Abstract
Tissue engineering and regenerative techniques targeting bone include a broad range of strategies and approaches to repair, augment, replace or regenerate bone tissue. Investigations that are aimed at optimization of these strategies until clinical translation require control of systemic factors as well as modification of a broad range of key parameters.
This article reviews a possible strategy using a tissue engineering approach and systematically describes a series of experiments evaluating the properties of an embroidered and surface coated polycaprolactone-co-lactide scaffold being considered as bone graft substitute for large bone defects. The scaffold design and fabrication, the scaffolds properties, as well as its surface modification and their influence in vitro are evaluated, followed by in vivo analysis of the scaffolds using orthotopic implantation models in small and large animals.
Keywords: bone substitute, tissue engineering, scaffold, polycaprolactone-co-lactide, collagen type I, chondroitin sulfate, critical size defect, nude rat, sheep
Introduction
Bone regeneration in large bone defects resulting from trauma, inflammation and tumor resection remains an important but unsolved problem in trauma and orthopedic surgery. Limitations of the established techniques, such as distraction osteogenesis and implantation of autografts or allografts include problems with storage, immune reaction, infection risk, pain and availability.1,2 Synthetic scaffolds have evolved as a vivid alternative for bone reconstruction.3 The scientific field of tissue engineering has emerged as an important approach for bone regeneration. Newly developed implant materials like hydroxyapatite, polymers and composites, partly in combination with growth factors, bone marrow or mesenchymal stem cells are studied as alternatives, but until now none of the synthetic bone graft materials has been generally accepted.4-6
This article reviews the characterization of embroidered and surface coated polycaprolactone-co-lactide (trade name: PCL, Catgut GmbH) scaffolds as a bone graft substitute in large bone defects. The scaffolds design and fabrication, its properties, as well as the surface modifications and their influence in vitro were evaluated, followed by a step by step analysis of the scaffolds in vivo, including orthotopic implantation in small and large animal models (Table 1).
Table 1. Survey of the in vitro and in vivo studies about embroidered and surface modified PCL scaffolds as bioartificial bone substitute.
| Study | Study design | Methods | Main results | Reference |
|---|---|---|---|---|
| In vitro studies |
Material: non-coated, NaOH treated, Coll I and Coll I/CS coated PCL scaffolds Scaffold design: round, 1 mm thick, 14 mm outer and 4 mm inner diameter or 19 mm outer and 10 mm inner diameter Cells: MSC Analysis: - Scaffold properties (structure, porosity, pore size), - Adherence, proliferation and differentiation of MSC |
- Micro computered tomography, - Scanning electron microscopy, - Contact angle measurement, - Quantification of CS and Coll I (toluidine blue and sirius red), - Measurement of lactate dehydrogenase and alkaline phosphatase, - Calcium measurement (o-cresolphthalein complexone) and histology (von Kossa), - Reverse transcriptase polymerase chain reaction (alkaline phosphatase, bone sialoprotein) |
- Adequate porosity and pore size - Coll I enhanced cell attachment and proliferation - Coll I/CS induced osteogenic differentiation of MSC without differentiation additives |
7, 8, 9 |
| In vivo study, orthotopic (femur), immunodeficient nude rat |
Material/groups: non-coated, Coll I and Coll I/CS coated and Coll I/CS coated/hMSC seeded PCL scaffolds, 5 animals per group Scaffold design: round, 0.5 mm thick, 5 mm diameter, stack of 10 scaffolds per defect Cells: hMSC undifferentiated Animal model: 5 mm critical size defect, duration12 weeks |
- Radiography, computered tomography and final bone volume quantification - Histology/immunohistology: estimation of new bone formation (trichrome masson-goldner, osteopontin, osteonectin, collagen II), quantification of vessel formation (smooth muscle actin), cells survival (human nuclei), quantification of matrix deposition (histomorphometry) |
- Coll I coating acts as matrix for cell adhesion and proliferation - Coll I/CS coating allowed recruiting of cells, osteogenic stimulation and induction of new bone formation - Additional cell seeding showed higher matrix accumulation and vascularization, but could not clearly improve new bone formation |
10 |
| Pilot in vivo study, orthotopic (tibia), sheep |
Material/groups: Coll I/CS coated PCL, scaffolds, 5 animals per group and time point Scaffold design: 1 mm thick, 19 mm outer, 10 mm inner diameter, stack of 30 scaffolds Animal model: 3 cm critical size defect, duration12 and 48 weeks |
- Radiography, computered tomography, micro computered tomography, - Bone volume quantification - Histology (trichrome masson-goldner), - Biomechanics |
- Appropriate network of pores to permit a complete vascularization and bone tissue formation - New bone formation at the proximal and distal tibia fragments increasing over time - Bridging the defect up to defect healing in 50 % of the animals |
Polycaprolactone-co-lactide (trade name: PCL, Catgut GmbH), Collagen I (Coll I), chondroitin sulfate A (CS), mesenchymal stem cells (hMSC).
Scaffold Design and Fabrication
Biodegradable scaffolds based on natural or synthetic polymers have received special attention in the field of tissue engineering and regenerative medicine. They can provide porous matrices that can temporarily support cells and guide their development.7,8 An ideal polymeric scaffold requires several structural and chemical properties to control and promote specific events at the cellular and tissue level: a target tissue adapted structure, a sufficient porosity as well as interconnected pores of a suited size, an appropriate surface chemistry, a defined degradation rate and an easy fabrication in a variety of shapes and sizes. However, designing a suitable scaffold for bone applications has become one of the most challenging issues in material sciences.7-9
In the last decade, bone formation on 3-dimensional scaffolds based on a variety of polymers was studied. Among the synthetic scaffolds, polyester of d,l-lactid, glycolid or ε-caprolactone and their copolymers were used intensely as they are approved by health authorities in various countries and are commonly studied materials for biomedical applications in bone and cartilage repair, as drug delivery systems, and as surgical sutures.10-15
A broad variety of manufacture technologies has been applied to process these biodegradable and bioresorbable materials into 3-dimensional scaffolds, including textile technologies, solvent casting and particulate leaching, gas forming, emulsion freeze drying, electro spinning, thermally induced phase separation, and rapid prototyping technologies. All of these processing techniques having advantages and disadvantages.7,16,17
The polycaprolactone-co-lactide used for this study was synthesized by ring-opening copolymerization of l-lactide and ε-caprolactone, with a molecular ratio of 75/25 (Gunze Ltd.). Melt spinning of the material resulted in a resorbable, monofilament fiber which is commercially available and approved as medical device (PCL, surgical suture, Catgut GmbH).14,15,18
A traditional manufacturing technique (embroidery) was used to produce PCL scaffolds in various sizes and shapes, in high quantity and quality, on electronically guided machines, including CAD techniques (Fig. 1A). In general, the embroidery technique allows the control of the size, the arrangement and the orientation of the fibers and is an effective tool to produce highly porous scaffolds, required for implants to allow cell ingrowth and an efficient transport of nutrients, oxygen, growth factors and waste products through a rich vascularization. Despite these advantages, only few authors describe this method of embroidering scaffolds.10,18-21
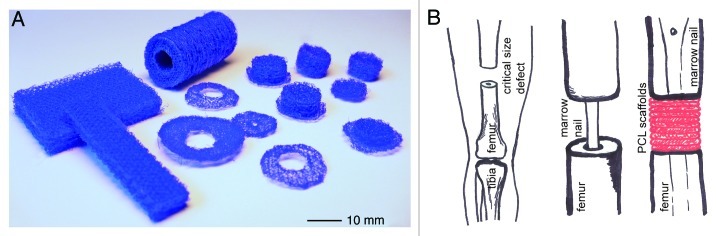
Figure 1. (A) Embroidering allows the fabrication of scaffolds in almost any size and shape. (B) Model of scaffold implantation in large bone defects. As many scaffolds as needed, could be piled up to create a real 3-dimensional implant that can be placed into the bone defect. New bone formation will take place during the scaffold resorption.
Adapted for the reconstruction of large bone defects the authors created round scaffolds of ca. 1 mm thickness having a triaxial structure (deposition of the thread in a triangular assembly; 0°/60°/120° netting) with a stitch length of 1.4 mm and a mesh spacing of 1.2 mm (Fig. 2A).18,20 The scaffolds can be piled up, depending on the defects size, to create a real 3-dimensional implant (Figs. 1B and 2B). The main advantages of the piling technique are the easy filling of any defect size as well as the possibility to provide scaffolds with different functionalities, e.g., coating and/or cell seeding within one implant. The created 3-dimensional PCL implant had a pore size of 0.2–1 mm, which represents a physiologically relevant range for bone tissue engineering. The 3-dimensional PCL scaffold showed an open and fully interconnected porosity of 87% (Fig. 2B and C).10,18,20
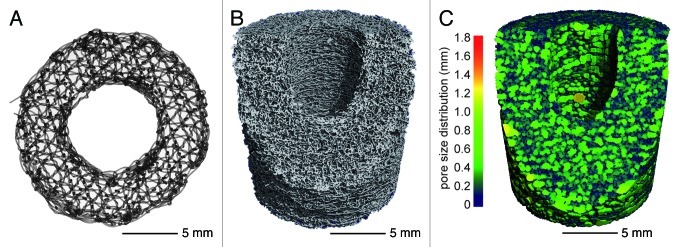
Figure 2. (A) Single embroidered scaffold designed for the reconstruction of large bone defects with a thickness of 1 mm and an outer diameter of 19 mm. The inner diameter of 10 mm provides space for an intramedullary nail. The triaxial structure had a stitch length of 1.4 mm and a mesh spacing of 1.2 mm. (B) The 3-dimensional reconstruction of the µCT analysis (Scanco vivaCT 75 system) of a 3 cm stack consisting of 30 single scaffolds shows an open porosity of 87%. (C) The analysis of the pore size distribution (Scanco vivaCT 75 system) shows homogeneously interconnected pores ranging between 0.1–0.8 mm distributed over the whole stack.
Porosity and pore size play a critical role in bone formation. Whereas lower porosities can enhance osteogenesis by suppressing cell proliferation and forcing cell aggregation, higher porosities and pore size result in greater and faster bone ingrowth. However, a pore size greater than 300 µm is generally accepted for enhancing bone ingrowth.22-24
Scaffold Surface
Besides the scaffold structure, the properties of the scaffold surface such as wettability, chemistry and roughness play an important role in biocompatibility of the material. Surface properties have a significant influence concerning initial protein interactions, which will mediate the cell response. Numerous studies have shown that surface roughness and a moderate wettability play a role in adhesion, proliferation and subsequent functionality of cells.25-27 However, for the construction of advanced bioartificial tissues, the material should not just be passively tolerated by the cells, but it should actively promote specific cell response.27
A treatment with NaOH turned the PCL polymer surface, described in this review, more hydrophilic which results in an increasing water contact angle. This promotes the collagen I (Coll I) surface coating on NaOH treated scaffolds (data not shown) and cell adherence after cell seeding on untreated, NaOH treated, NaOH treated and Coll I coated scaffolds (Fig. 3).18 Cell adhesion to artificial materials is mediated by molecules of the extra cellular matrix (ECM) e.g., fibronectin, vitronectin, collagen or fibrin which are normally adsorbed spontaneously to the material from different body fluids or are deposited by the cells themselves. On hydrophobic materials these molecules are adsorbed in a denatured and rigid state and their conformation is inappropriate for cells to bind.27 The hydrolysis of the polymer surface with NaOH resulted in the exposure of carboxylate and hydroxyl groups on the surface, directing more specific ionic interactions with the positively charged amino groups of proteins.18,28,29
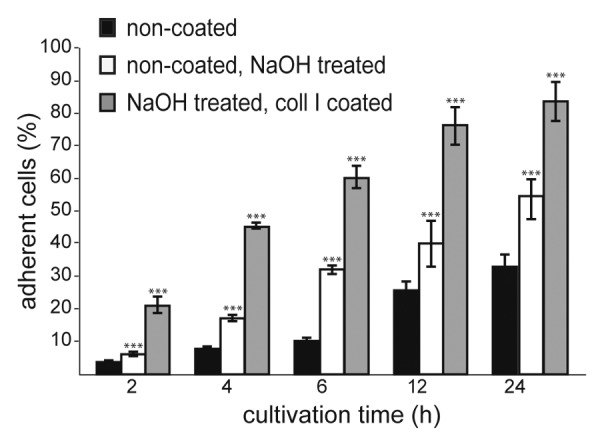
Figure 3. Effect of NaOH treatment (1 M NaOH in 50% methanol) on hMSC cell adherence. Two hundred thousand cells initially seeded per scaffold.
Surface Coating
Tissue engineering strategies include the transplantation of different kinds of cells alone or seeded on a variety of scaffolds and/or the use of biomolecules (growth factors, proteins, peptides or polysaccharides), which affect the cells of the target tissue.30,31 A considerable effort concerning tissue engineering strategies has been made in mimicking the ECM to guide morphogenesis and tissue repair.32 According to the function of the ECM in providing structural support, physical environment and bioactive molecules for cells to attach, grow and migrate, as well as for the regulation of their activity, the best scaffold for an engineered tissue should be adjusted to the ECM of the target tissue. Due to the fact that its function, complexity and dynamic nature make it difficult to imitate the ECM exactly, mimicry of the ECM on scaffolds should be aspired, at least partly.9,31 A step in the creation of an artificial ECM (aECM) is the immobilization of Coll I, the main component of the ECM, to the surface of scaffolds or implants.10,18,33-36
The function of Coll I can be ameliorated by glycosaminoglycans (GAG) like chondroitin sulfate (CS). CS is an important GAG inside the ECM of bone as a component of proteoglycans. It plays a key role in bone development, remodeling and healing by interacting with other molecules of the ECM, mediating cell adherence and providing the binding of different growth factors or cytokines on the ECM.10,18,34,37-40
The reticular PCL scaffolds used in this study were coated with Coll I or Coll I/CS. During the fibrillogenesis the Coll I (porcine skin, Medical Biomaterial Products) was adsorbed on the scaffold surface whereas CS (porcine trachea, Kraeber and Co. GmbH) was immobilized within the Coll I matrix (Fig. 4A and B).18,20
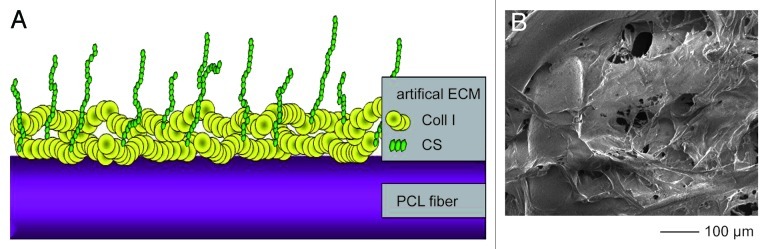
Figure 4. (A) The drawing presents schematically the Coll I and CS surface coating of a PCL scaffold showing immobilized Collagen fibrils on the polymer surface with incorporated CS chains. (B) The SEM micrograph shows the Coll I covering the polymer fiber and partly the pores. The addition of CS had no discernible influence on the resulting surface morphology.
In Vitro Experiments
In vitro assays using cell culture techniques are essential as the first step to discover the biological mechanisms and to characterize the effects of a material itself, as well as the material structure and surface properties, on isolated cells.
Non-coated, Coll I and Coll I/CS coated PCL scaffolds were seeded with mesenchymal stem cells (MSC) in a semi dynamic spinner system to investigate cell adherence, proliferation and differentiation.18,20,21 As a result, the coating with Coll I enhanced cell attachment and proliferation, as well as an osteogenic differentiation in differentiation medium, compared with uncoated scaffolds. The Coll I I/CS coating induced osteogenic differentiation of MSC in regular cultivation media (expansion medium) without the common differentiation additives, indicated through the increasing activity of the alkaline phosphatase (ALP) and large amounts of calcified matrix (Fig. 5A and B).18-20
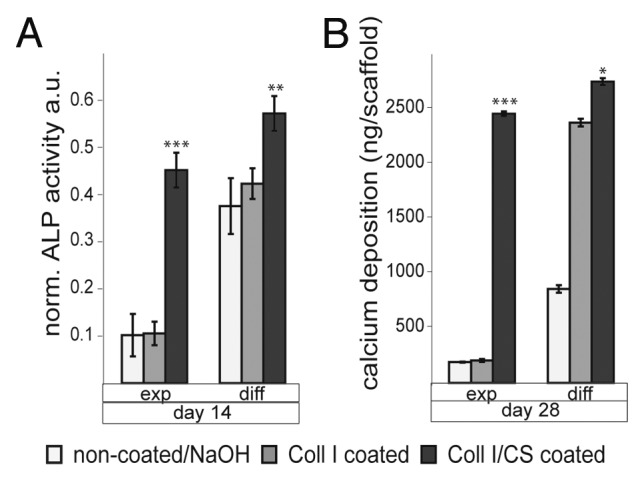
Figure 5. Differentiation of expanded or osteogenic differentiated hMSC on non-coated, Coll I coated, and Coll I/CS coated PCL scaffolds (exp, expansion medium; diff, differentiation medium). (A) alkaline phosphatase (ALP) activity, a.u. arbitrary units; (B) calcium deposition (significances: *p < 0.05, **p < 0.01, ***p < 0.001).18
Although in vitro assays using cell culture systems permit the characterization of the effects of the material and its properties on isolated cell functions, the capacity to display the complex in vivo situation is limited and not reliable in predicting the performance in vivo or in clinical applications. Animal models are required when in vitro systems have reached their limits.41
Orthotopic Analysis in a Small Animal Model
To establish a tissue engineering concept in a clinical setting, most of the feasibility and bioactivity testing are done in vitro and in small animal models like mice and rats. As an advantage, the outcomes can be determined after a relatively short period of time, animal-specific antibodies and probes are available, and the variation in radiography, imaging, histology and biochemical outcome is low.42
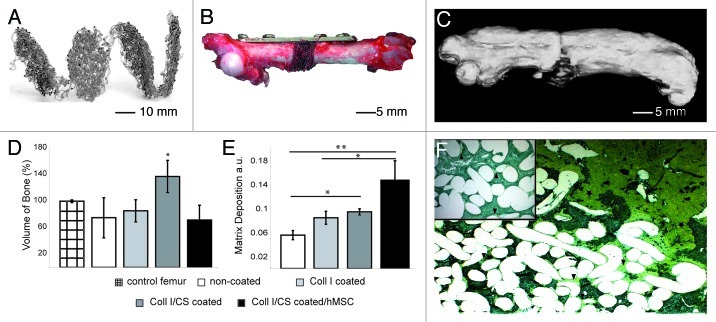
To evaluate the osteogenic potential of the embroidered, tissue-engineered PCL scaffolds for the application as a bone implant, a 5 mm long segmental mid-diaphysial femoral critical size defect was created in immunodeficient nude rats and stabilized with a mini-fragment plate. Ten piled-up PCL scaffolds were implanted into each defect to create a 3-dimensional scaffold, and four different conditions were investigated (group 1, non-coated; group 2, Coll I coated; group 3, Coll I/CS coated; group 4, Coll I/CS coated and hMSC seeded) (Fig. 6A and B).10
Figure 6. In vivo study small animals: created 5 mm orthotopic critical size defect (femur) in immunodeficient nude rat. Implantation of non-coated, Coll I or Coll I/CS coated, as well as Coll I/CS coated/hMSC seeded PCL scaffolds over 12 weeks, five animals per group. (A) Specially designed PCL scaffolds for rat femur critical size defects with a thickness of 0.5 mm and a diameter of 5 mm. (B) A 5-hole mini-fragment plate was fixed to the femur and a 5 mm long segmental mid-diaphysial osteotomy was performed. (C) 3-dimensional CT reconstruction of a rat femur (Coll I/CS group) showing callus formation along the femur. The new bone formed along and into the scaffold pad up to bridging the critical size defect. (D) Quantification of newly produced bone volume in the defect zone showed the highest amount of new bone in the Coll I/CS group (137%) compared with the non-coated (75%), Coll I (85%), or Coll I/CS/hMSC (72%) group. Non-operated contralateral femora were used as control (100%) (significance: *p < 0.05). (E) Quantification of the matrix deposition using a modified trichrome Masson-Goldner staining in the defect zone showing the highest matrix accumulation in the Coll I/CS/hMSC followed by the Coll I/CS group, the Coll I group and the non-coated group, a.u. arbitrary units (significances: *p < 0.05, **p < 0.01). (F) New bone formation occurred at the proximal and distal ends of the defect zone, localized around the scaffold pad and in the bordering scaffold areas (star, green coloring). The central part showed variable amounts of matrix aggregation (arrow, yellow coloring) depending on the surface modification of the scaffold (Coll I/CS/hMSC > Coll I/CS > Coll I > non-coated).10
Substantial differences in the in vivo bone healing between the differently coated scaffolds were observed. New bone formation took place in all groups, showing the significantly highest bone volume in the Coll I/CS group and the maximum matrix deposition in the Coll I/CS/hMSC group (Fig. 6C–E). Although the seeded cells could survive the observation period after implantation, the expected positive influence on bone healing of the previously seeded hMSC could not be proven.10 Due to these promising results and to clarify new bone formation under more specific, clinically relevant circumstances, further orthotopic investigations in large animals were realized.
Orthotopic Analysis in a Large Animal Model
A typical progression of animal experiments is an evolution from smaller to larger animals to mimic the process of bone healing, as well as the implants used and the associated surgical procedures as closely as possible to that in humans.41-43
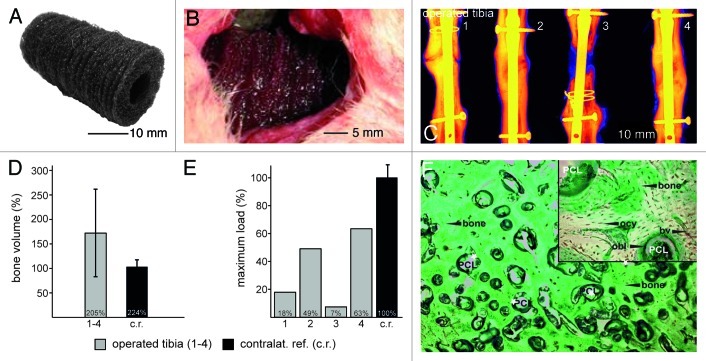
The embroidered 3-dimensional PCL scaffold stack (composed of 30 single scaffolds coated with Coll I/CS) was implanted in a 3 cm tibial critical size defect in sheep (n = 10) (Fig. 7A and B). New bone formation was determined over a period of 3 (n = 5) and 12 (n = 5) months by histological, radiological, CT-morphometric and biomechanical investigations.
Figure 7. In vivo study large animals: created 3 cm orthotopic critical size defect (tibia) in sheep. Implantation of Coll I/CS coated PCL scaffolds over 12 and 48 weeks, five animals per group and time point. (A) Thirty piled scaffolds forming a 3-dimensional implant. (B) Three centimeter long mid-diaphysial defect in the sheep tibia filled with 30 piled scaffolds. (C) Radiological investigation (false coloring) of four sheep tibia defect areas showing two tibial defects were bridged completely after 12 mo (2 and 4). One showed a hypertrophic non-union (1) and one an atrophic non-union (3). (D) The quantification of the bone volume ratio after 12 mo shows the newly formed bone averaged 172% (+/− 86%) as compared with the intact contra lateral tibiae used as a reference value (100%). (E) Biomechanical evaluation at 12 mo (maximum load until failure) demonstrated that two operated tibiae (1 and 4) reached 49% (2,880 N) and 63% (3,720 N) of the reference value for non-operated bone respectively (c.r., 100%, 5,875 N). The values in the other two animals (2 and 3) reached 18% (1,050 N) and 7% (428 N). (F) Trichrome Masson-Goldner staining. Bone formation took place directly around the scaffold fibers revealing no interconnected gaps. The newly formed lamellar bone inside the scaffolds presented osteons including Haversian canals suggesting regular bone formation. According to their natural localization, osteocytes (ocy) and osteoblasts (obl) could be localized within the bone or at the adjacent areas. The scaffold was completely vascularized (bv) and erosion of the PCL fibers was clearly visible. No inflammatory reaction was evident around the implant material after 12 mo.
The scaffold handling, using the piling technique, was uncomplicated and allowed creating a 3-dimensional implant with fully interconnected pores and a completely coated surface. The future clinical user will be able to handle the implant technique following a simple instructions protocol, not requiring any new or expensive instruments. The surgeon will be flexible to adapt the number of scaffolds to the defect size. Scaffolds can be produced in any size to fit any diameter of long bone.
Microscopically the PCL scaffold could still be observed after 3 mo of implantation and was completely intermingled with firm connective tissue, cartilage and bone. New bone formation was mostly localized at the peripheral zone of the scaffold stack. Small islets of new bone matrix were present at the inner parts of the scaffolds, straight around the scaffold fibers. The new bone matrix enclosed the scaffold fibers without any fibrous interface. After 12 mo two out of four tibia defects were bridged (Fig. 7C, E and F). New bone formation occurred from both proximal and distal tibial fragments until completely bridging the 3 cm critical size defect. One animal showed a hypertrophic nonunion and one an atrophic nonunion after 12 mo. Both animals had to be stabilized with a cerclage wire after nail insertion during surgery (see radiograph in Fig. 7C). Additional fractures, treated with a cerclage, and probably unknown micro-fractures may be responsible for both observed nonunions. There was no inflammation or necrosis noticeable at both time points. The 3-dimensional PCL scaffold stack did not result in any tissue free or non-vascularized areas after 3 or 12 mo (Fig. 7F).
Bone quantification was performed and revealed a mean new bone formation of 63% after 3 mo and 172% after 12 mo (Fig. 7D) compared with the contralateral tibiae. Nevertheless, the quantity of bone is not the only important factor of healing. The bone quality, including cortical and trabecular structure, their thickness and spacing provide evidences for the apparent mechanical properties.44 Reaching a biomechanical stability of 63% and 49% (in the two bridged defects) compared with the non-operated tibia after 12 mo, these preliminary data were considered as encouraging to plan additional large animal studies using a larger numbers of animals (Fig. 7E).
Conclusions
In vitro and in vivo studies with small and large animal models have demonstrated that embroidered and biologically modified Coll I/CS PCL scaffolds provide an appropriate network of interconnecting pores to act as a temporary matrix for cell adherence, migration, proliferation and differentiation. The 3-dimensional scaffolds allowed in vivo the reconstruction of a completely vascularized defect zone, presenting new bone formation by direct and endochondral ossification. However, to become clinically relevant a rigorous demonstration of the level of therapeutic benefit in preclinical models is important. Large animal pilot studies should be followed by additional preclinical investigations using an adequate number of animals over a clinically relevant time schedule, to create an environment that is as close as possible to the clinical setting in which a therapy will be used. From a scientific point of view the reported data are encouraging for these future experiments.
Data analysis
All statistical analyses were done using the Student’s t-test.
Glossary
Abbreviations:
- ECM
extra cellular matrix
- aECM
artificial ECM
- PCL
polycaprolactone-co-lactide
- Coll I
collagen I
- CS
chondroitin sulfate A
- MSC
mesenchymal stem cells
- hMSC
human mesenchymal stem cells
- exp
expansion medium
- diff
differentiation medium
- CAD
computer-aided design
- NaOH
sodium hydroxide
- SEM
scanning electron microscope
- ALP
alkaline phosphatase
- au
arbitrary units
- CT
computed tomography
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical Statement
The animal studies have been licensed by the regional board of Dresden (24-9168.11-1-2004-31, 24D-9168.11-1-2006-17). All animals were cared for according to the European guidelines for the care and use of laboratory animals (Directive 24.11.1986, 86/609/CEE).
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/21931
References
- 1.Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240–50. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Drosse I, Volkmer E, Capanna R, De Biase P, Mutschler W, Schieker M. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury. 2008;39:9–20. doi: 10.1016/S0020-1383(08)70011-1. [DOI] [PubMed] [Google Scholar]
- 3.Calori GM, Mazza E, Colombo M, Ripamonti C. The use of bone-graft substitutes in large bone defects: any specific needs? Injury. 2011;42(Suppl 2):S56–63. doi: 10.1016/j.injury.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Blokhuis TJ, Lindner T. Allograft and bone morphogenetic proteins: an overview. Injury. 2008;39(Suppl 2):S33–6. doi: 10.1016/S0020-1383(08)70013-5. [DOI] [PubMed] [Google Scholar]
- 5.Kruyt M, De Bruijn J, Rouwkema J, Van Bliterswijk C, Oner C, Verbout A, et al. Analysis of the dynamics of bone formation, effect of cell seeding density, and potential of allogeneic cells in cell-based bone tissue engineering in goats. Tissue Eng Part A. 2008;14:1081–8. doi: 10.1089/ten.tea.2007.0111. [DOI] [PubMed] [Google Scholar]
- 6.Betz OB, Betz VM, Abdulazim A, Penzkofer R, Schmitt B, Schröder C, et al. The repair of critical-sized bone defects using expedited, autologous BMP-2 gene-activated fat implants. Tissue Eng Part A. 2010;16:1093–101. doi: 10.1089/ten.tea.2009.0656. [DOI] [PubMed] [Google Scholar]
- 7.Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007;59:249–62. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Gloria A, De Santis R, Ambrosio L. Polymer-based composite scaffolds for tissue engineering. J Appl Biomater Biomech. 2010;8:57–67. [PubMed] [Google Scholar]
- 9.Kim YH, Jyoti MA, Youn MH, Youn HS, Seo HS, Lee BT, et al. In vitro and in vivo evaluation of a macro porous beta-TCP granule-shaped bone substitute fabricated by the fibrous monolithic process. Biomed Mater. 2010;5:035007. doi: 10.1088/1748-6041/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 10.Rentsch C, Rentsch B, Breier A, Spekl K, Jung R, Manthey S, et al. Long-bone critical-size defects treated with tissue-engineered polycaprolactone-co-lactide scaffolds: a pilot study on rats. J Biomed Mater Res A. 2010;95:964–72. doi: 10.1002/jbm.a.32878. [DOI] [PubMed] [Google Scholar]
- 11.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–27. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 12.Vergroesen PP, Kroeze RJ, Helder MN, Smit TH. The use of poly(L-lactide-co-caprolactone) as a scaffold for adipose stem cells in bone tissue engineering: application in a spinal fusion model. Macromol Biosci. 2011;11:722–30. doi: 10.1002/mabi.201000433. [DOI] [PubMed] [Google Scholar]
- 13.Bramfeldt H, Sarazin P, Vermette P. Characterization, degradation, and mechanical strength of poly(D,L-lactide-co-ε-caprolactone)-poly(ethylene glycol)-poly(D,L-lactide-co-ε-caprolactone) J Biomed Mater Res A. 2007;83:503–11. doi: 10.1002/jbm.a.31300. [DOI] [PubMed] [Google Scholar]
- 14.Tomihata K, Suzuki M, Oka T. Ikada Y. A new resorbable monofilament suture. Polym Degrad Stabil. 1998;51:13–8. doi: 10.1016/S0141-3910(97)00183-3. [DOI] [Google Scholar]
- 15.Tomihata K, Suzuki M, Tomita N. Handling characteristics of poly(L-lactide-co-epsilon-caprolactone) monofilament suture. Biomed Mater Eng. 2005;15:381–91. [PubMed] [Google Scholar]
- 16.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90(Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 17.Tanner KE. Bioactive composites for bone tissue engineering. Proc Inst Mech Eng H. 2010;224:1359–72. doi: 10.1243/09544119JEIM823. [DOI] [PubMed] [Google Scholar]
- 18.Rentsch B, Hofmann A, Breier A, Rentsch C, Scharnweber D. Embroidered and surface modified polycaprolactone-co-lactide scaffolds as bone substitute: in vitro characterization. Ann Biomed Eng. 2009;37:2118–28. doi: 10.1007/s10439-009-9731-0. [DOI] [PubMed] [Google Scholar]
- 19.Hess R, Douglas T, Myers KA, Rentsch B, Rentsch C, Worch H, et al. Hydrostatic pressure stimulation of human mesenchymal stem cells seeded on collagen-based artificial extracellular matrices. J Biomech Eng. 2010;132:021001. doi: 10.1115/1.4000194. [DOI] [PubMed] [Google Scholar]
- 20.Rentsch C, Hess R, Rentsch B, Hofmann A, Manthey S, Scharnweber D, et al. Ovine bone marrow mesenchymal stem cells: isolation and characterization of the cells and their osteogenic differentiation potential on embroidered and surface-modified polycaprolactone-co-lactide scaffolds. In Vitro Cell Dev Biol Anim. 2010;46:624–34. doi: 10.1007/s11626-010-9316-0. [DOI] [PubMed] [Google Scholar]
- 21.Wollenweber M, Domaschke H, Hanke T, Boxberger S, Schmack G, Gliesche K, et al. Mimicked bioartificial matrix containing chondroitin sulphate on a textile scaffold of poly(3-hydroxybutyrate) alters the differentiation of adult human mesenchymal stem cells. Tissue Eng. 2006;12:345–59. doi: 10.1089/ten.2006.12.345. [DOI] [PubMed] [Google Scholar]
- 22.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–65. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 23.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Hutmacher DW, Schantz JT, Lam CX, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245–60. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 25.Kay S, Thapa A, Haberstroh KM, Webster TJ. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002;8:753–61. doi: 10.1089/10763270260424114. [DOI] [PubMed] [Google Scholar]
- 26.Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28:3074–82. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv. 2011;29:739–67. doi: 10.1016/j.biotechadv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Park GE, Pattison MA, Park K, Webster TJ. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005;26:3075–82. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Atthoff B, Hilborn J. Protein adsorption onto polyester surfaces: is there a need for surface activation? J Biomed Mater Res B Appl Biomater. 2007;80:121–30. doi: 10.1002/jbm.b.30576. [DOI] [PubMed] [Google Scholar]
- 30.Hutmacher DW, Cool S. Concepts of scaffold-based tissue engineering--the rationale to use solid free-form fabrication techniques. J Cell Mol Med. 2007;11:654–69. doi: 10.1111/j.1582-4934.2007.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551–8. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Rammelt S, Schulze E, Witt M, Petsch E, Biewener A, Pompe W, et al. Collagen type I increases bone remodelling around hydroxyapatite implants in the rat tibia. Cells Tissues Organs. 2004;178:146–57. doi: 10.1159/000082245. [DOI] [PubMed] [Google Scholar]
- 34.Schneiders W, Reinstorf A, Biewener A, Serra A, Grass R, Kinscher M, et al. In vivo effects of modification of hydroxyapatite/collagen composites with and without chondroitin sulphate on bone remodeling in the sheep tibia. J Orthop Res. 2009;27:15–21. doi: 10.1002/jor.20719. [DOI] [PubMed] [Google Scholar]
- 35.Stadlinger B, Bierbaum S, Grimmer S, Schulz MC, Kuhlisch E, Scharnweber D, et al. Increased bone formation around coated implants. J Clin Periodontol. 2009;36:698–704. doi: 10.1111/j.1600-051X.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- 36.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27:5561–71. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Manton KJ, Leong DF, Cool SM, Nurcombe V. Disruption of heparan and chondroitin sulfate signaling enhances mesenchymal stem cell-derived osteogenic differentiation via bone morphogenetic protein signaling pathways. Stem Cells. 2007;25:2845–54. doi: 10.1634/stemcells.2007-0065. [DOI] [PubMed] [Google Scholar]
- 39.Volpi N. Quality of different chondroitin sulfate preparations in relation to their therapeutic activity. J Pharm Pharmacol. 2009;61:1271–80. doi: 10.1211/jpp.61.10.0002. [DOI] [PubMed] [Google Scholar]
- 40.Vandrovcová M, Douglas T, Hauk D, Grössner-Schreiber B, Wiltfang J, Bač´ková L, et al. Influence of collagen and chondroitin sulfate (CS) coatings on poly-(lactide-co-glycolide) (PLGA) on MG 63 osteoblast-like cells. Physiol Res. 2011;60:797–813. doi: 10.33549/physiolres.931994. [DOI] [PubMed] [Google Scholar]
- 41.Muschler GF, Raut VP, Patterson TE, Wenke JC, Hollinger JO. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2010;16:123–45. doi: 10.1089/ten.teb.2009.0658. [DOI] [PubMed] [Google Scholar]
- 42.O’Loughlin PF, Morr S, Bogunovic L, Kim AD, Park B, Lane JM. Selection and development of preclinical models in fracture-healing research. J Bone Joint Surg Am. 2008;90(Suppl 1):79–84. doi: 10.2106/JBJS.G.01585. [DOI] [PubMed] [Google Scholar]
- 43.Histing T, Garcia P, Holstein JH, Klein M, Matthys R, Nuetzi R, et al. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone. 2011;49:591–9. doi: 10.1016/j.bone.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Mittra E, Rubin C, Qin YX. Interrelationship of trabecular mechanical and microstructural properties in sheep trabecular bone. J Biomech. 2005;38:1229–37. doi: 10.1016/j.jbiomech.2004.06.007. [DOI] [PubMed] [Google Scholar]