Abstract
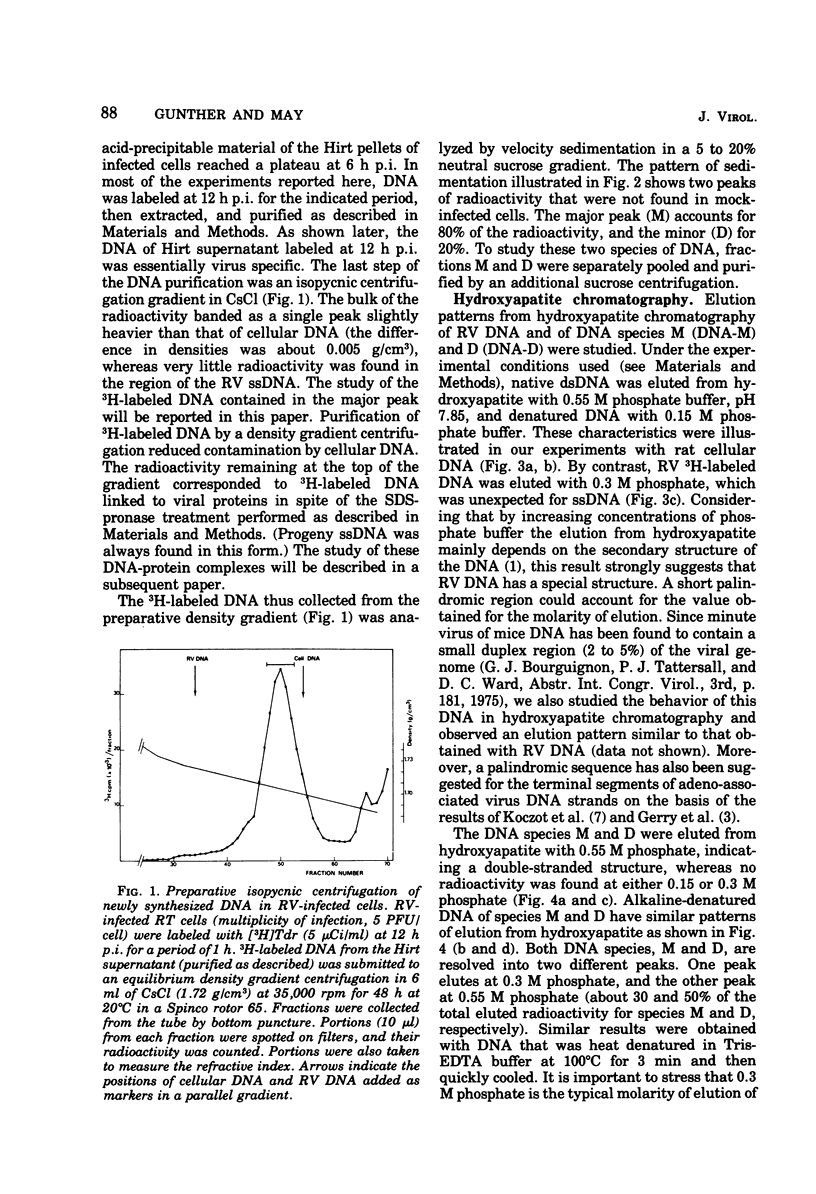
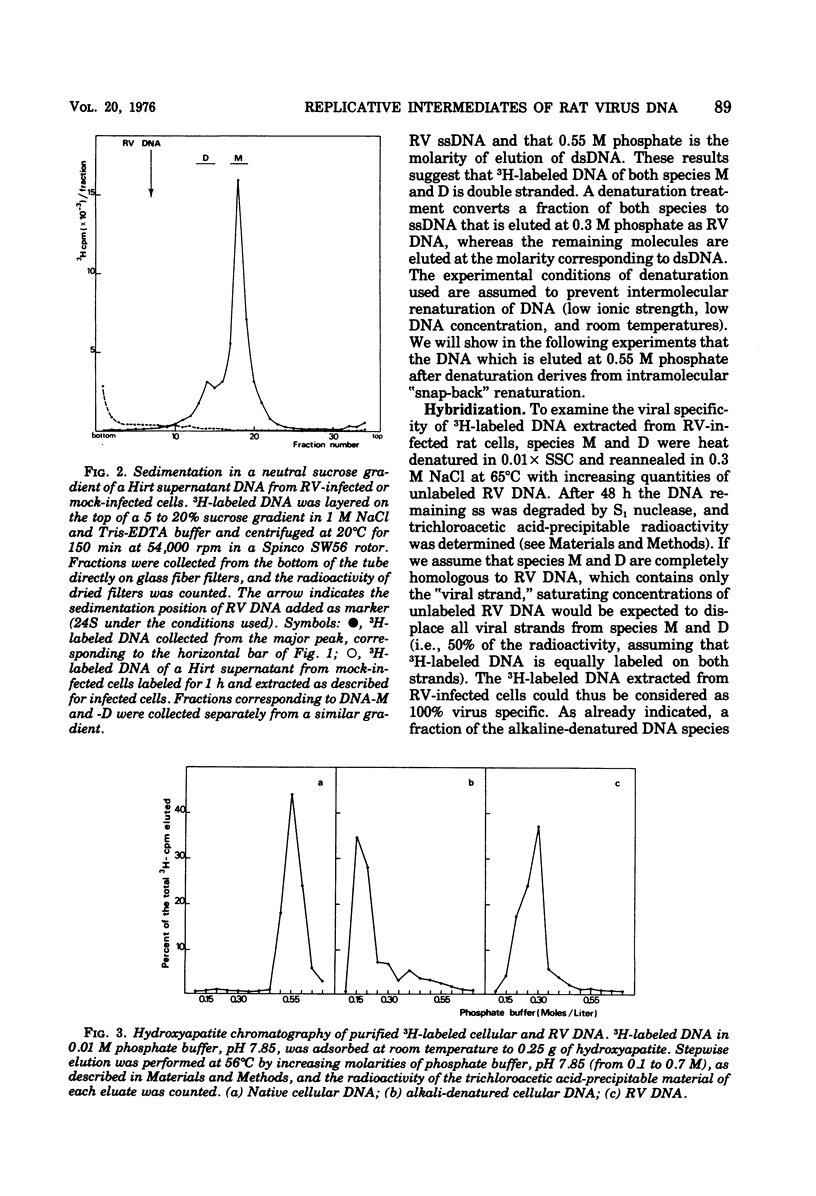
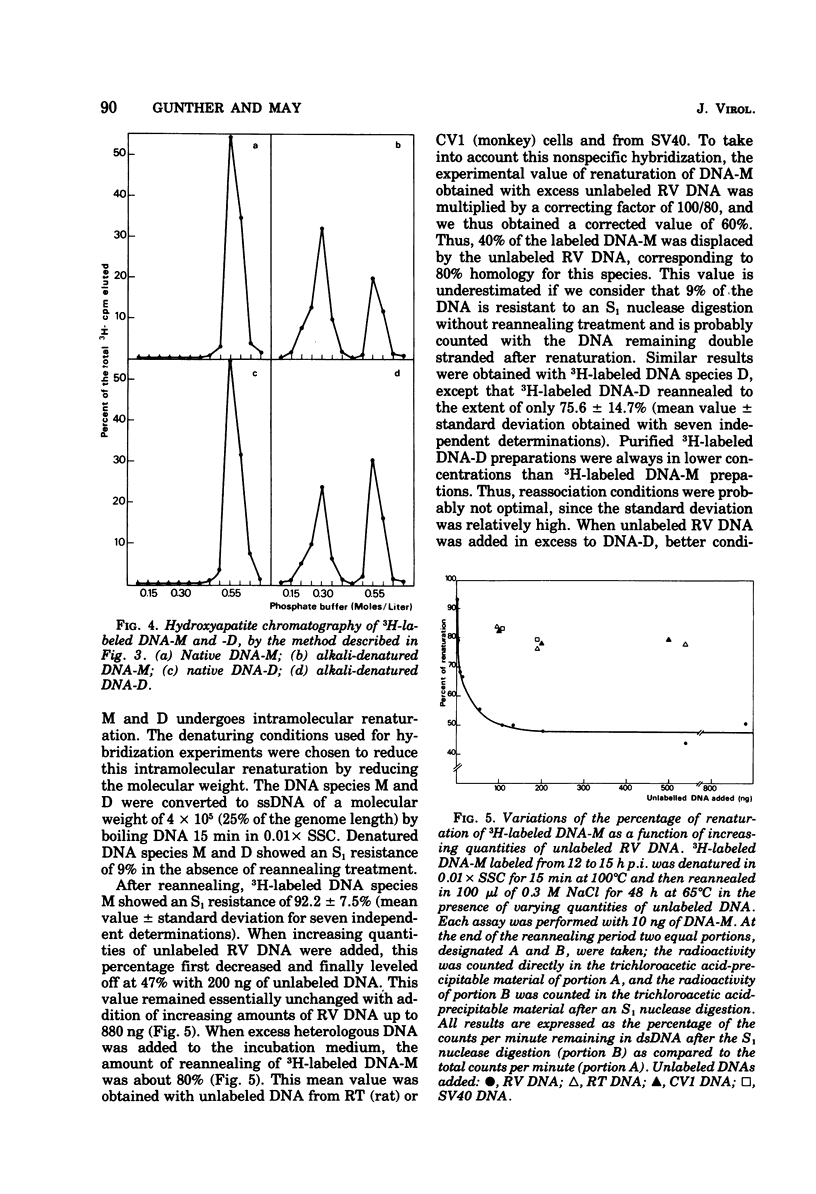
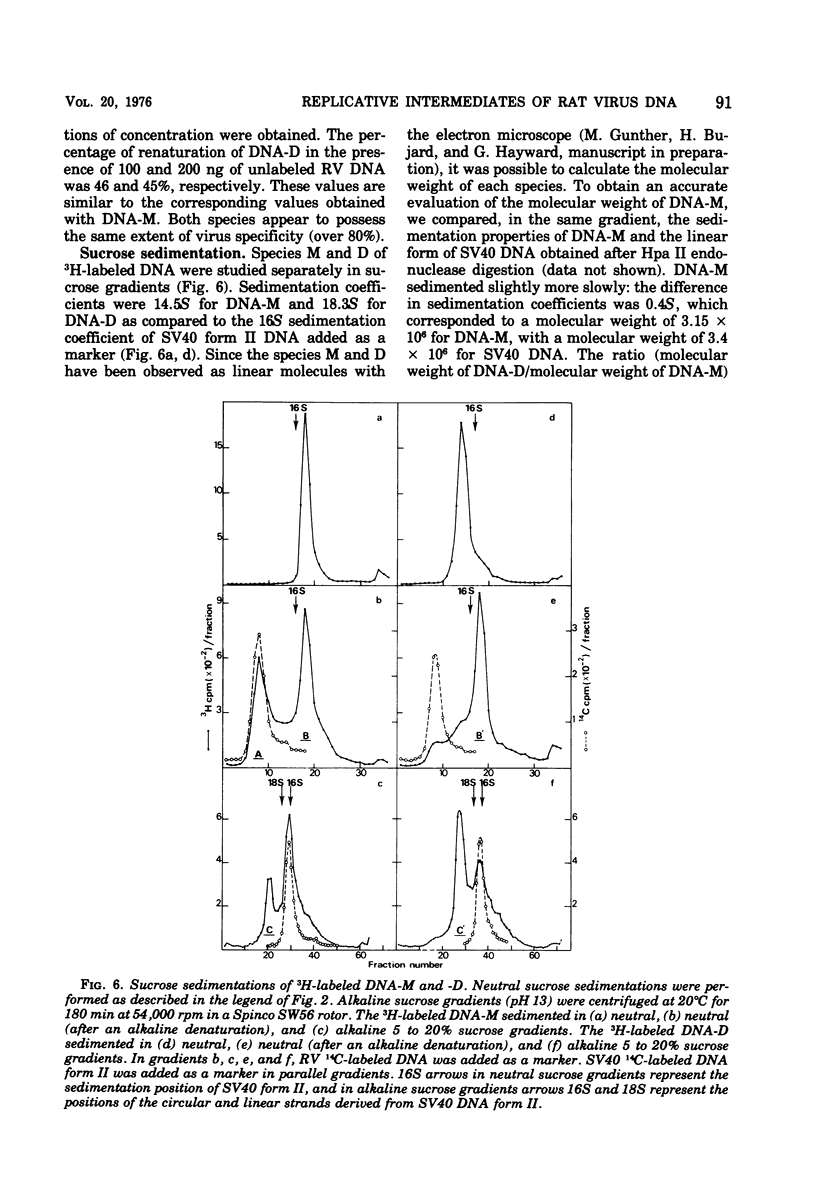
Two virus-specific species of newly synthesized DNA were isolated from rat fibroblast cell cultures infected with the Kilham rat virus (RV). These two DNA species were purified; their behavior on hydroxyapatite chromatography and their sedimentation coefficients in sucrose gradients were determined. One of the two species corresponds to the linear double-stranded form of the RV DNA, and the other corresponds to the dimeric duplex form. After denaturation, a fraction of both species showed an intramolecular renaturation; these molecules are composed of viral strand covalently linked to complementary strand. Models for the structure of both species are posposed. Both species may be considered as double-strand replicative intermediates of the single-stranded RV DNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. Nature. 1965 May 22;206(4986):779–783. doi: 10.1038/206779a0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Hallauer C., Kronauer G., Siegl G. Parvoiruses as contaminants of permanent human cell lines. I. Virus isolation from 1960-1970. Arch Gesamte Virusforsch. 1971;35(1):80–90. doi: 10.1007/BF01249755. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- KILHAM L., OLIVIER L. J. A latent virus of rats isolated in tissue culture. Virology. 1959 Apr;7(4):428–437. doi: 10.1016/0042-6822(59)90071-6. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., May E. The DNA of kilham rat virus. J Gen Virol. 1970 Mar;6(3):437–439. doi: 10.1099/0022-1317-6-3-437. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. M., Hetrick F. M. Single-stranded DNA from the Kilham rat virus. J Gen Virol. 1969 Mar;4(2):269–281. doi: 10.1099/0022-1317-4-2-269. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Jori L. A. Characterization of the Kilham rat virus. J Virol. 1970 Feb;5(2):114–122. doi: 10.1128/jvi.5.2.114-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., Kakefuda T. Linear, single-stranded deoxyribonucleic acid isolated from Kilham rat virus. J Virol. 1971 Jun;7(6):830–835. doi: 10.1128/jvi.7.6.830-835.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. In vivo conversion of the single-stranded DNA of the kilham rat virus to a double-stranded form. J Virol. 1973 Feb;11(2):299–305. doi: 10.1128/jvi.11.2.299-305.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H., Monier M. N., Shaool D., Harel J. Distribution of repetitious sequences in chick nuclear DNA. Nucleic Acids Res. 1974 Feb;1(2):309–322. doi: 10.1093/nar/1.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Crawford L. V., Shatkin A. J. Replication of the parvovirus MVM. II. Isolation and characterization of intermediates in the replication of the viral deoxyribonucleic acid. J Virol. 1973 Dec;12(6):1446–1456. doi: 10.1128/jvi.12.6.1446-1456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Hand R. E., Jr Requirement of cellular synthesis for Kilham rat virus replication. Virology. 1970 Dec;42(4):1054–1063. doi: 10.1016/0042-6822(70)90353-3. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]


