Abstract
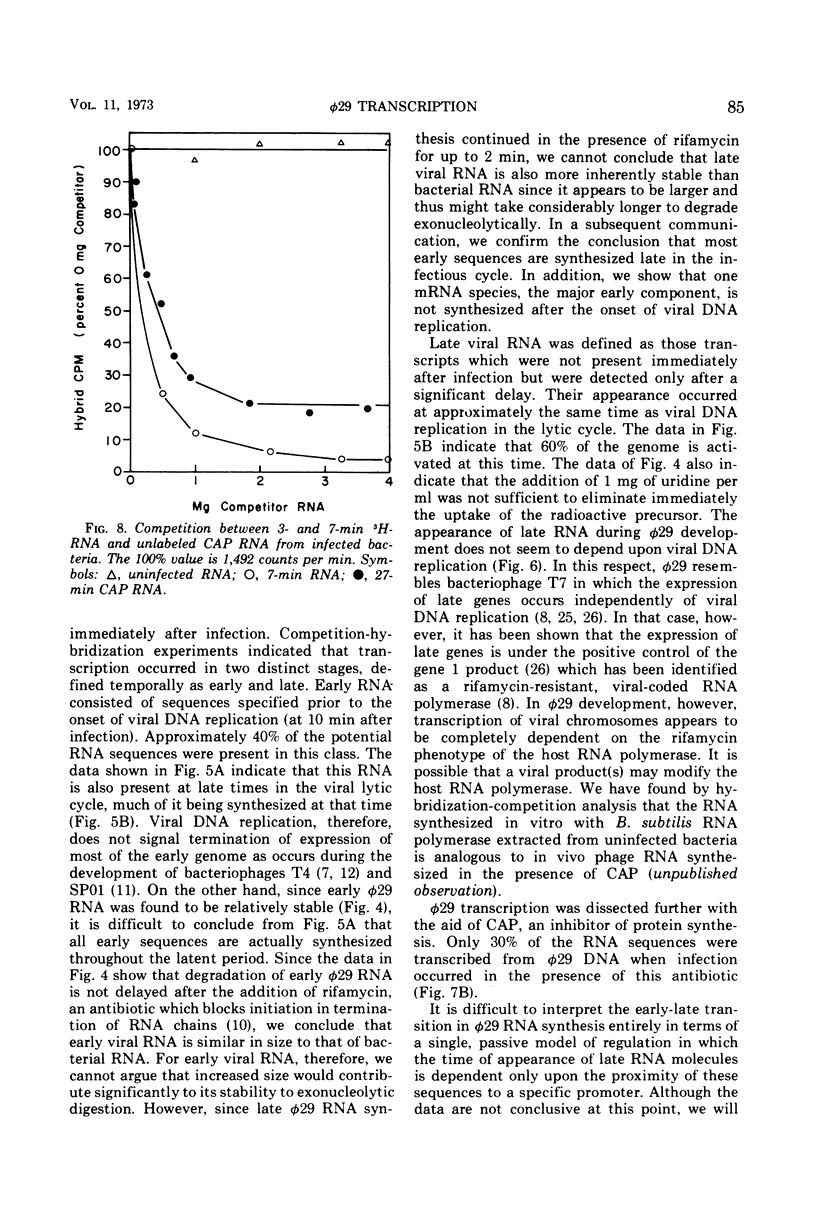
The ribonucleic acid (RNA) specified by bacteriophage φ29 was analyzed to determine its composition at various times in the viral lytic cycle. Although viral-specific RNA was detected immediately after infection, a large increase in the rate was observed at 10 min when DNA synthesis began. φ29 was found to resemble other viruses in that gene expression occurred in two stages which could be defined temporally as “early” and “late.” Early RNA appeared before the onset of viral deoxyribonucleic acid (DNA) replication and accounted for approximately 40% of the viral genetic potential. This RNA was also present late in the infectious cycle because of the slow turnover rate of φ29-specific RNA (approximately 10 min half-life) and the continued synthesis of much early viral RNA throughout infection. Late RNA was first detected at approximately the same time as viral DNA replication, although late transcription was not dependent upon DNA synthesis. This RNA was only partially displaced by early RNA in the appropriate competition experiments, suggesting that it contained sequences not present in the early class. Expression of viral genes was sensitive to rifamycin throughout the lytic cycle, the sensitivity resulting from a dependence upon the rifamycin phenotype of the host RNA polymerase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C., Handschumacher R. E. Inhibition of the synthesis of deoxyribonucleic acid in bacteria by 6-(p-hydroxyphenylazo)-2,4-dihydroxypyrimidine. I. Metabolic studies in Streptococcus fecalis. J Biol Chem. 1966 Jul 10;241(13):3083–3089. [PubMed] [Google Scholar]
- Calendar R. The regulation of phage development. Annu Rev Microbiol. 1970;24:241–296. doi: 10.1146/annurev.mi.24.100170.001325. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. Formation of ribosylthymine in Escherichia coli. Studies on pulse labeling with thymine and thymidine. J Biol Chem. 1969 May 25;244(10):2710–2715. [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriphage SPO1 development. II. Some modulations and prerequisites of the transcription program. Virology. 1971 Apr;44(1):200–210. doi: 10.1016/0042-6822(71)90165-6. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Haselkorn R. Messenger RNA. Annu Rev Biochem. 1969;38:647–676. doi: 10.1146/annurev.bi.38.070169.003243. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Sklar J. Continual requirement for a host RNA polymerase component in a bacteriophage development. Nature. 1969 Mar 1;221(5183):833–836. doi: 10.1038/221833a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hagen E. W., Zeece V. M., Anderson D. L. A genetic study of temperature-sensitive mutants of the Bacillus subtilis bacteriophage phi 29. Virology. 1971 Mar;43(3):561–568. doi: 10.1016/0042-6822(71)90281-9. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi G., Brody E. N., Grau O., Geiduschek E. P. Transcriptions of the bacteriophage T4 template in vitro: separation of "delayed early" from "immediate early" transcription. Proc Natl Acad Sci U S A. 1970 May;66(1):181–188. doi: 10.1073/pnas.66.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharrafa E. T., Schachtele C. F., Reilly B. E., Anderson D. L. Complementary Strands of Bacteriophage phi29 Deoxyribonucleic Acid: Preparative Separation and Transcription Studies. J Virol. 1970 Dec;6(6):855–864. doi: 10.1128/jvi.6.6.855-864.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez E., Ramírez G., Salas M., Viñuela E. Structural proteins of bacteriophage phi 29. Virology. 1971 Sep;45(3):567–576. doi: 10.1016/0042-6822(71)90172-3. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Siegel R. B., Summers W. C. The process of infection with coliphage T7. 3. Control of phage-specific RNA synthesis in vivo by an early phage gene. J Mol Biol. 1970 Apr 14;49(1):115–123. doi: 10.1016/0022-2836(70)90380-3. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Control of template specificity of E. coli RNA polymerase by a phage-coded protein. Nature. 1969 Sep 13;223(5211):1111–1113. doi: 10.1038/2231111a0. [DOI] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]


