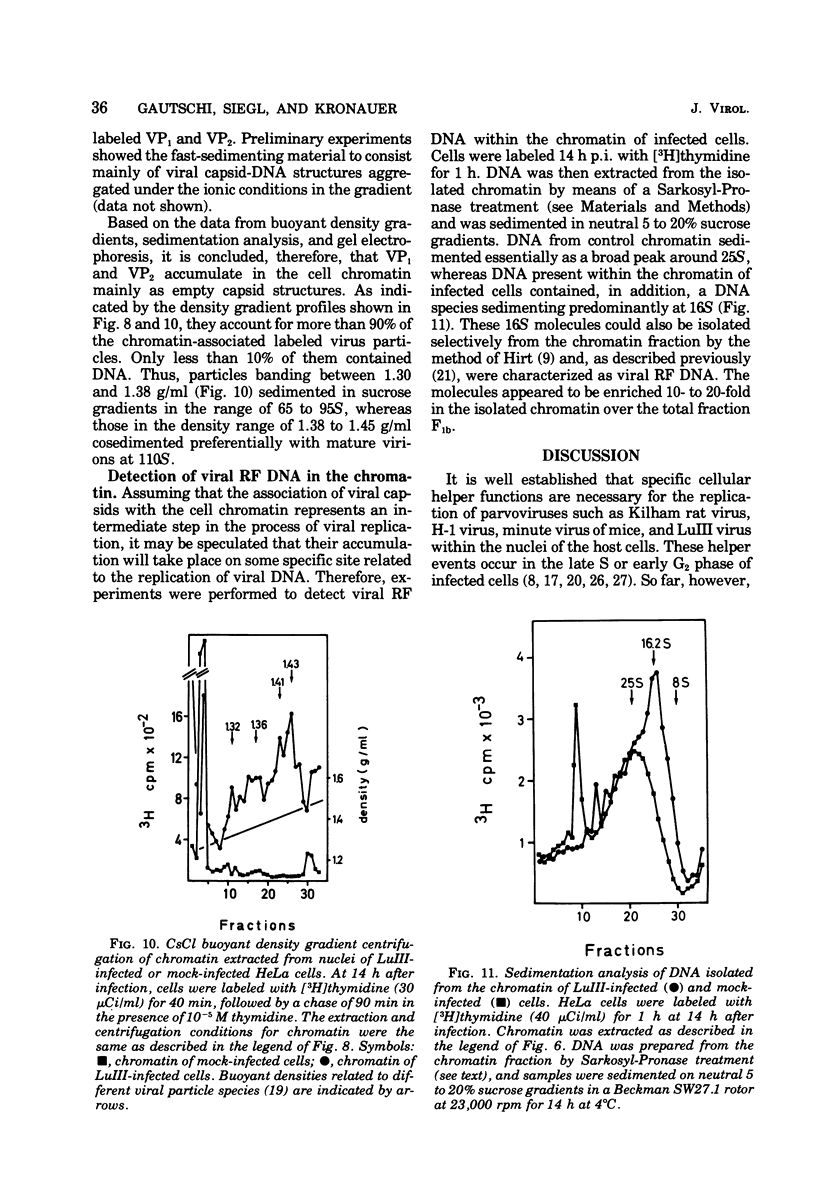
Newly synthesized structural polypeptides of parvovirus LuIII, VP1 (62,000 daltons) and VP2 (74,000 daltons), were detected in nuclei of synchronized, infected HeLa cells at 11 to 12 h postinfection, i.e., after cells had passed through the S phase of the cell cycle. At this time, most of intranuclear viral polypeptides were associated with the chromatin acidic proteins. However, 13 to 14 h postinfection, about one-third of intranuclear VP1 and VP2 also could be extracted in the fraction containing nuclear sap proteins. According to pulse-chase experiments, VP1 and VP2 accumulated in the chromatin with a time lag of 20 to 30 min. About 90% of these chromatin-associated viral polypeptides represented empty viral capsids. In addition, chromatin prepared at 14 h postinfection contained 90 to 95% of the total intranuclear viral 16S replicative-form DNA. Since viral replicative-form DNA and empty viral capsids seem to be associated specifically with cellular chromatin, we assume that this subnuclear structure is the site of the synthesis of progeny viral DNA and the formation of complete virions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhorjee J. S., Pederson T. Nonhistone chromosomal proteins in synchronized HeLa cells. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3345–3349. doi: 10.1073/pnas.69.11.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Fujitani H., Holoubek V. Similarity of the 0.35 M NaCl soluble nuclear proteins and the nonhistone chromosomal proteins. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1300–1305. doi: 10.1016/0006-291x(73)91129-7. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Clarkson J. M. Discontinuous DNA replication in mouse P-815 cells. Eur J Biochem. 1975 Jan 2;50(2):403–412. doi: 10.1111/j.1432-1033.1975.tb09816.x. [DOI] [PubMed] [Google Scholar]
- Gautschi M., Siegl G. Structural proteins of parvovirus Lu 3. Evidence for only two protein components within infectious virions. Arch Gesamte Virusforsch. 1973;43(4):326–333. [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. The metabolism of histone fractions. IV. Synthesis of histones during the G1-phase of the mammalian life cycle. Arch Biochem Biophys. 1972 Feb;148(2):633–641. doi: 10.1016/0003-9861(72)90182-8. [DOI] [PubMed] [Google Scholar]
- Hampton E. G. H-1 virus growth in synchronized rat embryo cells. Can J Microbiol. 1970 Apr;16(4):266–268. doi: 10.1139/m70-049. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johns E. W., Forrester S. Studies on nuclear proteins. The binding of extra acidic proteins to deoxyribonucleoprotein during the preparation of nuclear proteins. Eur J Biochem. 1969 Apr;8(4):547–551. doi: 10.1111/j.1432-1033.1969.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K. The acid extraction of histones from calf thymus deoxyribonucleoprotein. J Mol Biol. 1966 Feb;15(2):409–419. doi: 10.1016/s0022-2836(66)80116-x. [DOI] [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Tegtmeyer P. Synthesis and assembly of simian virus 40. II. Synthesis of the major capsid protein and its incorporation into viral particles. J Virol. 1972 Jan;9(1):52–60. doi: 10.1128/jvi.9.1.52-60.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. Multiplication of parvovirus LuIII in a synchronized culture system. III. Replication of viral DNA. J Virol. 1976 Mar;17(3):841–853. doi: 10.1128/jvi.17.3.841-853.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Siegl G. Physicochemical characteristics of the DNA of parvovirus Lu 3. Arch Gesamte Virusforsch. 1973;43(4):334–344. doi: 10.1007/BF01556150. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Toolan H. W. Ultrastructural studies of H-1 parvovirus replication. I. Cytopathology produced in human NB epithelial cells and hamster embryo fibroblasts. Virology. 1975 May;65(1):40–54. doi: 10.1016/0042-6822(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Matthews D. E. Nonhistone chromosomal protein synthesis: utilization of preexisting and newly transcribed messenger RNA's. Science. 1973 Jul 6;181(4094):71–73. doi: 10.1126/science.181.4094.71. [DOI] [PubMed] [Google Scholar]
- Sundquist B., Everitt E., Philipson L., Hoglund S. Assembly of adenoviruses. J Virol. 1973 Mar;11(3):449–459. doi: 10.1128/jvi.11.3.449-459.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Roblin R., Dulbecco R. Protein synthesis in Simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1972 Apr;69(4):921–924. doi: 10.1073/pnas.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]


