Colony-stimulating factor 1 and IL-34 protect against and partially reverse neurodegeneration in mice in part via promoting CREB signaling.
Abstract
Colony-stimulating factor 1 (CSF1) and interleukin-34 (IL-34) are functional ligands of the CSF1 receptor (CSF1R) and thus are key regulators of the monocyte/macrophage lineage. We discovered that systemic administration of human recombinant CSF1 ameliorates memory deficits in a transgenic mouse model of Alzheimer’s disease. CSF1 and IL-34 strongly reduced excitotoxin-induced neuronal cell loss and gliosis in wild-type mice when administered systemically before or up to 6 h after injury. These effects were accompanied by maintenance of cAMP responsive element–binding protein (CREB) signaling in neurons rather than in microglia. Using lineage-tracing experiments, we discovered that a small number of neurons in the hippocampus and cortex express CSF1R under physiological conditions and that kainic acid–induced excitotoxic injury results in a profound increase in neuronal receptor expression. Selective deletion of CSF1R in forebrain neurons in mice exacerbated excitotoxin-induced death and neurodegeneration. We conclude that CSF1 and IL-34 provide powerful neuroprotective and survival signals in brain injury and neurodegeneration involving CSF1R expression on neurons.
CSF1, also known as M-CSF, regulates the survival, proliferation, differentiation, and chemotaxis of cells of the monocyte/macrophage lineage (Pixley and Stanley, 2004; Hamilton, 2008). It is produced by multiple cell types, including monocytes/macrophages, endothelial cells, fibroblasts, and bone marrow stromal cells (Pixley and Stanley, 2004; Chitu and Stanley, 2006; Hamilton, 2008). The biological effects of CSF1 are mediated by a single CSF1 receptor (CSF1R), which is encoded by the c-fms proto-oncogene (Sherr et al., 1985). The receptor is largely restricted to mononuclear phagocytes, although it has been detected on oocytes, trophoblasts, and certain lymphocytes (Pixley and Stanley, 2004; Chitu and Stanley, 2006; Hamilton, 2008). Ligand binding to CSF1R in macrophages triggers multiple signal transduction pathways resulting in activation of AKT and cAMP responsive element–binding protein (CREB) and mitogen-activated protein kinase (Hamilton, 1997; Pixley and Stanley, 2004).
The importance of CSF1 has been demonstrated by studies with the CSF1-null mutant osteopetrotic (Csf1op/op) mouse (Wiktor-Jedrzejczak et al., 1990; Yoshida et al., 1990). Homozygous Csf1op/op mice lack functional CSF1 and display pleiotropic phenotypes, including osteopetrosis and reduced numbers of tissue macrophages. These phenotypes are rescued by expression of a CSF1 transgene (Ryan et al., 2001), confirming that the absence of CSF1 is responsible for the abnormalities. Furthermore, targeted ablation of CSF1R largely recapitulates the pathology seen in Csf1op/op mice (Dai et al., 2002), indicating that the effects of CSF1 are mediated by this single receptor. More recently, IL-34 was identified as a second ligand for CSF1R (Lin et al., 2008), but little is known about its biology and capacity to substitute for CSF1. In cultured macrophages, IL-34 shows an equivalent ability to support cell growth and survival as CSF1; however, it may interact with distinct regions of CSF1R (Garceau et al., 2010) and initiate different biological activities and signal activation (Chihara et al., 2010; Wei et al., 2010; Liu et al., 2012).
CSF1 levels are increased in brains from patients with Alzheimer’s disease (AD), HIV-1 encephalitis, or brain tumors and in several experimental brain injury models (Imai and Kohsaka, 2002; Chitu and Stanley, 2006), but few studies have dissected the functional role of this factor in central nervous system (CNS) disease. Consistent with its role in regulating the monocyte/macrophage lineage, CSF1R is expressed in microglia in the CNS (Raivich et al., 1998), and CSF1 is crucial for maturation of these cells (Imai and Kohsaka, 2002). Indeed, CSF1-null Csf1op/op mice have fewer microglia and show impaired microglial activation in response to injury (Berezovskaya et al., 1995). CSF1R expression is enhanced in activated microglia surrounding plaques in AD and in transgenic mouse models of AD (Murphy et al., 2000), and brain CSF1 levels were reported to be higher in AD compared with nondemented controls (Du Yan et al., 1997). Although these studies imply that CSF1 may have a role in CNS function and disease, its mode of action remains unclear. Because CSF1 synergizes with fibrillar Aβ to induce neurotoxicity in co-cultures of microglia with primary neurons (Li et al., 2004), CSF1 has been suggested to promote detrimental inflammatory processes in the AD brain (Murphy et al., 1998) and in a mouse model of amyotrophic lateral sclerosis (Gowing et al., 2009). In contrast, CSF1 enhances survival of Purkinje cells and cortical neurons cultured in serum-free medium (Murase and Hayashi, 1998; Wang et al., 1999) and reduces N-methyl-d-aspartic acid (NMDA)–induced neuronal cell death in hippocampal slices (Vincent et al., 2002). Recombinant CSF1 delivered in implanted microcapsules in the peritoneum protects against ischemic injury (Berezovskaya et al., 1996). Similarly, in an AD mouse model, treatment with recombinant CSF1 improved cognitive performance (Boissonneault et al., 2009). This opens the question of whether CSF1 has neuroprotective functions and targets cells other than microglia in the CNS. In support of this notion, CSF1R mRNA has been detected in cultured astrocytes, oligodendrocytes, and neurons using RT-PCR and in situ hybridization (Sawada et al., 1993; Murase and Hayashi, 1998; Wang et al., 1999). However, the presence of CSF1R protein in these cells in vivo has not been demonstrated conclusively, and results from immunohistochemical studies are inconsistent and contradictory (Murase and Hayashi, 1998; Raivich et al., 1998; Wang et al., 1999; Murphy et al., 2000). Furthermore, no functional data supporting an in vivo role of CSF1R in neurons are available. In spite of cell culture studies showing CSF1 production in different CNS cells, the source of CSF1 in the CNS has not been unequivocally located.
Using CSF1R reporter mice and genetic deletion of CSF1R in neurons, we demonstrate a critical protective and survival function of this receptor in neurons in a model of excitotoxic neurodegeneration. Importantly, systemic CSF1 and IL-34 administration is sufficient to exert similar beneficial effects even hours after the neuronal insult, supporting their potential importance in recovery from neuronal injury.
RESULTS
Systemic administration of CSF1 improves cognitive function in human amyloid precursor protein (hAPP) transgenic mice, independent of Aβ pathology
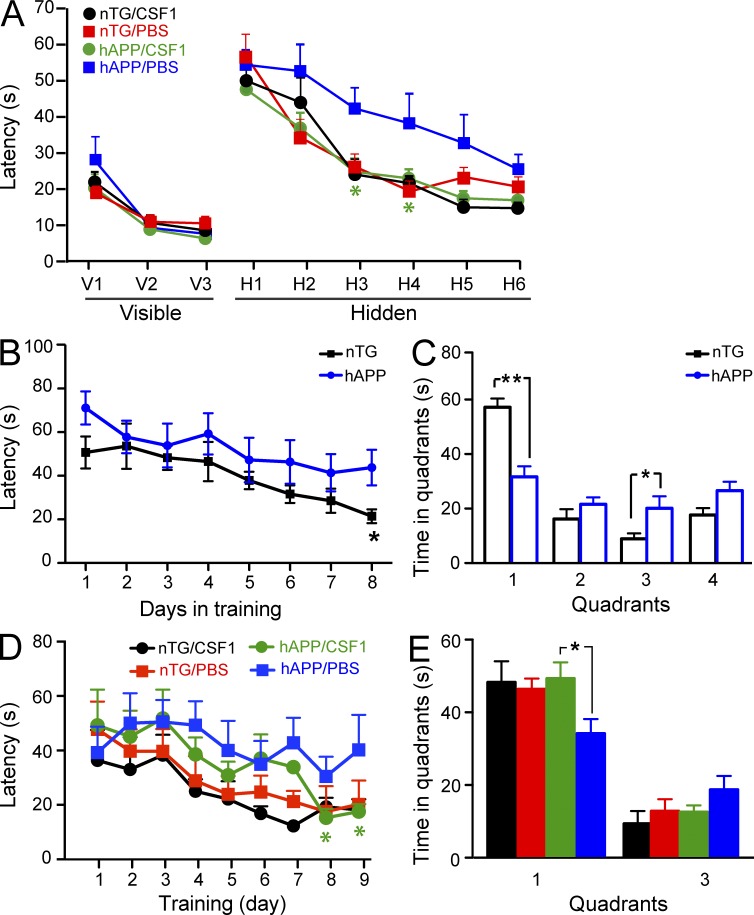
We have previously reported that CSF1 was reduced in AD plasma (Ray et al., 2007; Britschgi et al., 2011), and CSF1 has been shown to reduce AD-like disease in mice (Boissonneault et al., 2009). To better understand the role of CSF1 in neurodegeneration, we injected human CSF1 or PBS i.p. into 6-mo-old hAPP transgenic mice (Rockenstein et al., 2001) and their nontransgenic (nTG) littermates at a dose of 800 µg/kg body weight, similar to what has been used clinically in bone marrow transplantation patients (Nemunaitis et al., 1993). After 10 wk of treatment, mice were assessed for learning and memory function using the Morris water maze. CSF1-treated hAPP mice showed significantly better behavioral outcomes than PBS-injected hAPP mice, as indicated by shorter escape latencies in the hidden platform tests (Fig. 1 A). In addition, to determine whether CSF1 could improve memory function in animals already exhibiting behavioral deficits (Fig. 1, B and C) and whether a shorter period of treatment exerts similar effects, we injected CSF1 or PBS into 18–20-mo-old hAPP and nTG mice for 4 wk. CSF1 treatment significantly reduced memory deficits in hAPP mice in the hidden platform test (Fig. 1 D) and the probe trial (Fig. 1 E). Thus, CSF1 treatment ameliorates learning and memory deficits in hAPP transgenic mice.
Figure 1.
Systemic CSF1 improves cognitive function in hAPP transgenic mice. (A) hAPP transgenic mice and their nTG littermates (n = 9–10 mice per genotype, age 5.5–6.5 mo) were injected with 800 µg/kg CSF1 or PBS three times a week. After 10 wk of treatment, spatial cognitive function in mice was assessed using the Morris water maze. (B–E) hAPP mice and nTG littermates (18–20 mo old) were assessed by water maze in hidden platform tests (B) and a probe trial 24 h later (C). The mice were then randomly divided into CSF1 or PBS groups (n = 6–8 mice per genotype). After 1 mo of treatment, mice were tested again with a water maze hidden platform test (D) and a probe trial (E). The target quadrant was quadrant 1 in C and E. Bars are mean ± SEM. *, P < 0.05; **, P < 0.01 compared by ANOVA and Bonferroni post-hoc test. Each experiment was performed once.
To determine whether CSF1 exerts its effect by altering Aβ accumulation or aggregation in the brain, we measured Aβ levels by immunoreactivity and amyloid by thioflavin S staining (sections from the 6-mo-old hAPP mice that were treated with CSF1 for 10 wk, as shown in Fig. 1 A). No significant differences were observed in the hippocampus in either of these measures between CSF1- or PBS-treated hAPP mice (percentage of area occupied by Aβ immunoreactivity [anti–Aβ1-5] was 4.670 ± 0.811% in CSF1-treated hAPP mice vs. 4.141 ± 0.874% in PBS-injected group, P = 0.333 by Student’s t test; percentage of area covered by thioflavin S was 0.703 ± 0.143% in CFS1-treated and 0.792 ± 0.146% in PBS-injected animals, P = 0.335 by Student’s t test). Furthermore, we measured both soluble and insoluble levels of Aβ1-x and Aβ1-42 by ELISA and observed no significant changes in hippocampus or cortex of hAPP mice after CSF1 treatment (not depicted). These findings show that in contrast to previous findings in another AD mouse model (Boissonneault et al., 2009), the beneficial effects of CSF1 on cognitive function in the hAPP mice tested here are likely independent of Aβ accumulation.
Systemic administration of CSF1 reduces kainic acid (KA)–induced neuroinflammation and neurodegeneration
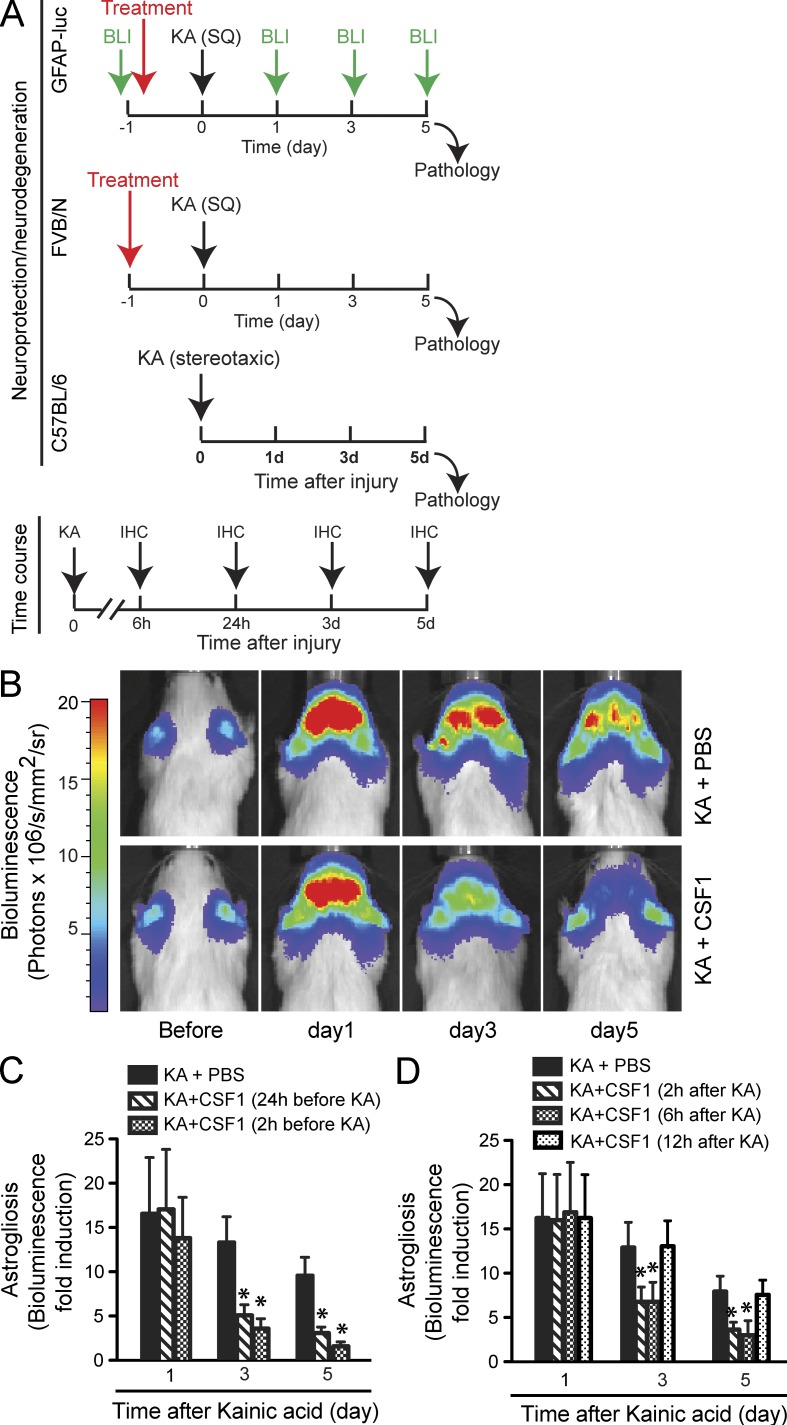
Because CSF1 did not seem to affect Aβ load in hAPP mice, we asked whether it might exert protective effects on neurons and thereby ameliorate cognitive deficits. To explore this possibility, we used a model of excitotoxicity, which is thought to represent a common pathway of neuronal demise and has been implicated in various neurodegenerative diseases including AD (Mattson, 2004). We administered CSF1 systemically in a mouse model of KA-induced excitotoxicity. To induce consistent excitotoxic neurodegeneration, KA was administered either systemically (subcutaneously) in FVB/N mice or stereotaxically into the hippocampus in C57BL/6 mice (Fig. 2 A). We first used bioluminescence imaging, which enabled us to follow astrocyte activation (astrogliosis) and neurodegeneration in reporter mice expressing luciferase under the control of a glial fibrillary acidic protein (GFAP) promoter (GFAP-luc mice; Zhu et al., 2004; Luo et al., 2007). Neuronal injury is closely tied to astrogliosis, and KA-induced bioluminescence in GFAP-luc mice correlates significantly with hippocampal cell death (Zhu et al., 2004). We used this technique to take advantage of testing a relatively large number of potentially therapeutic conditions in a medium throughput manner while studying individual mice throughout the course of injury (Fig. 2 A). KA lesion (subcutaneous administration) in the reporter mice led to a reproducible, significant increase in bioluminescence in the brain (Fig. 2, B and C). Notably, systemic CSF1 pretreatment (800 µg/kg body weight) at 24 or 2 h before KA administration significantly inhibited astrogliosis at days 3 and 5 (Fig. 2, B and C). To determine the potential clinical relevance of our findings, we tested whether systemic administration of CSF1 could be used to reduce neurodegeneration after an injurious insult had occurred. Mice receiving CSF1 (800 µg/kg body weight) at 2 or 6 h (but not 12 h) after KA showed similar and significant reduction of astrogliosis (Fig. 2 D).
Figure 2.
Systemic administration of CSF1 inhibits KA-induced astrogliosis in reporter mice. (A) Experimental design and injury models of excitotoxicity. To induce excitotoxic neurodegeneration, KA was administered systemically (subcutaneously, SQ) in FVB/N and stereotaxically into the hippocampus in C57BL/6 mice. Neurodegeneration was analyzed 5 d later. To determine the effective time window of CSF1 and IL-34 application, CSF1 and IL-34 (single-bolus) were administered at different time points in relation to KA injury, 2 and 24 h before and 2, 6, and 12 h after KA administration. An example of one treatment (24 h before) is shown (top two panels). To study the time course of expression of endogenous CSF1 or its receptor, CSF1R, mice were analyzed at 6 h, 24 h, 3 d, and 5 d (bottom). BLI, bioluminescence imaging. (B–D) GFAP-luc mice (FVB/N background, 2 mo old) were lesioned with 20 mg/kg KA, and bioluminescence was recorded longitudinally at the indicated time points in each mouse. (B) Representative images from two independent experiments showing increased bioluminescence signals over the brain after KA injury (top) and the reduction by CSF1 treatment (applied 24 h before KA; bottom). (C and D) Bioluminescence is expressed as fold induction over baseline in mice treated with CSF1 at 2 or 24 h before KA (C) or at 2, 6, or 12 h after KA (D). Baseline was measured 1 d before KA administration for each mouse. Bars are mean ± SEM (n = 4–7 mice). *, P < 0.05 compared by ANOVA and Bonferroni post-hoc test. Each experiment (condition) was performed once in D.
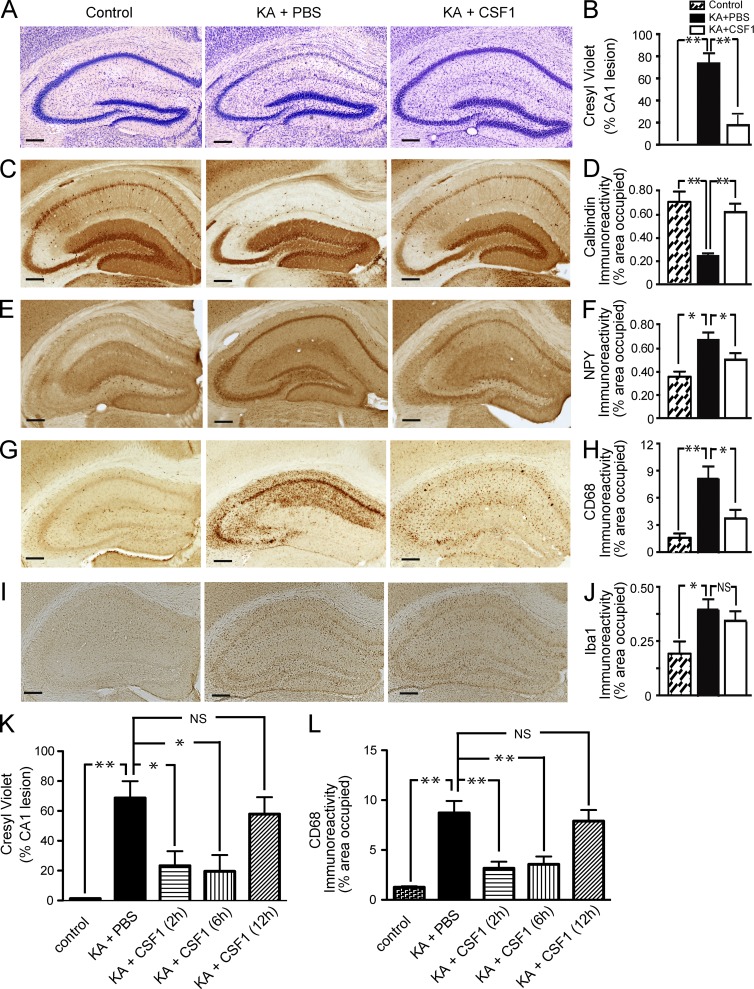
Reduced neurodegeneration with CSF1 treatment was confirmed by pathological analysis of brains, with examples shown for CSF1 applied before or after KA injection (Fig. 3). KA (subcutaneous administration) resulted in significant degeneration of neurons in the pyramidal layer (Fig. 3 A) and reduced hippocampal calbindin immunoreactivity (Fig. 3 C) upon postmortem pathological examination, consistent with a previous study (Luo et al., 2006). In contrast, mice injected i.p. with recombinant human CSF1 24 h before KA showed little hippocampal cell loss (Fig. 3, A and B) and calbindin reduction (Fig. 3, C and D), although they suffered from similar seizure activity (highest seizure score 6.2 ± 1.7 in CSF1-treated group vs. 6.4 ± 1.3 in PBS-treated group). In line with these findings, systemic CSF1 administration significantly reduced the increase in levels of neuropeptide Y (NPY) in the hippocampus associated with KA lesioning (Fig. 3, E and F). Consistent with in vivo imaging, CSF1 administered up to 6 h after KA led to similar and significant reduction of neurodegeneration (Fig. 3, K and L). Thus, systemic administration of CSF1 attenuates KA-induced excitotoxic neurodegeneration and provides significant neuroprotection.
Figure 3.
Systemic CSF1 attenuates KA-induced neurodegeneration and microgliosis. (A–J) 2-mo-old FVB/N mice were lesioned with 20 mg/kg KA (subcutaneous injection) or injected with PBS as control and sacrificed 5 d later. Recombinant 800 µg/kg CSF1 was injected i.p. once 24 h before KA. KA-induced neuronal injury was assessed by cresyl violet staining (A and B), calbindin immunostaining (C and D), and NPY immunostaining (E and F), and microglial activation was assessed by CD68 (G and H) or Iba-1 (I and J) immunostaining. Representative images from three independent experiments are shown from hippocampi of mice: control (nonlesioned, left) or KA lesioned and treated with PBS (middle) or CSF1 (right). Bars, 200 µm. (K and L) Systemic administration of CSF1 after KA lesion reduces neurodegeneration. KA-lesioned mice were treated with CSF1 at 2, 6, and 12 h after injury. The mice were sacrificed at day 5. Excitotoxic injury was assessed by cresyl violet staining (K) and CD68 immunostaining (L). Bars are mean ± SEM (n = 4–7 mice/group). *, P < 0.05; **, P < 0.01 compared by ANOVA and Bonferroni post-hoc test. Similar results were obtained from two independent experiments.
Strong neuroprotective effects of systemically administered IL-34
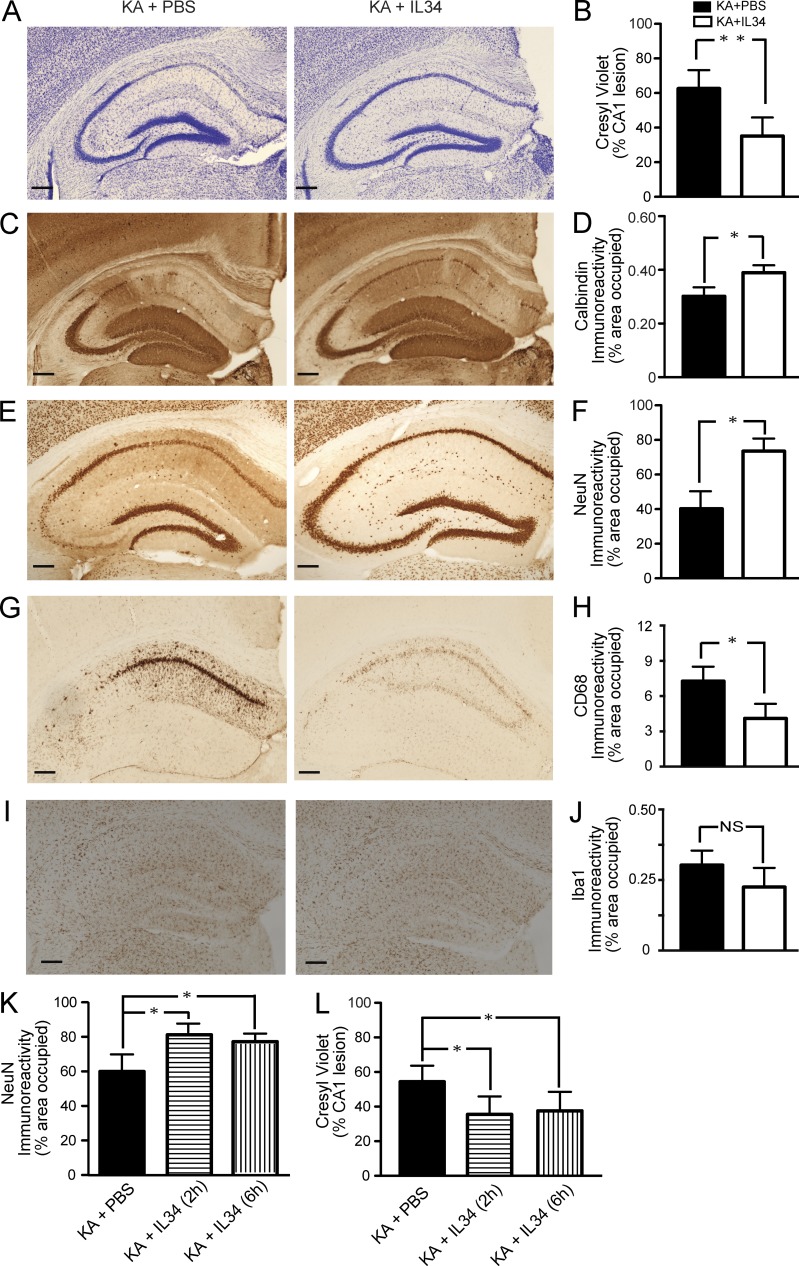
Recently, IL-34 has been identified as a second ligand for CSF1R (Lin et al., 2008). To determine whether IL-34 provides neuroprotection against excitotoxic injury as well we administered recombinant IL-34 (100 µg/kg) systemically in FVB/N mice lesioned by KA (subcutaneous administration). Mice receiving IL-34 showed significantly reduced neuronal cell loss (Fig. 4, A, B, E, F) and calbindin reduction (Fig. 4, C and D) in the pyramidal cell layer of the hippocampus. Interestingly, IL-34 administered 2 or 6 h after KA also provided significant reduction of neurodegeneration (Fig. 4, K and L). These results demonstrate that systemic administration of recombinant IL-34 attenuates excitotoxic injury and provides similar neuroprotection as recombinant CSF1.
Figure 4.
Systemically administered IL-34 attenuates KA-induced neurodegeneration and microgliosis. (A–J) 2-mo-old FVB/N mice were lesioned with 20 mg/kg KA (subcutaneous injection) and sacrificed 5 d later. 100 µg/kg IL-34 was injected i.p. once 2 h before KA. KA-induced neuronal injury was assessed by cresyl violet staining (A and B), calbindin (C and D), and NeuN (E and F) immunostaining, and microglial activation was assessed by CD68 (G and H) or Iba-1 (I and J) immunostaining. Representative images from two independent experiments are shown from hippocampi of mice treated with PBS (left) or IL-34 (right). Bars, 200 µm. Bars in B, D, F, H, and J are mean ± SEM (n = 4 mice/group) from one out of two independent experiments. *, P < 0.05; **, P < 0.01 compared by Student’s t test. (K and L) KA-lesioned mice were treated with IL-34 at 2 and 6 h after injury. The mice were sacrificed at day 5. Excitotoxic injury was assessed by NeuN immunostaining (K) and cresyl violet staining (L). The experiment was performed once. Bars are mean ± SEM (n = 5 mice/group). *, P < 0.05 compared by ANOVA and Bonferroni post-hoc test.
CSF1 inhibits KA-induced microgliosis but does not induce the infiltration of peripheral (myeloid) cells into the brain
CSF1 is the primary regulator of the survival, proliferation, differentiation, and function of cells of the monocyte/macrophage lineage (Pixley and Stanley, 2004; Hamilton, 2008). To investigate how microglia respond to KA injury and to CSF1 treatment, the activation of microglia was analyzed as a function of CD68 expression (Luo et al., 2006). KA injection caused massive activation of microglia in the hippocampus, which was almost completely prevented by i.p. application of CSF1 (Fig. 3, G and H). Similar results were obtained when microglial activation was quantified with an antibody against the activation marker CD11b (immunoreactivity assessed by percentage of occupied area was 3.948 ± 0.1997% in KA/CSF1 vs. 4.664 ± 0.04571% in KA/PBS group, P = 0.001 by Student’s t test). No significant difference was found in immunoreactivity for Iba-1, a marker which seems less sensitive to activation changes in microglia (Fig. 3, I and J; P = 0.569, KA/CSF1 vs. KA/PBS group). Similarly, no significant difference was found in Iba-1 immunoreactivity after IL-34 treatment (Fig. 4, I and J).
To determine whether activated microglia originated from local resident cells or from the periphery and whether CSF1 induced the infiltration of peripheral (myeloid) cells into the brain, we made use of parabiosis in which two mice share a common blood supply after being joined surgically at their flanks (Villeda et al., 2011). Wild-type or hAPP transgenic mice were paired with actin-EGFP transgenic mice (Okabe et al., 1997) and were analyzed for GFP+ cells in the brain 6 wk later. In the control parabionts, which received no KA injury, there were few GFP+ cells in the brain (12.83 ± 2.18 GFP+ cells/section), consistent with previous studies (Ajami et al., 2007; Villeda et al., 2011), and CSF1 treatment did not significantly increase the number of these cells (14.33 ± 2.43 GFP+ cells/section, P > 0.05). Likewise, no significant difference was detected in the numbers of GFP+ cells in KA-injected mice, with or without CSF1 treatment (15.50 ± 2.42 GFP+ cells/section without CSF1 vs. 14.89 ± 3.41 GFP+ cells/section with CSF1, P > 0.05). These results are in agreement with previous publications using different methods (Dommergues et al., 2003) and using mice with a different genetic background (Ajami et al., 2007; Villeda et al., 2011). In addition, similar results were obtained from hAPP parabionts. The number of GFP+ cells in the PBS-injected hAPP brain was 12.33 ± 1.60/section, and that in the CSF1-treated brain was 11.33 ± 2.42/section (P > 0.05). In aggregate, our findings suggest that CSF1 does not exert its protective effects by recruiting peripheral myeloid or other cells to the brain in the hAPP transgenic mice and the 5-d excitotoxicity paradigm.
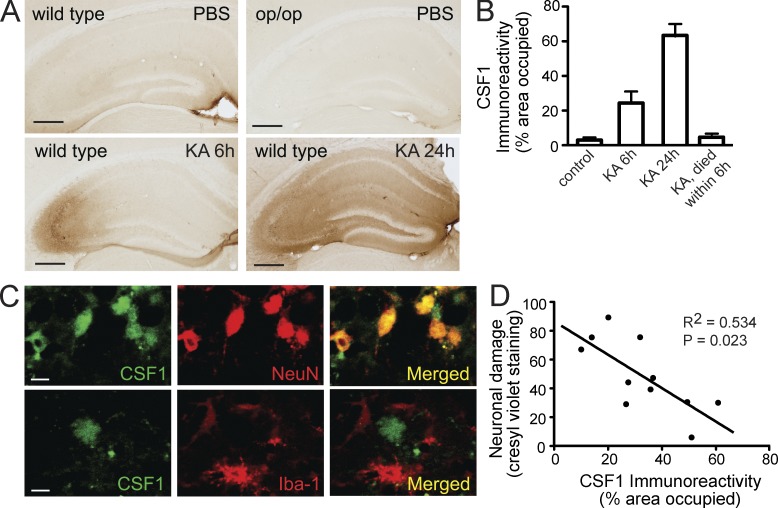
Endogenous CSF1 is up-regulated in neurons after injury
To identify the potential target cell responsible for beneficial effects of CSF1 in the excitotoxicity model, we examined expression of endogenous CSF1 in the uninjured and injured brain. In the uninjured brain, we observed faint CSF1 immunoreactivity throughout the brain, which was absent in CSF1-deficient Csf1op/op mice (Fig. 5 A), thus confirming specificity of the antibody. In contrast, KA administration led to a progressive increase of CSF1 immunoreactivity in the hippocampus, first in CA3 at 6 h and then throughout the hippocampus at 24 h (Fig. 5, A and B). Interestingly, CSF1 immunoreactivity was localized mostly to neurons (Fig. 5 C, 6 h after KA). Although CSF1 expression varied significantly among individual animals, immunoreactivity showed a remarkable inverse correlation with neuronal cell loss at day 3 (R = −0.731, P = 0.023; Fig. 5 D). In agreement with this inverse correlation between CSF1 expression and neurodegeneration, we found a striking lack of CSF1 immunoreactivity in mice that died within 6 h after KA administration and were immediately dissected for analysis (n = 6 mice; Fig. 5 B). Together, these results are consistent with the possibility that up-regulation of local CSF1 in the brain serves to protect neurons from degeneration and cell death.
Figure 5.
Endogenous CSF1 is up-regulated in neurons after excitotoxic brain injury. (A–C) Wild-type FVB/N or CSF1-null Csf1op/op mice (2 mo old) were lesioned with 20 mg/kg KA (subcutaneous injection) and sacrificed 6 h, 1 d, and 3 d later. Brain sections were immunostained with an antibody against CSF1. (A) Representative images from wild-type controls (no injury), KA lesioned and sacrificed at 6 h, KA lesioned and sacrificed at 24 h, or Csf1op/op mice. (B) Quantification of CSF1 immunoreactivity in the hippocampus after KA injury (n = 4–6 mice/group). Error bars indicate SEM. (C) Colocalization of CSF1 and neuronal marker NeuN but not microglial marker (Iba-1) after KA lesion (6 h). Bars: (A) 200 µm; (C) 20 µm. (D) Inverse correlation (Pearson correlation) of CSF1 immunoreactivity with excitotoxic injury assessed by cresyl violet staining. Results are from one out of two independent experiments.
Figure 6.
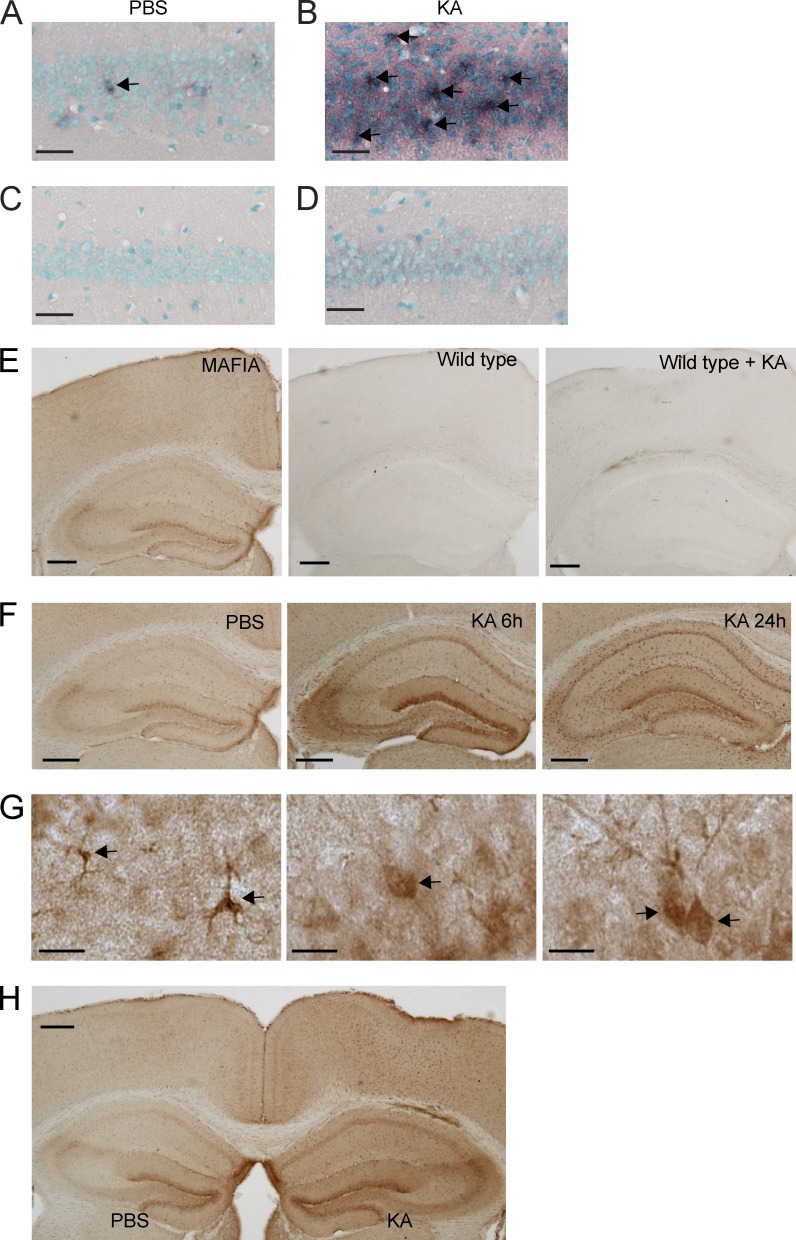
Expression of CSF1R in neurons. (A–D) Detection of Csf1r mRNA in the hippocampus using in situ hybridization. Wild-type FVB/N mice were lesioned with 20 mg/kg KA (subcutaneous injection) or injected with PBS as a control and sacrificed 24 h later. Brain sections were hybridized with DIG-labeled antisense (A and B) or sense (C and D) probes for Csf1r and developed with an anti-DIG antibody. No signal was detected with the sense probe (C and D). Arrows point to cells expressing CSF1R, as detected by in situ hybridization. (E–H) CSF1R reporter mice (MAFIA mice, 2 mo of age) were lesioned with 20 mg/kg KA (subcutaneous injection) or injected with PBS as control and sacrificed 6 and 24 h later. These mice express an EGFP tag under the control of the Csf1r promoter so that the expression of CSF1R can be detected by GFP immunostaining. (E) To test the specificity of the antibody against GFP, brain sections from MAFIA mice (not injured, left), wild-type mice (middle), and wild-type mice with KA injury (right) were immunostained. (F) Representative images of different brain regions from MAFIA mice injected with PBS and sacrificed 24 h later (left) or injected with KA and sacrificed at 6 (middle) or 24 (right). (G) High-magnification images showing GFP immunoreactivity in microglia (left) and neurons (middle and right), determined by morphology. Arrows point to cells expressing CSF1R, as detected by immunohistochemistry for the reporter gene. (H) 2-mo-old MAFIA mice received unilateral stereotaxic injections of 50 ng KA (right hemibrain) or PBS (left hemibrain). Mice were sacrificed 6 h later, and brain sections were analyzed for reporter gene expression by GFP immunostaining. The data are representative of two independent experiments with n = 3 mice/group. Bars: (A–D) 50 µm; (E, F, and H) 200 µm; (G) 10 µm.
CSF1R is expressed in neurons and up-regulated after injury
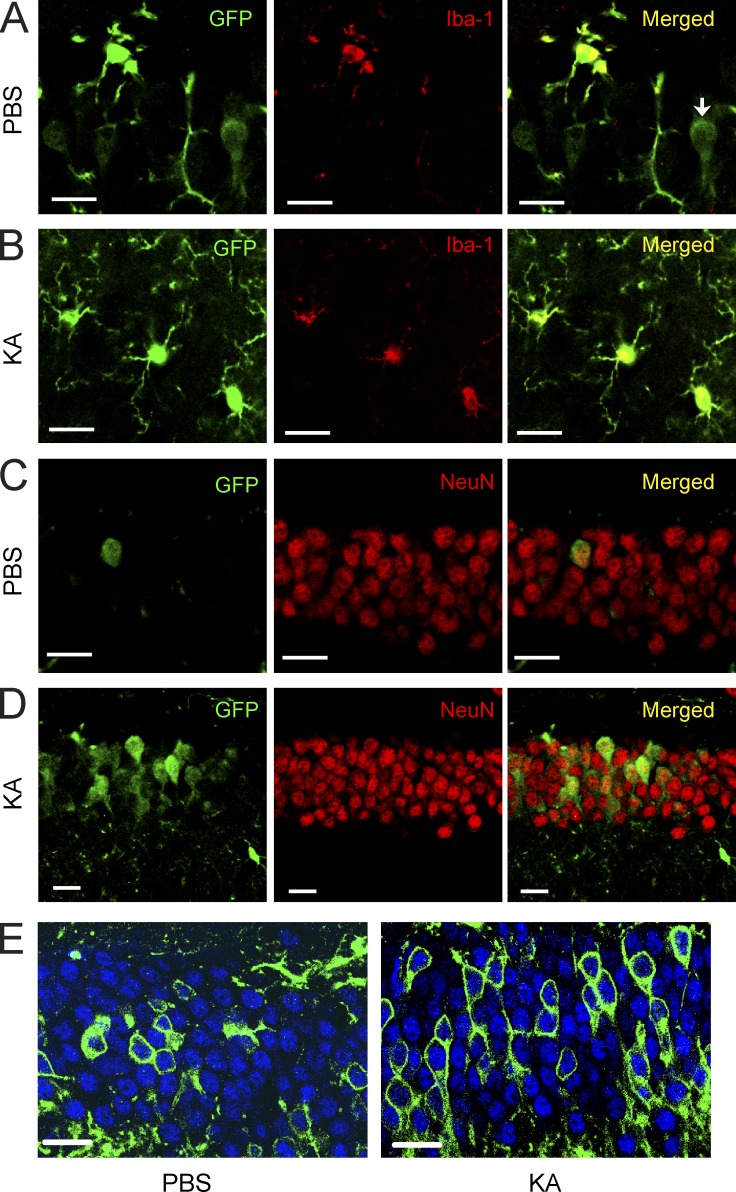
Next, we studied the expression of CSF1R in the brain. We first tested six different commercially available CSF1R antibodies (for a list see Materials and methods), some of which had been used in the literature to stain brain tissues in the past, but found that none of them produced specific staining that was absent in CSF1R knockout mice (Li et al., 2006). We therefore used in situ hybridization to detect Csf1r mRNA. Using antisense probes to Csf1r, we found scattered stainings throughout the normal, uninjured mouse brain, including in the hippocampal CA1 and CA3 regions (Fig. 6 A). Notably, there was a prominent induction of Csf1r mRNA 24 h after KA injury in the neuron-dense pyramidal cell layer (Fig. 6 B), suggesting expression of Csf1r mRNA in cell types other than microglia. Importantly, no hybridization signal was observed in the sense control sections (Fig. 6, C and D). To identify the cell types that express CSF1R, we used a transgenic reporter mouse that expresses EGFP under control of the Csf1r promoter (Burnett et al., 2004). The expression of EGFP was detected with immunostaining using an anti-GFP antibody, which produced no immunostaining in brains of wild-type mice (Fig. 6 E). In contrast, we observed a broad, predominantly microglial expression pattern of the reporter gene throughout the normal, uninjured mouse brain (Fig. 6 E), in agreement with reports in the literature (Sherr et al., 1985; Raivich et al., 1998). Consistent with the mRNA in situ staining, weaker but discernable reporter immunoreactivity was also seen in few, scattered neurons in the hippocampus (1.639 ± 0.217%) throughout the brain (n = 3 mice/group), consistent with a recent publication using immunohistochemistry (Nandi et al., 2012). In the hippocampus, these neurons were observed in the CA2/3 regions and in the dentate gyrus (Fig. 6 F). To determine whether CSF1R expression is increased after injury, we lesioned mice with KA and found that systemic or intrahippocampal administration of the glutamate receptor agonist leads to prominent up-regulation of the Csf1r reporter (Fig. 6, F–H). At 6 h after KA administration, reporter expression was increased not only in microglia but clearly also in neurons (36.52 ± 7.125%; n = 3 mice/group; Fig. 7, A–D). Although reporter expression continued to increase in microglia at 24 h and 5 d, it decreased in neurons (not depicted). The expression of CSF1R in neurons was also observed in separate lineage-tracing studies using crosses between Csf1r-iCre (Deng et al., 2010) and mTmG mice (Fig. 7 E; Muzumdar et al., 2007) or ROSA-stopflox-CFP mice (Srinivas et al., 2001; not depicted). The Csf1r-iCre (Tg(Csf1r-icre)1Jwp) mice express an improved Cre (iCre) sequence under control of the Csf1r promoter (Deng et al., 2010). In the Csf1r-iCre and mTmG double transgenic reporter mice, cre recombinase expression in cells with an active Csf1r promoter results in expression of the membrane-targeted EGFP. Again, small numbers of neurons throughout the brain showed clear reporter gene expression (Fig. 7 E). The expression of Csf1r mRNA in neurons was further confirmed in cultured primary hippocampal neurons (see Fig. 9 A). Together, these results demonstrate that the CSF1R gene is expressed in neurons and up-regulated after excitotoxic injury.
Figure 7.
CSF1R reporter gene is expressed in neurons and up-regulated after excitotoxic brain injury. (A–D) CSF1R reporter mice (MAFIA mice, 2 mo of age) were lesioned with 20 mg/kg KA (subcutaneous injection) or injected with PBS as control and sacrificed 6 h later. Representative confocal microscopy images from control (PBS; A and C) and KA-lesioned mice (B and D) double immunolabeled with antibodies against GFP and cell type–specific markers Iba-1 (microglia; A and B) and NeuN (neurons; C and D). The reporter gene–expressing cells appear yellow after superimposition. An Iba-1 immuno-negative cell that expresses the reporter gene is shown (arrow) in A. Up-regulation of the reporter gene in neurons is shown after KA lesion (D) compared with control (C). The data are representative of two independent experiments with n = 3 mice/group. (E) Csf1r-iCre mice were crossed with mTmG mice. Cre recombinase expression driven by the endogenous Csf1r promoter leads to expression of EGFP in double transgenic mice. Representative confocal microscopy images from control (PBS; left) and KA-lesioned mice (right) 6 h after injury double immunolabeled with antibodies against GFP (green) and neuron-specific marker NeuN (blue). This experiment was performed once with n = 5 mice/group. Bars, 20 µm.
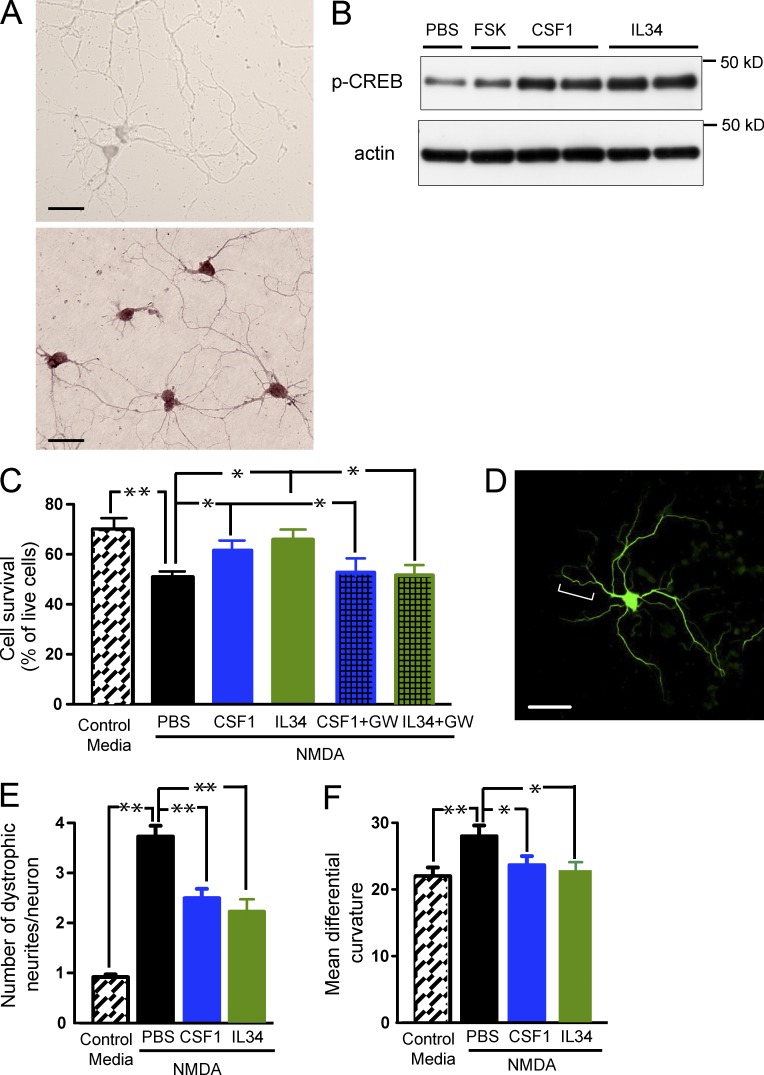
Figure 9.
Recombinant CSF1 and IL-34 inhibit excitotoxic injury and activate the CREB pathway in vitro. (A) Detection of Csf1r mRNA by in situ hybridization in cultured primary neurons. Primary hippocampal neurons were isolated from 16-d-old CF1 mouse embryos, aged for 6–7 d and hybridized with DIG-labeled Csf1r sense (top) or antisense (bottom) probes. (B–F) Primary hippocampal neurons were isolated from 16-d-old CF1 mouse embryos and were aged for 6–7 d (B and C) or 21–22 d (D–F). (B) Primary neurons were exposed to 1 or 100 ng/ml IL-34, 1 or 100 ng/ml CSF1, 10 µM Forskolin (FSK, as a positive control), or PBS (as a negative control) for 30 min. The cells were lysed and subjected to Western blot analysis for p-CREB. (C–F) Primary neurons were challenged with 100 µM NMDA in the presence and absence of CSF1 or IL-34 (both at 10 ng/ml) and assayed for neurotoxicity (C) or neuritic dystrophy (D–F), respectively. For inhibitor experiments, 10 µM GW2580 was coincubated with CSF1 or IL-34. (C) Live and dead cells were counted according to their morphologies determined by phase-contrast microscopy. Results are expressed as percentage of live cells. (D–F) Neurons were fixed and immunostained for MAP-2 to visualize dendrites. Dystrophic neurites in NMDA-treated cultures show increased tortuosity, exhibiting multiple abrupt turns (D, bracket). The number of dystrophic neurites (E) and their mean differential curvature (F) are reduced with CSF1 or IL-34 treatment. Error bars indicate SEM. Bars, 20 µm. *, P < 0.05; **, P < 0.01 compared by ANOVA and Tukey’s post-hoc test. The experiments were independently performed five times for B and twice for the rest with triplicates of each group.
CSF1 maintains neuronal CREB phosphorylation
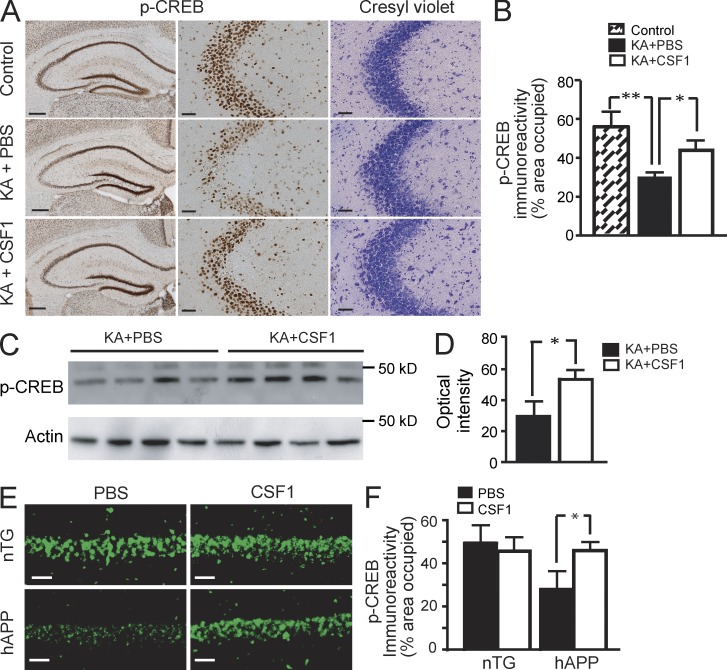
We next asked whether CSF1 might activate CSF1R-coupled intracellular pathways. Of these, CREB signaling appears to play a major role in mediating CSF1’s biological effects in macrophages (Casals-Casas et al., 2009). Importantly, KA injury was shown to selectively decrease phosphorylation of CREB (p-CREB) in vulnerable brain regions, but the cause for this decrease was not identified (Ferrer et al., 2002). Because CREB has a key function in neuronal survival (Walton and Dragunow, 2000), we reasoned that CSF1 might help maintain or restore CREB phosphorylation in neurons as well. Indeed, at 6 h after KA administration, p-CREB immunoreactivity was reduced in CA3 neurons (Fig. 8, A and B), notably without obvious cell loss at this early time point (Fig. 8 A). Systemic treatment with CSF1 significantly prevented the loss of p-CREB immunoreactivity (Fig. 8, A and B) and p-CREB protein as measured by Western blot from hippocampal lysates (Fig. 8, C and D). Likewise, CSF1-treated hAPP mice showed significantly higher p-CREB immunoreactivity in pyramidal neurons compared with PBS-treated hAPP mice (Fig. 8, E and F). These results show that CSF1 can maintain CREB phosphorylation in neurons.
Figure 8.
CSF1 maintains CREB phosphorylation. (A–D) CSF1 maintains CREB phosphorylation after KA lesion. Wild-type FVB/N mice (n = 4 per group, 2 mo of age) were lesioned with 20 mg/kg KA (subcutaneous injection) or injected with PBS as control. For treatment, CSF1 or PBS was applied 24 h before KA. Mice were sacrificed 6 h after KA injection. One hemibrain was fixed for immunohistochemistry with an antibody against p-CREB (A, left and middle), and p-CREB immunoreactivity was quantified as percentage of area occupied (B). The opposite hippocampi were isolated and subjected to Western blot analysis for p-CREB (C), which was quantified as optical intensity (D). Results are from one out of two independent experiments. (E and F) CSF1 restores CREB signaling in hAPP mice. hAPP mice and their nTG littermates (Fig. 1 A) were treated with 800 µg/kg CSF1 or PBS three times a week. 10 wk later, the mice were sacrificed after water maze behavior test, and one hemibrain was analyzed by immunohistochemistry for p-CREB immunoreactivity. The experiment was performed once. Error bars indicate SEM. Bars: (A, left) 200 µm; (A [middle and right] and E) 50 µm. *, P < 0.05; **, P < 0.01 compared by ANOVA and Bonferroni post-hoc test (B and F) or Student’s t test (D).
CSF1 and IL-34 activate the CREB pathway in primary neurons and protect against excitotoxic injury
The aforementioned results open the possibility that CSF1 might act in part through neurons in our paradigm. To test the involvement of neuronal CSF1R signaling, we investigated whether its ligands, CSF1 and IL-34 possess the capacity to protect neurons from excitotoxicity in cell culture, in the absence of microglia. Indeed, incubation with CSF1 or IL-34 significantly increased p-CREB in primary neuronal culture as measured by Western blotting of cell lysates (Fig. 9 B). Importantly, NMDA-induced excitotoxic cell death was significantly reduced by CSF1 or IL-34 (Fig. 9 C). Treatment of cells with GW2580 (Conway et al., 2005), a CSF1R kinase inhibitor, significantly blocked CSF1- or IL-34–mediated protection (Fig. 9 C). Moreover, exposure of hippocampal primary neurons to NMDA caused neuritic dystrophy, characterized by the presence of varicosities and excessive tortuosity (Fig. 9 D). Assessment of dystrophy by visual criteria (Fig. 9 E) and by quantification of the neurite mean differential curvature, a measure of tortuosity (Fig. 9 F; Yang et al., 2008; Knowles et al., 2009), showed that CSF1 and IL-34 effectively blocked NMDA-induced dystrophy. Collectively, these results show that CSF1R ligands can activate CREB signaling and protect neurons against NMDA excitotoxic injury in vitro.
Depleting CSF1R in neurons increases susceptibility to excitotoxic injury
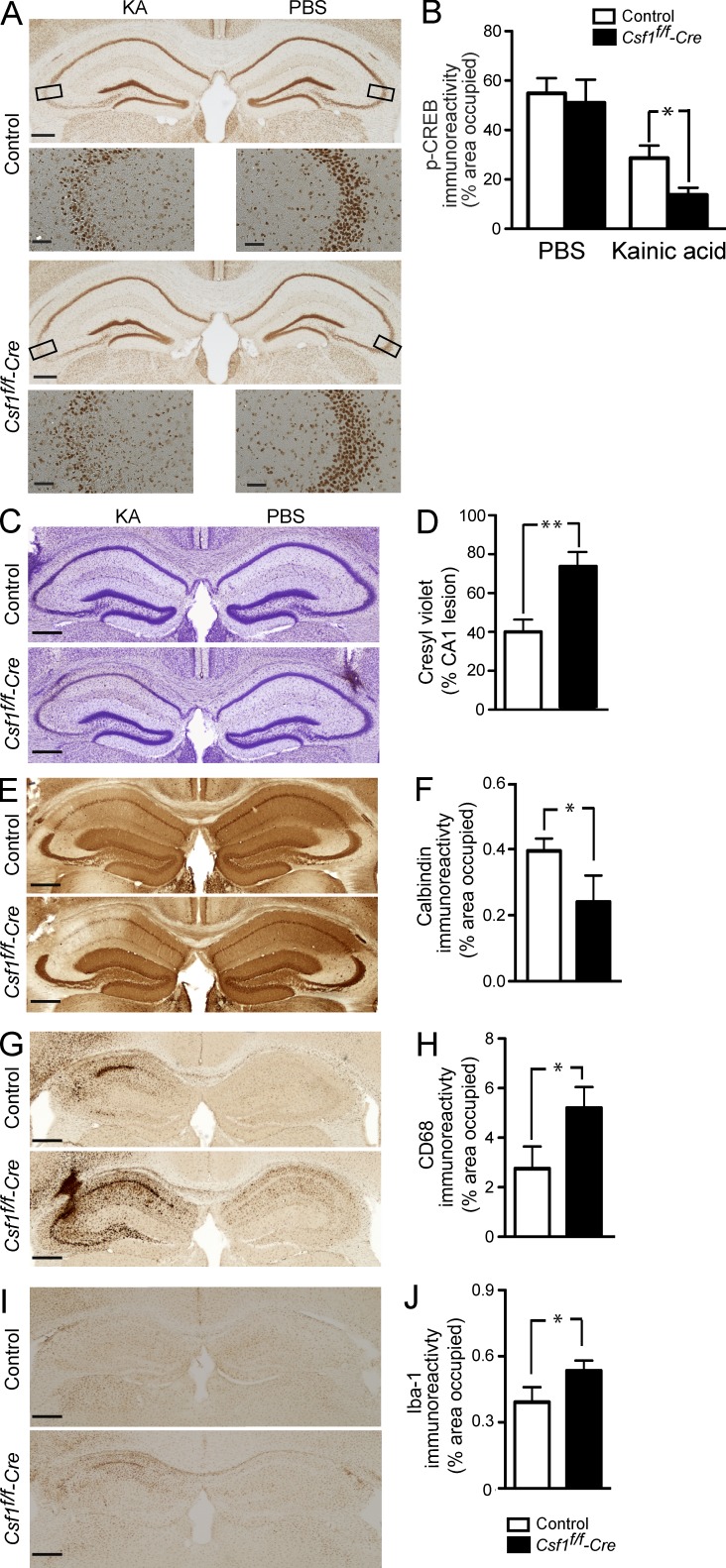
To further study the potential significance of neuronal CSF1R signaling in vivo, we deleted the receptor specifically in forebrain neurons. We generated CSF1R-null mutant mice (Csf1rf/f-cre) by breeding mice carrying a floxed exon 5 allele of the Csf1r gene (Csf1rf/f; Li et al., 2006) with CaMKIIα-cre transgenic mice (Fan et al., 2001). We first compared CREB phosphorylation in Csf1rf/f-cre mice and their control littermates. We did not observe significant differences in p-CREB immunoreactivity between uninjured Csf1rf/f-cre and littermate control brains (not depicted). To determine whether p-CREB immunoreactivity changes in response to KA injury, we stereotaxically injected PBS into the right and 50 ng KA into the left hippocampus. Mutant mice showed significantly reduced p-CREB immunoreactivity in the KA-injured side, whereas there was no difference in the PBS-injected side (Fig. 10, A and B). To investigate whether reduced CSF1R signaling is associated with increased susceptibility to neurodegeneration, we compared Csf1rf/f-cre mice and their control littermates 5 d after stereotaxic injection of KA into the hippocampus. In spite of similar seizure severity between Csf1rf/f-cre and control mice (highest seizure score was 4.6 ± 1.5 in control vs. 4.9 ± 1.7 in mutant mice), mutant mice died at twice the rate of control littermates (mortality was 16% in control vs. 30% in mutant, P = 0.042). Moreover, surviving Csf1rf/f-cre mice showed significantly more neurodegeneration and neuroinflammation than control littermates (Fig. 10). Although cell loss was restricted to the pyramidal cell layer of the CA3 region in control littermates (Fig. 10 C), it was more profound and widespread, spanning the whole CA3 and CA1 regions in Csf1rf/f-cre mice (Fig. 10, C and D). Similarly, calbindin immunoreactivity was depleted more severely in the CA1 subfield in KA-injected Csf1rf/f-cre compared with control littermates (Fig. 10, E and F). This increase in susceptibility to excitotoxic neurodegeneration in Csf1rf/f-cre mice was mirrored by an increase in the microglial response. Microglial activation measured by CD68 and Iba-1 immunoreactivity was markedly increased in Csf1rf/f-cre compared with control mice (Fig. 10, G–J). In control mice, microgliosis was observed only on the ipsilateral side, whereas in Csf1rf/f-cre mice, it was also observed on the contralateral hippocampus (Fig. 10 G). In summary, mice lacking CSF1R in neurons are more susceptible to death and neurodegeneration after excitotoxic injury, supporting a role for neuronal CSF1R signaling in their survival.
Figure 10.
Deletion of CSF1R in neurons reduces p-CREB immunoreactivity and increases susceptibility to excitotoxic injury. Csf1rf/f-cre mice and their control littermates (2 mo of age) were lesioned with a unilateral stereotaxic injection of 50 ng KA or PBS as control into the hippocampus. (A and B) Mice were sacrificed 24 h later, and brain sections were analyzed for p-CREB immunoreactivity, quantified as percentage of area occupied (n = 3 mice/group). The boxes in A indicate the corresponding hippocampal areas shown in the panel below (with higher magnification). (C–J) Mice were sacrificed 5 d later, and brain sections were analyzed for neuronal injury by cresyl violet staining (C and D), calbindin immunostaining (E and F), and CD68 or Iba-1 immunostaining (microglial activation; G–J). In C, E, G, and I, mice from the control (top) and Csf1rf/f-cre mice (bottom) are shown. Bars, 200 µm. Bars are mean ± SEM (n = 3–4 mice/group) from one out of two independent experiments. *, P < 0.05; **, P < 0.01 compared with control by Student’s t test.
DISCUSSION
The present study demonstrates that CSF1R is up-regulated in injured neurons and contributes to the protection and increased survival of these cells in vitro and possibly in vivo. We show that CSF1R ligands CSF1 and IL-34 are able to protect against excitotoxin-induced neurodegeneration and that CSF1 ameliorates APP/Aβ-mediated cognitive impairments. We also show that deletion of CSF1R in neurons exacerbates excitotoxic injury and subsequent lethality and that up-regulation of endogenous CSF1 inversely correlates with neurodegeneration and neuroinflammation. Thus, CSF1R signaling supports the health and survival of neurons in our paradigms.
Our study provides several lines of evidence in support of a novel function for CSF1R signaling in neurons. First, CSF1R is expressed in neurons and up-regulated after brain injury. It was long held that the expression of CSF1R is restricted to cells of the monocyte/macrophage lineage and to microglia in the brain (Sherr et al., 1985; Raivich et al., 1998), and recent studies using 7.2fms-EGFP reporter mice (C57BL/6N.Gn-Tg(Csf1r-EGFP)hume) lent further support to this notion (Sierra et al., 2007; Erblich et al., 2011). Although Csf1r mRNA was detected by in situ hybridization in the brain (Murase and Hayashi, 1998; Wang et al., 1999), no functional expression of the receptor had been documented. Efforts to localize CSF1R protein expression in the brain using immunohistochemistry generated largely inconsistent and contradictory results (Murase and Hayashi, 1998; Raivich et al., 1998; Wang et al., 1999; Murphy et al., 2000). We think this was mainly caused by a lack of specific CSF1R antibodies as the six commercially available anti-CSF1R antibodies we tested failed to produce specific staining in the brain when tested in CSF1R knockout control mice. Instead, we demonstrate neuronal CSF1R expression using two distinct reporter mouse models: one harboring a transgene consisting of the Csf1r promoter fused to EGFP (Figs. 6 and 7) and the other an icre recombinase inserted downstream of the Csf1r promoter and crossed with EGFP reporter mice (Fig. 7). Both mouse models show expression of CSF1R in roughly 1–2% of neurons, but this expression was up-regulated after injury (Figs. 6 and 7). In addition, primary neurons express Csf1r mRNA based on in situ hybridization. These results are consistent with a recent publication showing that CSF1R is expressed in immature neurons during early postnatal development but is reduced in adult brain (Nandi et al., 2012).
Second, reducing neuronal CSF1R signaling by deleting CSF1R specifically in neurons resulted in increased neurodegeneration and mortality after excitotoxic injury (Fig. 10). Because CSF1 is known as a primary regulator for the monocyte/macrophage lineage, the function of CSF1 in the brain was typically assigned to microglial regulation, and its role in neurons has not been established. Only a few studies proposed effects of CSF1 on neurons based either on cell culture experiments in which CSF1 enhanced survival of Purkinje cells and reduced apoptotic neuron death (Murase and Hayashi, 1998; Wang et al., 1999) or on using a model of stroke whereby peripheral administration of CSF1 reduced infarct size and increased neuronal survival (Berezovskaya et al., 1996). CSF1-deficient Csf1op/op mice showed increased neuronal vulnerability to ischemic and chemical-induced damage (Berezovskaya et al., 1995; Penkowa et al., 2002). In addition, the Csf1op/op and CSF1R knockout mice showed abnormal brain development (Michaelson et al., 1996; Erblich et al., 2011), but it remained unclear whether these phenotypes involved CSF1R signaling in neurons. Deleting CSF1R in neural progenitor cells partially recapitulated the forebrain phenotypes of Csf1op/op and CSF1R knockout mice (Nandi et al., 2012), suggesting that lacking CSF1R signaling in immature neurons contributes to CSF1R-mediated brain developmental abnormalities of the Csf1op/op and CSF1R knockout mice. Our genetic experiments using the cre-lox system allowed us to specifically ablate the CSF1R in forebrain neurons, thus further linking CSF1R signaling to mature neurons.
Third, besides CSF1R itself, the ligand CSF1 also increases in response to excitotoxic injury in neurons. 6 h after KA injection, strong induction of CSF1 is seen in CA3, the most sensitive region known to harbor neurons at risk of dying in this paradigm. Importantly, induction of CSF1 after injury seems to serve as a protective mechanism: mice that died within 6 h after KA administration showed no CSF1 immunostaining, whereas mice that survived showed high levels of CSF1 expression. Interestingly, up-regulation of CSF1 immunoreactivity in neurons has been noted before in response to Aβ and to focal brain injury (Du Yan et al., 1997; Takeuchi et al., 2001). Therefore, it is likely that CSF1 is produced in response to injury and may function as a protective system in neurons. Our experiments further suggest that at least part of this protective response involves signaling via CSF1R in injured neurons in addition to indirect neuroprotective effects mediated by microglia and other cells.
Fourth, IL-34, which is an independent, newly discovered CSF1R ligand, provides similarly potent neuroprotection as CSF1. IL-34 has so far not been examined for its function on cell types other than macrophages. In the brain, IL-34 mRNA was strongly expressed at E11.5, before the expression of Csf1 mRNA (Wei et al., 2010). Interestingly, levels of IL-34 mRNA are higher than Csf1 mRNA in most regions of both the developing and adult brain (Wei et al., 2010), suggesting that IL-34 may have additional, nonoverlapping functions from CSF1 in the brain (Hamilton and Achuthan, 2012). Results from a recent cell fate mapping study support this notion and provide indirect evidence for an important role of IL-34 in the regulation of microglial homeostasis (Ginhoux et al., 2010). Interestingly, IL-34–deficient reporter mice revealed that neurons are the main sources of IL-34 (Wang et al., 2012). Intracerebroventricular administration of IL-34 ameliorated impairment of associative learning and reduced Aβ levels in an APP/PS1 transgenic mouse model of AD, although these effects were ascribed to microglia and direct effects on neurons were not studied (Mizuno et al., 2011).
Lastly, administration of recombinant CSF1 or IL-34 protected cultured primary neurons from excitotoxic injury and induced the phosphorylation of CREB, consistent with previous reports that CSF1 enhanced survival and neurite outgrowth of neurons cultured in serum-free medium (Michaelson et al., 1996; Murase and Hayashi, 1998; Wang et al., 1999). In addition, CSF1 administration reduced the loss of p-CREB in response to KA or APP/Aβ transgene in vivo. Because CREB signaling has been implicated in promoting neuronal survival and in memory formation and cognitive function (Kandel, 2001; Lonze and Ginty, 2002), the induction and activation of the CREB pathway in neurons may explain at least part of the robust survival effects of CSF1 and IL-34. How CSF1 and IL-34 activate the CREB pathway in neurons needs further investigation. In macrophages, CSF1-coupled signaling leads to activation of PKA and cAMP (Majumdar et al., 2007), both of which can induce CREB phosphorylation, and it is conceivable that CSF1 activates the CREB pathway through similar mechanisms in neurons.
Although our experiments suggest a novel role for CSF1R signaling in protecting neurons against injury, it is likely that independent or synergistic effects on other CNS cell types contribute to the potent in vivo effects of CSF1 and IL-34. For example, CSF1 may modulate inflammatory responses in microglia or astrocytes which play an important role in neurodegeneration (Wyss-Coray, 2006). Besides neurons and microglia, CSF1R mRNA has been detected in cultured astrocytes and oligodendrocytes using RT-PCR (Sawada et al., 1993), although CSF1R was not up-regulated in these cells in the reporter mice in our paradigm (Figs. 6 and 7).
A key finding from our study and one of potential clinical relevance is that CSF1 and IL-34 produce strong neuroprotective and survival effects in the brain even if administered systemically several hours after a neuronal insult. Using in vivo bioluminescence imaging, we demonstrate that CSF1 administered 2 or 6 h after injury exerts similar benefits to neuronal survival as CSF1 delivered before injury (Fig. 2). In addition, in vivo imaging of astrocyte activation showed that the initial injury/insult was similar (shown by similar levels of astrogliosis at day 1) whether CSF1 was given before or after injury but that the outcome was affected (shown by reduced levels of astrogliosis at days 3 and 5). These results suggest that CSF1 may promote the postinjury survival of neurons rather than reducing the initial insult. How exactly systemic CSF1 reaches CNS neurons remains to be determined, but it is possible that the blood–brain barrier is impaired early after KA injury (Saija et al., 1992; Pont et al., 1995).
Our findings place the hematopoietic factors CSF1 and IL-34 among several important neuroprotective factors and support the growing view that hematopoietic and other classical immune factors may have equally important functions in the CNS (Maurer et al., 2008). The findings provide a genetically based, mechanistic framework on how CSF1, a classical immune factor assigned to macrophage function, protects neurons. Because CSF1 is approved for human use in clinical trials (Douglass et al., 2008; Hume and MacDonald, 2012), it may be an attractive therapeutic candidate for brain injury and neurodegenerative disorders.
MATERIALS AND METHODS
Mice.
The following transgenic mouse lines were used: hAPP mice (line 41) expressing mutated (London V717I and Swedish K670M/N671L) human APP751 under the control of the mouse Thy1 promoter (Rockenstein et al., 2001), GFAP-luc mice (Caliper Life Science; Zhu et al., 2004), CSF1-null Csf1op/Csf1op mice (The Jackson Laboratory; Wiktor-Jedrzejczak et al., 1990; Yoshida et al., 1990), actin-EGFP (C57BL/6-Tg(ACTB-EGFP)1Osb/J) mice expressing EGFP under the control of a chicken β-actin promoter (The Jackson Laboratory; Okabe et al., 1997), CaMKIIα-cre mice (obtained from R. Jaenisch, Massachusetts Institute of Technology, Cambridge, MA; Fan et al., 2001), Csf1r-iCre (Tg(Csf1r-icre)1Jwp) mice expressing an iCre sequence under control of the Csf1r promoter (Deng et al., 2010), ROSA-stopflox-CFP mice (Srinivas et al., 2001), mTmG mice (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP); The Jackson Laboratory; Muzumdar et al., 2007), Csf1rf/f mice (Csf1rtm1Lex5jwp, carrying a floxed exon 5 allele of the Csf1r gene; Li et al., 2006), and Csf1r-deficient mice (deleted in exon 5 of the Csf1r gene; Li et al., 2006). Csf1r f/f mice were crossed with CaMKIIα-cre mice to generate forebrain neuron–specific CSF1R-null mutant mice (CaMKIIα-cre+; Csf1r f/f, named Csf1rf/f-cre in this study). Csf1r-iCre mice were crossed with ROSA-stopflox-CFP mice or mTmG mice to label Csf1r-expressing cells. CSF1R reporter (C57BL/6J-Tg(Csf1r-EGFP-NGFR/FKBP1A/TNFRSF6)2Bck/J, synonym: macrophage Fas–induced apoptosis [MAFIA]) mice (Burnett et al., 2004) expressing EGFP under control of the Csf1r promoter were purchased from the Jackson Laboratory under agreement with Ariad Pharmaceuticals. The hAPP, Csf1rf/f, CaMKIIα-cre, and MAFIA mice were on a C57BL/6 genetic background, and GFAP-luc mice were on an FVB/N background. The following mice were used as controls for mutants: nTG littermates for hAPP and actin-EGFP, wild-type C57BL/6 for Csf1op/op, and CaMKIIα-cre-;Csf1r f/f littermates (Cre-) for Csf1rf/f-cre. Wild-type FVB/N or C57BL/6 mice were purchased from the Jackson Laboratory. Animal handling was performed in accordance with institutional guidelines and approved by a local Institutional Animal Care and Use Committee.
KA-induced excitotoxicity.
For FVB/N mice, KA (Tocris Bioscience) was dissolved in distilled water and injected subcutaneously (20 or 30 mg/kg) to induce neurodegeneration (Luo et al., 2006). Seizure activity was monitored every 15 min for 1 h after KA administration, using a scoring system from 0 to 8, with 0 showing no behavioral changes and 8 showing death (Janumpalli et al., 1998). For C57BL/6 mice, 0.50 µl KA (0.1 µg/µl) was injected stereotaxically unilaterally into the hippocampus (coordinates from bregma: A = −2.0 mm and L = −1.8 mm; from brain surface: H = −2.0 mm) under isoflurane anesthesia. KA was injected over 2 min using a 5-µl Hamilton syringe. After injection, the needle was maintained in situ for an additional 2 min to limit reflux along the injection track. The skin was closed using adhesive surgical block, and each mouse was injected subcutaneously with Buprenex as directed for pain relief. Animals were examined 5 d after KA injection. In some experiments, to study how expression of endogenous CSF1 and the receptor changes in response to KA, animal mice were analyzed at different time points (6 h, 24 h, 3 d, and 5 d) after KA administration.
CSF1 and IL-34 treatment.
Recombinant human CSF1 was provided by Biogen Idec. Recombinant mouse IL-34 was purchased from R&D Systems. CSF1 was injected i.p. at 800 µg/kg body weight, a dose previously used clinically in bone marrow transplantation patients (Nemunaitis et al., 1993). IL-34 was injected i.p. at 100 µg/kg body weight. The same volume of PBS was used as a control treatment. For hAPP mice, CSF1 was injected i.p. three times a week. For kainate injury, CSF1 or IL-34 was injected i.p. once at different time points in relation to kainate injury.
Behavioral tests for hAPP mice.
We used the Morris water maze to assess the effect of CSF1 on spatial learning and memory (Adlard et al., 2005). In brief, after training the mice with a visible platform, animals were subjected to 6 d of place discrimination training using a hidden platform, with four trials per day, followed by a probe trial 24 h later to assess retention of the task. Data were analyzed using the Ethovision automated tracking system (Noldus Information Technology).
In vivo bioluminescence imaging.
Bioluminescence was detected with the In Vivo Imaging System (IVIS Spectrum; Caliper Life Science; Lin et al., 2005; Luo et al., 2006, 2007). Mice were injected i.p. with 150 mg/kg d-luciferin 10 min before imaging and anesthetized with isoflurane during imaging. Photons emitted from living mice were acquired as photons/s/cm2/steridian (sr) using LIVINGIMAGE software (version 3.1) and integrated over 3 min. For photon quantification, a region of interest was manually selected and kept constant for all experiments; the signal intensity was converted into photons/s/mm2/sr. For longitudinal comparison of bioluminescence, baseline imaging was performed 24 h before KA was administered, and bioluminescence was expressed as fold induction over baseline levels for each mouse.
Parabiosis.
Actin-EGFP mice were surgically connected with their nTG littermates or hAPP mice to produce parabiosis, as previously described (Villeda et al., 2011). In brief, pairs of mice were anesthetized and prepared for surgery. Mirror-image incisions at the left and right flanks, respectively, were made through the skin. Shorter (∼1 cm) incisions were made through the abdominal wall. The abdominal openings were sutured together, and the skin of each mouse was stapled (9-mm Autoclip; Clay Adams) to the skin of its parabiont, thereby closing the incision. Each mouse was injected subcutaneously with Baytril antibiotic and Buprenex as directed for pain and monitored during recovery. Blood circulation was established after ∼2 wk, and the non-GFP–expressing (nTG or hAPP) parabionts were analyzed for GFP+ cells in the brain at 6 wk after surgery. To study the effects of CSF1, CSF1 or PBS was administered separately into both parabionts of each pair starting at week 2 (hAPP parabionts received CSF1 for 5 wk). For KA injury, actin-EGFP mice were crossed with FVB/N mice to increase susceptibility, and F1 mice were used for experiments. Both parabionts of each pair were lesioned with KA at 5 wk after surgery. CSF1 was injected (i.p.) 24 h before KA.
Tissue processing.
Mice were anesthetized with 400 mg/kg chloral hydrate (Sigma-Aldrich) and transcardially perfused with 0.9% saline (Luo et al., 2006, 2007). Brains were removed and divided sagittally. One hemibrain was postfixed in phosphate-buffered 4% paraformaldehyde (PFA), pH 7.4, at 4°C for 48 h and sectioned at 40 µm with a Vibratome 2000 (Leica) and stored in cryoprotective medium; the other hemibrain was snap frozen and stored at −80°C for biochemical analysis (Luo et al., 2006, 2007).
Western blotting.
Snap-frozen hippocampi were lysed in 200 µl RIPA lysis buffer (500 mM Tris, pH 7.4, 150 mM NaCl, 0.5% Na deoxycholate, 1% NP-40, 0.1% SDS, and complete protease inhibitors; Roche; Lin et al., 2005). Tissue or 20 µl of cell lysates was mixed with 4× NuPage LDS loading buffer (Invitrogen) and loaded on a 3–12% SDS-polyacrylamide gradient gel and subsequently transferred onto a polyvinylidene fluoride membrane. The blot was incubated with rabbit polyclonal antibodies against p-CREB (1:1,000; Cell Signaling Technology) and an HRP-conjugated secondary antibody (GE Healthcare). Protein signals were detected using an ECL kit (GE Healthcare).
ELISA analysis of Aβ.
For cerebral Aβ levels, snap-frozen hippocampi and cortices were homogenized in RIPA buffer followed by 70% formic acid at 0.1 mg weight tissue per 1 ml. Aβ peptides were quantified by ELISA as described previously (Pickford et al., 2008) using 5 µg/ml antibody 266 (Aβ13–28; Elan Pharmaceuticals) as the capture antibody for total Aβ1-x or 5 µg/ml antibody 21F12 (Aβ37–42; Elan Pharmaceuticals) as the capture antibody for Aβx–42 and 2 µg/ml of biotinylated 3D6 (Aβ1–5; Elan Pharmaceuticals) as the detection antibody. After incubation with the secondary antibody, samples were incubated with avidin-HRP (diluted 1:4,000; Vector Laboratories), and the signal was developed using 1-step Turbo TMB ELISA solution (Thermo Fisher Scientific).
Cresyl violet staining.
Brain sections were mounted on Superfrost plus slides (Thermo Fisher Scientific), air-dried, rehydrated, stained with 0.02% cresyl violet (Sigma-Aldrich) in acetate buffer, pH 3.2, then dehydrated through a series of alcohols, cleared in xylene, and coverslipped (Luo et al., 2006). Neuronal damage/loss was assessed based on the appearance of gaps or thinning and disappearance of the Nissl substance in the CA1 and CA3 pyramidal cell layers. The lesion area was quantified with MetaMorph Imaging software (Molecular Devices).
Immunohistochemistry, image analysis, and confocal microscopy.
Immunohistochemistry was performed on free-floating sections according to standard procedures (Luo et al., 2006, 2007). Primary antibodies were against 3D6 (biotinylated; Elan Pharmaceuticals), Calbindin (1:10,000; EMD Millipore), CD68 (1:50; AbD Serotec), Iba-1 (1:2,500; Wako Chemicals USA), CD11b (1:200; Abcam), NPY (1:200; EMD Millipore), CSF1 (1:2,000; R&D Systems), p-CREB (Ser 133; 1:1,000; EMD Millipore), and GFP (1:1,000; Invitrogen). Six anti-CSF1R antibodies were obtained (two from Santa Cruz Biotechnology, Inc., two from EMD Millipore, one from Cell Signaling Technology, and one from Biogen Idec, generated by New England Peptide, sequence Ac-DPESPGSTC-amide) and tested with brain sections from Csf1r knockout mice (Li et al., 2006). After overnight incubation, primary antibody staining was revealed using biotinylated secondary antibodies and the ABC kit (Vector Laboratories) with diaminobenzidine (Sigma-Aldrich) or fluorescence-conjugated secondary antibodies. Photographs were acquired using a BX51 microscope (Olympus) and a SPOT Flex shifting pixel charge-coupled device camera with SPOT Advanced software (SPOT Imaging Solutions). The immunoreactivity was quantified as the percentage of area covered by MetaMorph software (version 7) as previously described (Luo et al., 2006, 2007). For each staining, a total of three hippocampal brain sections per mouse were analyzed. Fluorescent images and colocalization of two antigens were analyzed under an LSM 510 confocal microscope (Carl Zeiss) and viewed with LSM Image Browser software (Carl Zeiss).
Primary neuron culture.
Primary hippocampal neurons were isolated from 16-d-old CF1 embryos (Yang et al., 2008; Knowles et al., 2009). 24-well culture plates were coated with 10 mg/ml poly-l-lysine (Sigma-Aldrich). Cells were seeded overnight at a density of 30,000 cells/well in DMEM/F-12 medium supplemented with 10% FBS and penicillin/streptomycin and subsequently maintained in Neurobasal medium containing 2% B27 supplement (Invitrogen). They were aged for 6–7 d or 21–22 d, then challenged with 100 µM NMDA (Sigma-Aldrich) for 24 h in the presence and absence of CSF1 or IL-34, and assayed for neurotoxicity or neuritic dystrophy, respectively. For inhibitor experiments, 10 µM GW2580 was coincubated with CSF1 or IL-34. At the end of treatment, equal volume of 4% PFA was added, and plates were incubated at room temperature for 15 min and then washed three times with PBS. Cells were kept in the last wash of PBS at 4°C until being counted or stained. For the neurotoxicity assay, live and dead cells were counted according to their morphologies determined by phase-contrast microscopy (Yang et al., 2008; Knowles et al., 2009). Results were expressed as percentage of live cells. For quantification of neuritic dystrophy, the fixed cells were immunostained with a microtubule-associated protein 2 (MAP-2) monoclonal antibody (1:5,000; Sigma-Aldrich). After overnight incubation, primary antibody staining was revealed by an Alexa Fluor 488–conjugated secondary antibody (Invitrogen). MAP-2–positive dendrites were observed under an IX71 invert fluorescence microscope (Olympus). Dendrites were considered dystrophic when they showed a persistent pattern of increased tortuosity (multiple abrupt turns). To quantify neurite curvature, neurite courses were digitized and approximated by a series of connected line segments using ImageJ (National Institutes of Health). The angle of each segment was determined using a SigmaPlot macro, and the results were averaged to give the mean differential curvature (Yang et al., 2008; Knowles et al., 2009). This parameter reflects the degree of neurite curvature, with an increasing value indicating increased curvature. Neurite counting and quantification were performed in randomly selected fields (five fields/well) in a completely blinded manner.
In situ hybridization.
In situ hybridization on brain sections was performed as previously described (Pan et al., 2006). In brief, mice were perfused transcardially with 0.9% NaCl prepared in diethylpyrocarbonate-treated water, followed by 4% PFA. Brains were immediately removed, postfixed for 24 h at 4°C in the same fixative, cryoprotected by immersion in 30% sucrose overnight, and kept frozen. 12-µm cryostat sections were fixed in 4% PFA, washed in PBS, and acetylated with acetic anhydride. The slides were prehybridized at 58°C for 2 h and hybridized in hybridization buffer (50% formamide, 5× SSC, 5× Denhardt’s, and 1 mg/ml sperm DNA) containing digoxigenin (DIG)-labeled antisense or sense RNA probes at 58°C overnight. The slides were subsequently digested with RNase A and incubated with anti-DIG antibody. Finally, the slides were developed with 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indoyl-phosphate (Roche). For visualization, the slides were counterstained with 1% methyl green.
For primary neurons (6–7 d in culture), a 48-mer DNA oligonucleotide probe complementary to bases 904–951 of Csf1r (Rothwell and Rohrschneider, 1987) was used. The antisense oligonucleotide sequence is 5′-GTTCATGGTGGCCGTGCGTGTGCCAACATCATTGCTGGCCACACAAGA-3′. The probe was labeled at the 3′ end with DIG. After fixation, the cells were washed with PBS and exposed to proteinase K. Prehybridization was performed for 2 h at 42°C with hybridization solution (Dako). Hybridization (probe concentration 1 µg/ml) was performed in a humidified chamber at 42°C overnight. The hybridization signal was detected by a DIG Nucleic Acid Detection kit (both from Roche). The corresponding sense oligonucleotide probe was used as a control.
Data and statistical analysis.
Data are presented as mean ± SEM. Data were analyzed using a two-tailed Student’s t test for comparing two groups or ANOVA for multiple groups. Bonferroni or Tukey’s post-hoc test was used to compare pairs of groups after ANOVA. Statistical analysis was performed with Prism software (version 5; GraphPad Software). P < 0.05 was considered statistically significant.
Acknowledgments
We thank C. Ishikawa for animal husbandry and genotyping and A. Okada for technical assistance.
This work was supported by grants from the National Institutes of Health (AG23708 and AG20603 to T. Wyss-Coray) and Biogen Idec.
G. Wong and J. Relton were employed by Biogen Idec. A provisional patent has been filed in relation to this work. The authors have no other conflicting financial interests.
Footnotes
Abbreviations used:
- AD
- Alzheimer’s disease
- CNS
- central nervous system
- CREB
- cAMP responsive element–binding protein
- CSF1R
- CSF1 receptor
- DIG
- digoxigenin
- GFAP
- glial fibrillary acidic protein
- hAPP
- human amyloid precursor protein
- iCre
- improved Cre
- KA
- kainic acid
- MAFIA
- macrophage Fas–induced apoptosis
- NMDA
- N-methyl-d-aspartic acid
- NPY
- neuropeptide Y
- nTG
- nontransgenic
- PFA
- paraformaldehyde
References
- Adlard P.A., Perreau V.M., Pop V., Cotman C.W. 2005. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 25:4217–4221 10.1523/JNEUROSCI.0496-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M. 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10:1538–1543 10.1038/nn2014 [DOI] [PubMed] [Google Scholar]
- Berezovskaya O., Maysinger D., Fedoroff S. 1995. The hematopoietic cytokine, colony-stimulating factor 1, is also a growth factor in the CNS: congenital absence of CSF-1 in mice results in abnormal microglial response and increased neuron vulnerability to injury. Int. J. Dev. Neurosci. 13:285–299 10.1016/0736-5748(95)00013-7 [DOI] [PubMed] [Google Scholar]
- Berezovskaya O., Maysinger D., Fedoroff S. 1996. Colony stimulating factor-1 potentiates neuronal survival in cerebral cortex ischemic lesion. Acta Neuropathol. 92:479–486 10.1007/s004010050550 [DOI] [PubMed] [Google Scholar]
- Boissonneault V., Filali M., Lessard M., Relton J., Wong G., Rivest S. 2009. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 132:1078–1092 10.1093/brain/awn331 [DOI] [PubMed] [Google Scholar]
- Britschgi M., Rufibach K., Huang S.L., Clark C.M., Kaye J.A., Li G., Peskind E.R., Quinn J.F., Galasko D.R., Wyss-Coray T. 2011. Modeling of pathological traits in Alzheimer’s disease based on systemic extracellular signaling proteome. Mol. Cell. Proteomics. 10:M111: 008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S.H., Kershen E.J., Zhang J., Zeng L., Straley S.C., Kaplan A.M., Cohen D.A. 2004. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol. 75:612–623 10.1189/jlb.0903442 [DOI] [PubMed] [Google Scholar]
- Casals-Casas C., Alvarez E., Serra M., de la Torre C., Farrera C., Sánchez-Tilló E., Caelles C., Lloberas J., Celada A. 2009. CREB and AP-1 activation regulates MKP-1 induction by LPS or M-CSF and their kinetics correlate with macrophage activation versus proliferation. Eur. J. Immunol. 39:1902–1913 10.1002/eji.200839037 [DOI] [PubMed] [Google Scholar]
- Chihara T., Suzu S., Hassan R., Chutiwitoonchai N., Hiyoshi M., Motoyoshi K., Kimura F., Okada S. 2010. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 17:1917–1927 10.1038/cdd.2010.60 [DOI] [PubMed] [Google Scholar]
- Chitu V., Stanley E.R. 2006. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18:39–48 10.1016/j.coi.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Conway J.G., McDonald B., Parham J., Keith B., Rusnak D.W., Shaw E., Jansen M., Lin P., Payne A., Crosby R.M., et al. 2005. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc. Natl. Acad. Sci. USA. 102:16078–16083 10.1073/pnas.0502000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.M., Ryan G.R., Hapel A.J., Dominguez M.G., Russell R.G., Kapp S., Sylvestre V., Stanley E.R. 2002. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 99:111–120 10.1182/blood.V99.1.111 [DOI] [PubMed] [Google Scholar]
- Deng L., Zhou J.F., Sellers R.S., Li J.F., Nguyen A.V., Wang Y., Orlofsky A., Liu Q., Hume D.A., Pollard J.W., et al. 2010. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am. J. Pathol. 176:952–967 10.2353/ajpath.2010.090622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommergues M.A., Plaisant F., Verney C., Gressens P. 2003. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 121:619–628 10.1016/S0306-4522(03)00558-X [DOI] [PubMed] [Google Scholar]
- Douglass T.G., Driggers L., Zhang J.G., Hoa N., Delgado C., Williams C.C., Dan Q., Sanchez R., Jeffes E.W., Wepsic H.T., et al. 2008. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int. Immunopharmacol. 8:1354–1376 10.1016/j.intimp.2008.04.016 [DOI] [PubMed] [Google Scholar]
- Du Yan S., Zhu H., Fu J., Yan S.F., Roher A., Tourtellotte W.W., Rajavashisth T., Chen X., Godman G.C., Stern D., Schmidt A.M. 1997. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 94:5296–5301 10.1073/pnas.94.10.5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B., Zhu L., Etgen A.M., Dobrenis K., Pollard J.W. 2011. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE. 6:e26317 10.1371/journal.pone.0026317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G., Beard C., Chen R.Z., Csankovszki G., Sun Y., Siniaia M., Biniszkiewicz D., Bates B., Lee P.P., Kuhn R., et al. 2001. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 21:788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Blanco R., Carmona M., Puig B., Domínguez I., Viñals F. 2002. Active, phosphorylation-dependent MAP kinases, MAPK/ERK, SAPK/JNK and p38, and specific transcription factor substrates are differentially expressed following systemic administration of kainic acid to the adult rat. Acta Neuropathol. 103:391–407 10.1007/s00401-001-0481-9 [DOI] [PubMed] [Google Scholar]
- Garceau V., Smith J., Paton I.R., Davey M., Fares M.A., Sester D.P., Burt D.W., Hume D.A. 2010. Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J. Leukoc. Biol. 87:753–764 10.1189/jlb.0909624 [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., et al. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 330:841–845 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G., Lalancette-Hébert M., Audet J.N., Dequen F., Julien J.P. 2009. Macrophage colony stimulating factor (M-CSF) exacerbates ALS disease in a mouse model through altered responses of microglia expressing mutant superoxide dismutase. Exp. Neurol. 220:267–275 10.1016/j.expneurol.2009.08.021 [DOI] [PubMed] [Google Scholar]
- Hamilton J.A. 1997. CSF-1 signal transduction. J. Leukoc. Biol. 62:145–155 [DOI] [PubMed] [Google Scholar]
- Hamilton J.A. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533–544 10.1038/nri2356 [DOI] [PubMed] [Google Scholar]
- Hamilton J.A., Achuthan A. 2012. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 10.1016/j.it.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Hume D.A., MacDonald K.P. 2012. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 119:1810–1820 10.1182/blood-2011-09-379214 [DOI] [PubMed] [Google Scholar]
- Imai Y., Kohsaka S. 2002. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 40:164–174 10.1002/glia.10149 [DOI] [PubMed] [Google Scholar]
- Janumpalli S., Butler L.S., MacMillan L.B., Limbird L.E., McNamara J.O. 1998. A point mutation (D79N) of the alpha2A adrenergic receptor abolishes the antiepileptogenic action of endogenous norepinephrine. J. Neurosci. 18:2004–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R. 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 294:1030–1038 10.1126/science.1067020 [DOI] [PubMed] [Google Scholar]
- Knowles J.K., Rajadas J., Nguyen T.V., Yang T., LeMieux M.C., Vander Griend L., Ishikawa C., Massa S.M., Wyss-Coray T., Longo F.M. 2009. The p75 neurotrophin receptor promotes amyloid-beta(1-42)-induced neuritic dystrophy in vitro and in vivo. J. Neurosci. 29:10627–10637 10.1523/JNEUROSCI.0620-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen K., Zhu L., Pollard J.W. 2006. Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis. 44:328–335 10.1002/dvg.20219 [DOI] [PubMed] [Google Scholar]
- Li M., Pisalyaput K., Galvan M., Tenner A.J. 2004. Macrophage colony stimulatory factor and interferon-gamma trigger distinct mechanisms for augmentation of beta-amyloid-induced microglia-mediated neurotoxicity. J. Neurochem. 91:623–633 10.1111/j.1471-4159.2004.02765.x [DOI] [PubMed] [Google Scholar]
- Lin A.H., Luo J., Mondshein L.H., ten Dijke P., Vivien D., Contag C.H., Wyss-Coray T. 2005. Global analysis of Smad2/3-dependent TGF-beta signaling in living mice reveals prominent tissue-specific responses to injury. J. Immunol. 175:547–554 [DOI] [PubMed] [Google Scholar]
- Lin H., Lee E., Hestir K., Leo C., Huang M., Bosch E., Halenbeck R., Wu G., Zhou A., Behrens D., et al. 2008. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 320:807–811 10.1126/science.1154370 [DOI] [PubMed] [Google Scholar]
- Liu H., Leo C., Chen X., Wong B.R., Williams L.T., Lin H., He X. 2012. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim. Biophys. Acta. 1824:938–945 10.1016/j.bbapap.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze B.E., Ginty D.D. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 35:605–623 10.1016/S0896-6273(02)00828-0 [DOI] [PubMed] [Google Scholar]
- Luo J., Lin A.H., Masliah E., Wyss-Coray T. 2006. Bioluminescence imaging of Smad signaling in living mice shows correlation with excitotoxic neurodegeneration. Proc. Natl. Acad. Sci. USA. 103:18326–18331 10.1073/pnas.0605077103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Ho P.P., Buckwalter M.S., Hsu T., Lee L.Y., Zhang H., Kim D.K., Kim S.J., Gambhir S.S., Steinman L., Wyss-Coray T. 2007. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J. Clin. Invest. 117:3306–3315 10.1172/JCI31763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Cruz D., Asamoah N., Buxbaum A., Sohar I., Lobel P., Maxfield F.R. 2007. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell. 18:1490–1496 10.1091/mbc.E06-10-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. 2004. Pathways towards and away from Alzheimer’s disease. Nature. 430:631–639 10.1038/nature02621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M.H., Schäbitz W.R., Schneider A. 2008. Old friends in new constellations—the hematopoetic growth factors G-CSF, GM-CSF, and EPO for the treatment of neurological diseases. Curr. Med. Chem. 15:1407–1411 10.2174/092986708784567671 [DOI] [PubMed] [Google Scholar]
- Michaelson M.D., Bieri P.L., Mehler M.F., Xu H., Arezzo J.C., Pollard J.W., Kessler J.A. 1996. CSF-1 deficiency in mice results in abnormal brain development. Development. 122:2661–2672 [DOI] [PubMed] [Google Scholar]
- Mizuno T., Doi Y., Mizoguchi H., Jin S., Noda M., Sonobe Y., Takeuchi H., Suzumura A. 2011. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-β neurotoxicity. Am. J. Pathol. 179:2016–2027 10.1016/j.ajpath.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S., Hayashi Y. 1998. Expression pattern and neurotrophic role of the c-fms proto-oncogene M-CSF receptor in rodent Purkinje cells. J. Neurosci. 18:10481–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G.M., Jr, Yang L., Cordell B. 1998. Macrophage colony-stimulating factor augments beta-amyloid-induced interleukin-1, interleukin-6, and nitric oxide production by microglial cells. J. Biol. Chem. 273:20967–20971 10.1074/jbc.273.33.20967 [DOI] [PubMed] [Google Scholar]
- Murphy G.M., Jr, Zhao F., Yang L., Cordell B. 2000. Expression of macrophage colony-stimulating factor receptor is increased in the AbetaPP(V717F) transgenic mouse model of Alzheimer’s disease. Am. J. Pathol. 157:895–904 10.1016/S0002-9440(10)64603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis. 45:593–605 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Nandi S., Gokhan S., Dai X.M., Wei S., Enikolopov G., Lin H., Mehler M.F., Stanley E.R. 2012. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 367:100–113 10.1016/j.ydbio.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J., Shannon-Dorcy K., Appelbaum F.R., Meyers J., Owens A., Day R., Ando D., O’Neill C., Buckner D., Singer J. 1993. Long-term follow-up of patients with invasive fungal disease who received adjunctive therapy with recombinant human macrophage colony-stimulating factor. Blood. 82:1422–1427 [PubMed] [Google Scholar]
- Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. 1997. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407:313–319 10.1016/S0014-5793(97)00313-X [DOI] [PubMed] [Google Scholar]
- Pan H., Zhu L., Deng Y., Pollard J.W. 2006. Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology. 147:4904–4916 10.1210/en.2006-0140 [DOI] [PubMed] [Google Scholar]
- Penkowa M., Poulsen C., Carrasco J., Hidalgo J. 2002. M-CSF deficiency leads to reduced metallothioneins I and II expression and increased tissue damage in the brain stem after 6-aminonicotinamide treatment. Exp. Neurol. 176:308–321 10.1006/exnr.2002.7968 [DOI] [PubMed] [Google Scholar]
- Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P.A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. 2008. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 118:2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley F.J., Stanley E.R. 2004. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 14:628–638 10.1016/j.tcb.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Pont F., Collet A., Lallement G. 1995. Early and transient increase of rat hippocampal blood-brain barrier permeability to amino acids during kainic acid-induced seizures. Neurosci. Lett. 184:52–54 10.1016/0304-3940(94)11166-G [DOI] [PubMed] [Google Scholar]
- Raivich G., Haas S., Werner A., Klein M.A., Kloss C., Kreutzberg G.W. 1998. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J. Comp. Neurol. 395:342–358 [DOI] [PubMed] [Google Scholar]
- Ray S., Britschgi M., Herbert C., Takeda-Uchimura Y., Boxer A., Blennow K., Friedman L.F., Galasko D.R., Jutel M., Karydas A., et al. 2007. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat. Med. 13:1359–1362 10.1038/nm1653 [DOI] [PubMed] [Google Scholar]
- Rockenstein E., Mallory M., Mante M., Sisk A., Masliaha E. 2001. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1-42). J. Neurosci. Res. 66:573–582 10.1002/jnr.1247 [DOI] [PubMed] [Google Scholar]
- Rothwell V.M., Rohrschneider L.R. 1987. Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res. 1:311–324 [PubMed] [Google Scholar]
- Ryan G.R., Dai X.M., Dominguez M.G., Tong W., Chuan F., Chisholm O., Russell R.G., Pollard J.W., Stanley E.R. 2001. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 98:74–84 10.1182/blood.V98.1.74 [DOI] [PubMed] [Google Scholar]
- Saija A., Princi P., Pisani A., Santoro G., De Pasquale R., Massi M., Costa G. 1992. Blood-brain barrier dysfunctions following systemic injection of kainic acid in the rat. Life Sci. 51:467–477 10.1016/0024-3205(92)90023-I [DOI] [PubMed] [Google Scholar]
- Sawada M., Itoh Y., Suzumura A., Marunouchi T. 1993. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci. Lett. 160:131–134 10.1016/0304-3940(93)90396-3 [DOI] [PubMed] [Google Scholar]
- Sherr C.J., Rettenmier C.W., Sacca R., Roussel M.F., Look A.T., Stanley E.R. 1985. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 41:665–676 10.1016/S0092-8674(85)80047-7 [DOI] [PubMed] [Google Scholar]
- Sierra A., Gottfried-Blackmore A.C., McEwen B.S., Bulloch K. 2007. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 55:412–424 10.1002/glia.20468 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1:4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Miyaishi O., Kiuchi K., Isobe K. 2001. Macrophage colony-stimulating factor is expressed in neuron and microglia after focal brain injury. J. Neurosci. Res. 65:38–44 10.1002/jnr.1125 [DOI] [PubMed] [Google Scholar]
- Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A., et al. 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 477:90–94 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent V.A., Robinson C.C., Simsek D., Murphy G.M. 2002. Macrophage colony stimulating factor prevents NMDA-induced neuronal death in hippocampal organotypic cultures. J. Neurochem. 82:1388–1397 10.1046/j.1471-4159.2002.01087.x [DOI] [PubMed] [Google Scholar]
- Walton M.R., Dragunow I. 2000. Is CREB a key to neuronal survival? Trends Neurosci. 23:48–53 10.1016/S0166-2236(99)01500-3 [DOI] [PubMed] [Google Scholar]
- Wang Y., Berezovska O., Fedoroff S. 1999. Expression of colony stimulating factor-1 receptor (CSF-1R) by CNS neurons in mice. J. Neurosci. Res. 57:616–632 [DOI] [PubMed] [Google Scholar]
- Wang Y., Szretter K.J., Vermi W., Gilfillan S., Rossini C., Cella M., Barrow A.D., Diamond M.S., Colonna M. 2012. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13:753–760 10.1038/ni.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Nandi S., Chitu V., Yeung Y.G., Yu W., Huang M., Williams L.T., Lin H., Stanley E.R. 2010. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J. Leukoc. Biol. 88:495–505 10.1189/jlb.1209822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A.W., Jr, Ahmed-Ansari A., Sell K.W., Pollard J.W., Stanley E.R. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA. 87:4828–4832 10.1073/pnas.87.12.4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. 2006. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 12:1005–1015 [DOI] [PubMed] [Google Scholar]
- Yang T., Knowles J.K., Lu Q., Zhang H., Arancio O., Moore L.A., Chang T., Wang Q., Andreasson K., Rajadas J., et al. 2008. Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS ONE. 3:e3604 10.1371/journal.pone.0003604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L.D., Nishikawa S. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345:442–444 10.1038/345442a0 [DOI] [PubMed] [Google Scholar]
- Zhu L., Ramboz S., Hewitt D., Boring L., Grass D.S., Purchio A.F. 2004. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci. Lett. 367:210–212 10.1016/j.neulet.2004.06.020 [DOI] [PubMed] [Google Scholar]