Abstract
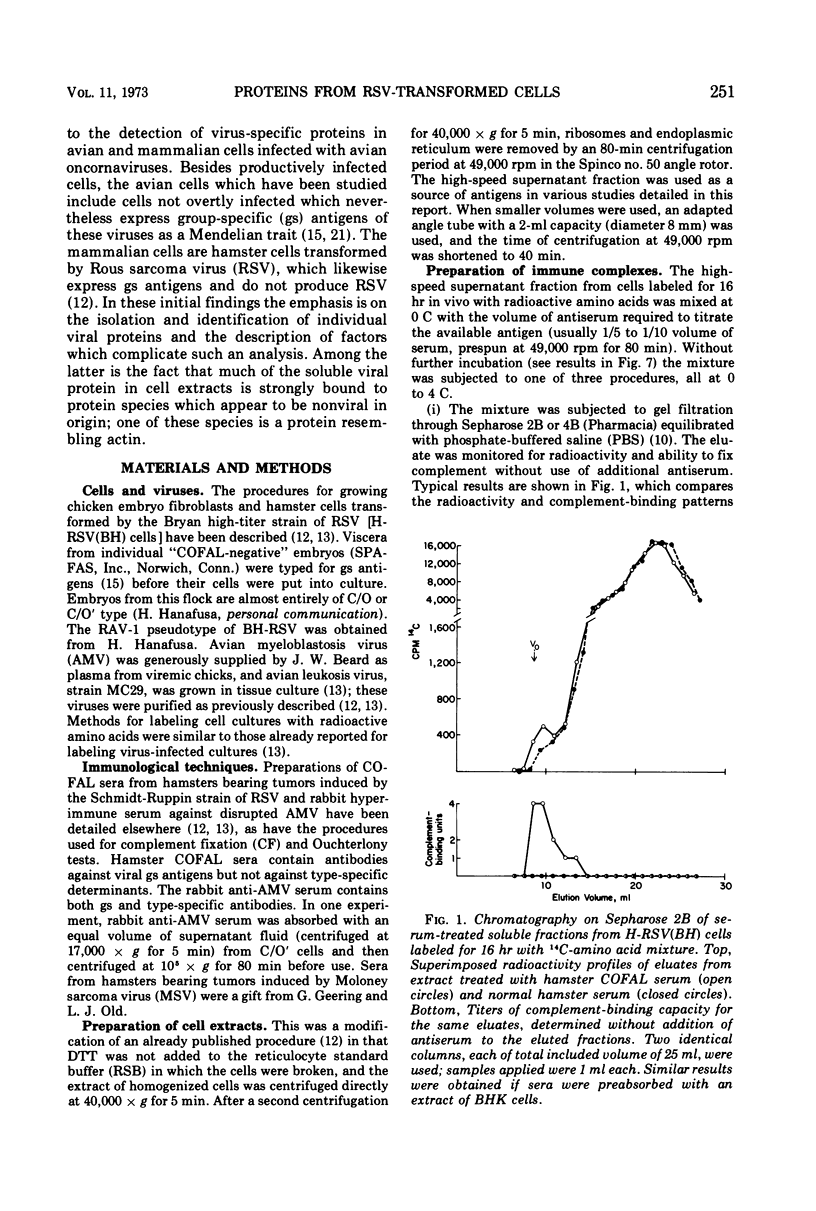
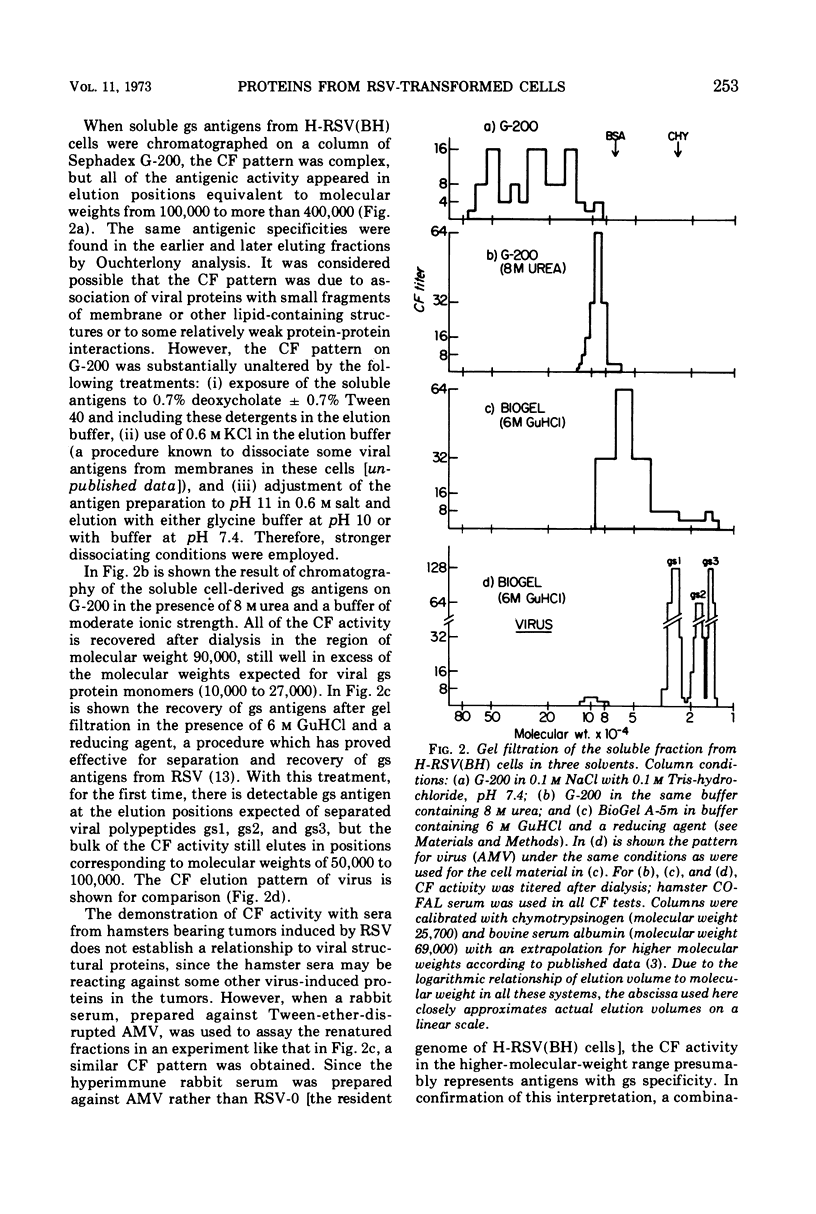
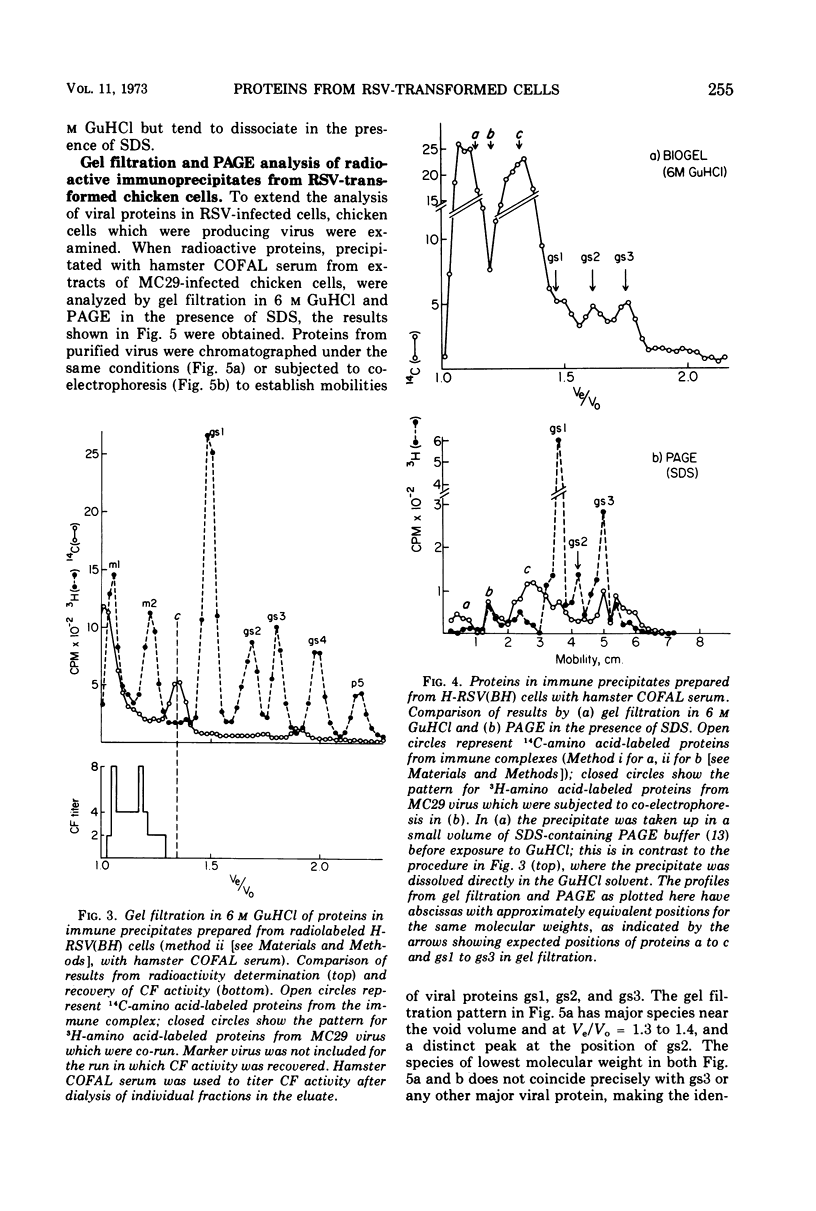
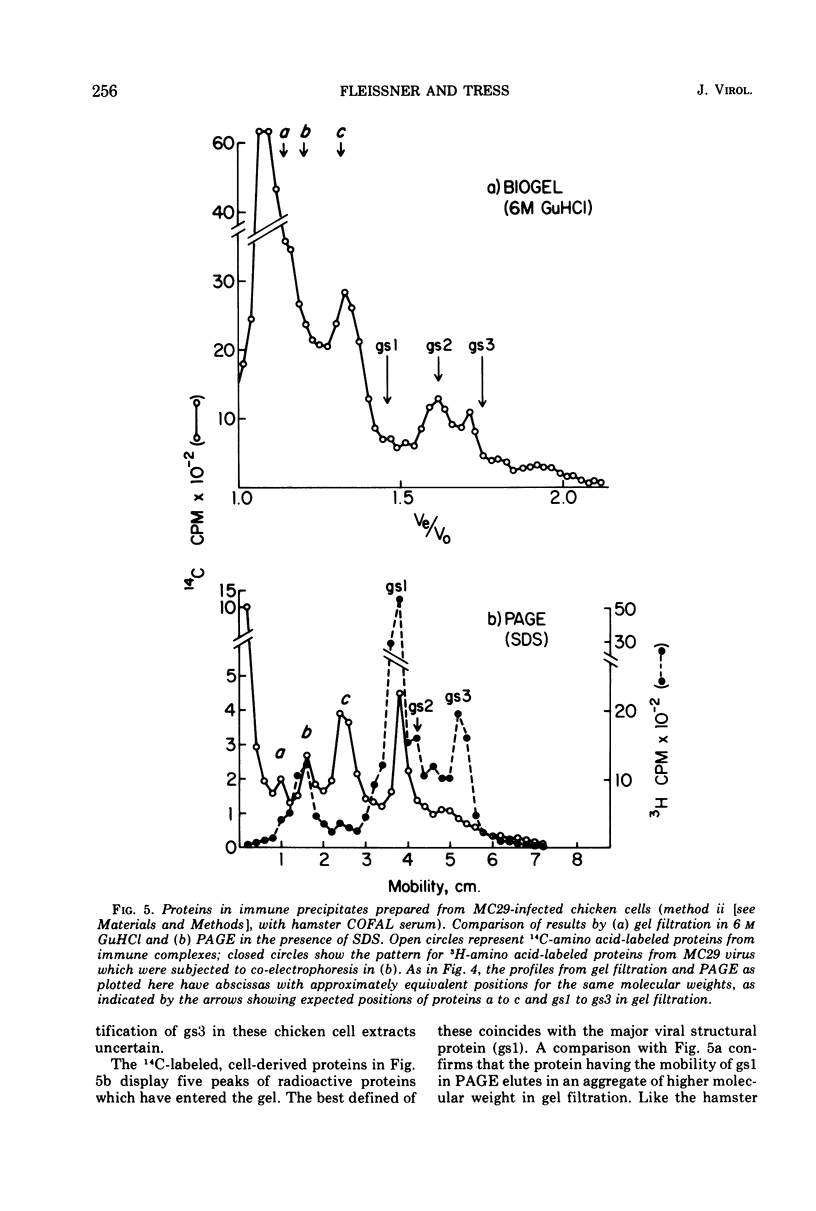
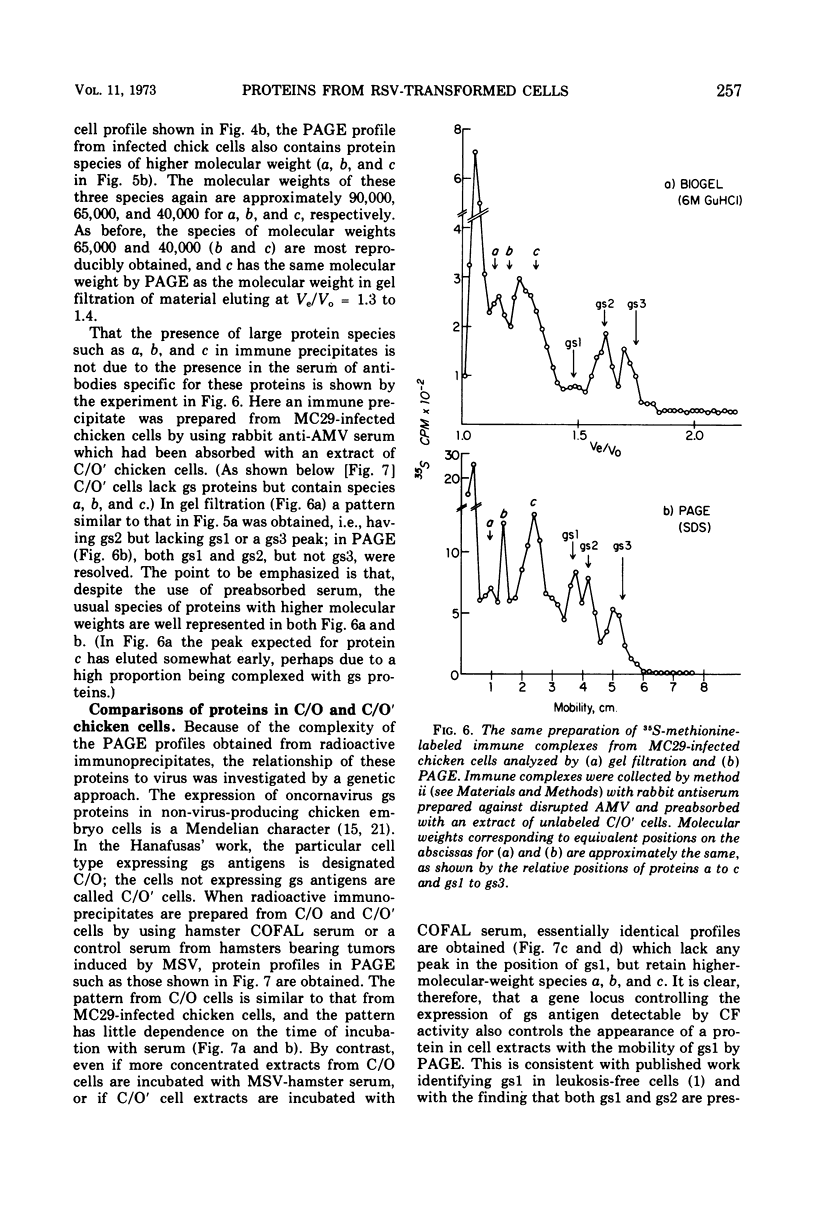
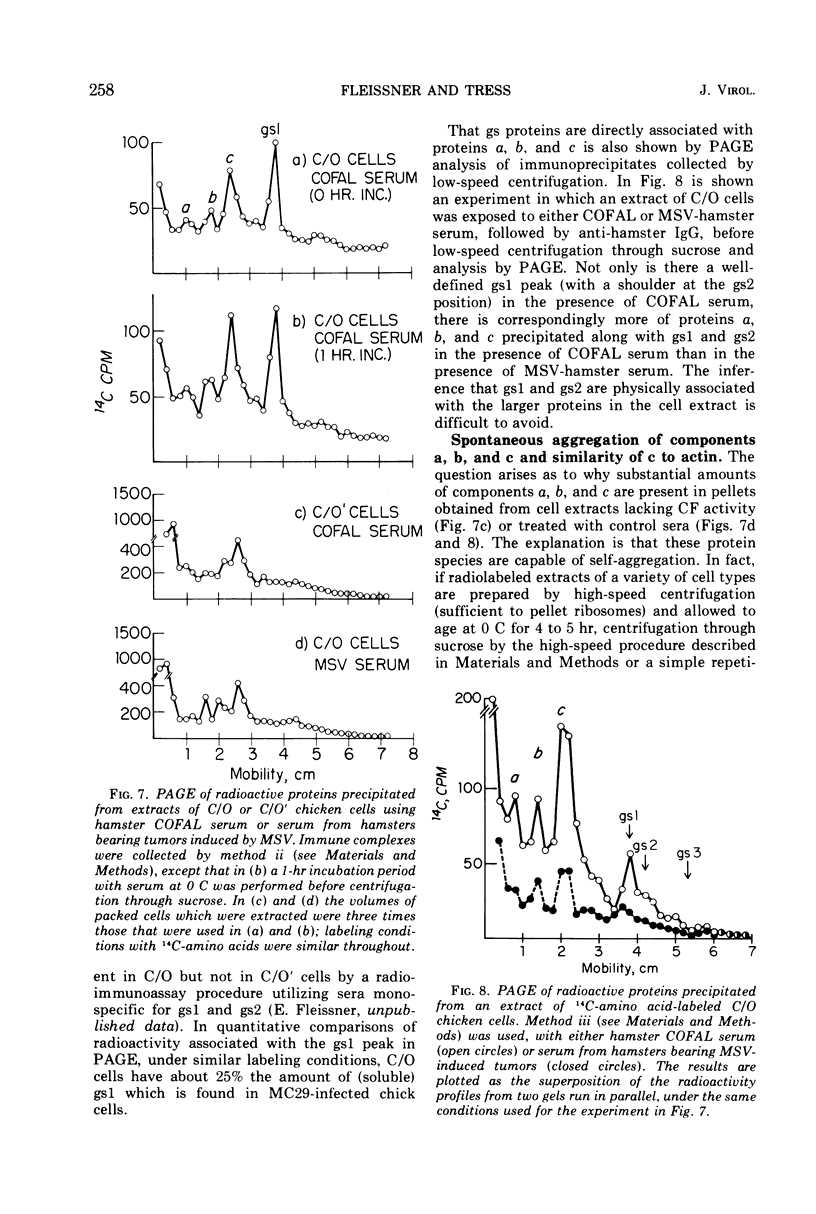
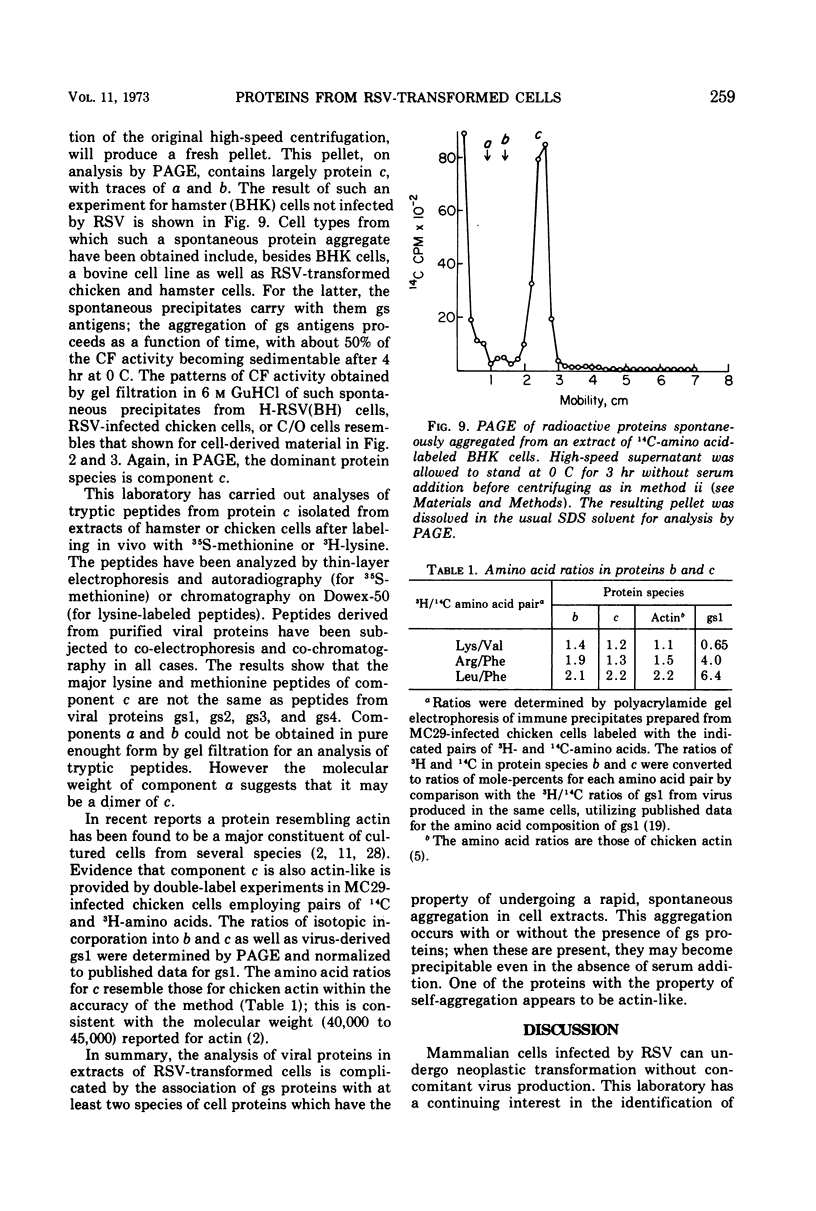
Several methods have been explored for the detection and characterization of viral proteins from soluble extracts of cells transformed by Rous sarcoma virus (RSV). Viral antigens have been analyzed after gel filtration in several solvents. In addition, immune complexes formed with virus-specific sera have been isolated by agarose gel filtration and by high- or low-speed centrifugation through sucrose solutions. Radioactive proteins from these immune complexes have been analyzed by gel filtration in 6 m guanidine hydrochloride or by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. Comparison with proteins from purified virus indicates the presence of two viral core proteins (gs1 and gs2) in the soluble fraction from virus-producing chicken cells. In the same fraction from RSV-transformed hamster cells (which do not produce virus), three gs proteins (gs1, gs2, and gs3) could be identified. The soluble viral gs proteins are strongly bound to at least two larger polypeptides in cell extracts. These polypeptides do not appear to be viral in origin and have the property of undergoing a time-dependent aggregation in the extracts. One of these cell-derived proteins, which is present in a variety of uninfected cell types, closely resembles actin.
Full text
PDF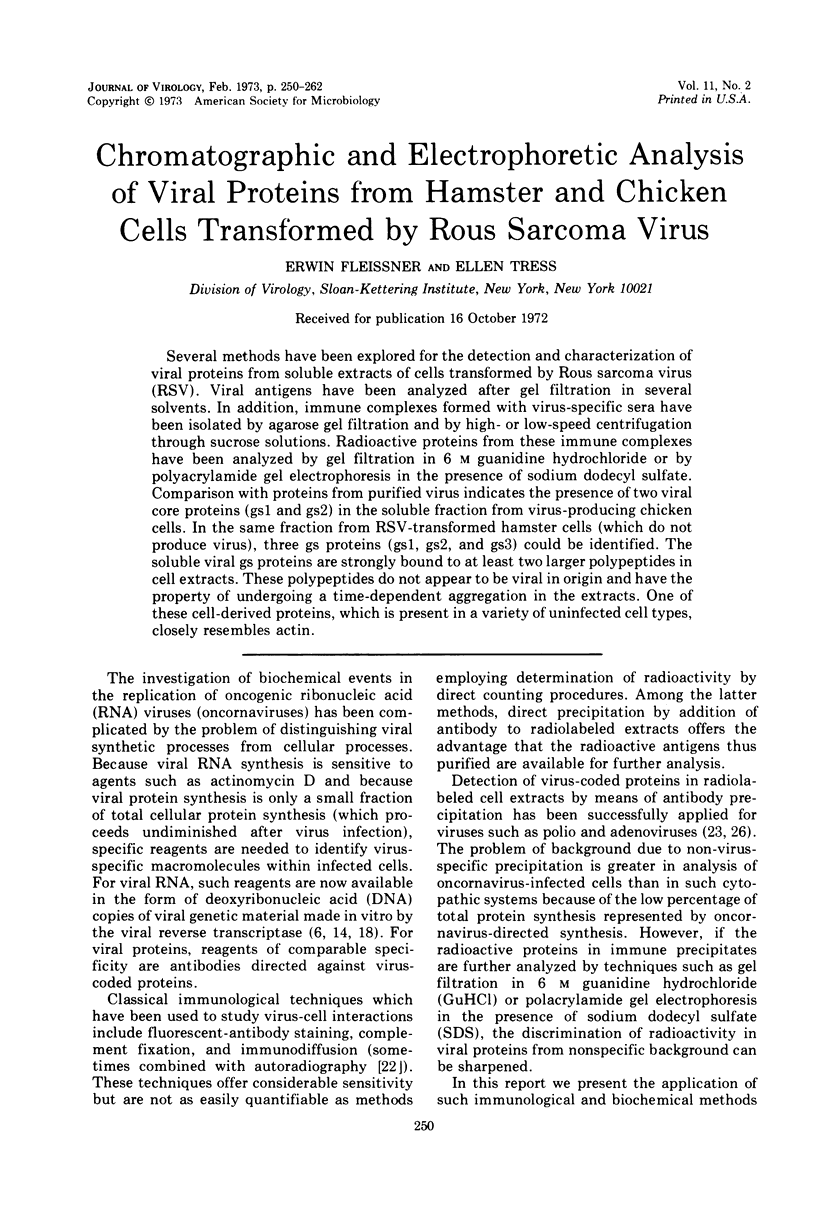





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Sarma P. S. Identification and localization of avian leukosis group-specific antigen within "leukosis-free" chick embryos. Virology. 1972 May;48(2):624–626. doi: 10.1016/0042-6822(72)90078-5. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Gesteland R. F. Pattern of protein synthesis in monkey cells infected by simian virus 40. J Virol. 1972 May;9(5):758–765. doi: 10.1128/jvi.9.5.758-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar R. A., Ishizaki R., Beard J. W. Immunoelectrophoretic analysis of avian ribonucleic acid tumor virus group-specific antigens. J Virol. 1972 Jan;9(1):90–95. doi: 10.1128/jvi.9.1.90-95.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSTEN M. E., KATZ A. M. ACTIN: A COMPARATIVE STUDY. Biochim Biophys Acta. 1964 Sep 4;90:534–541. doi: 10.1016/0304-4165(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Hybridization of Rous sarcoma virus deoxyribonucleic acid polymerase product and ribonucleic acids from chicken and rat cells infected with Rous sarcoma virus. J Virol. 1972 May;9(5):766–775. doi: 10.1128/jvi.9.5.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. D., Summers D. F., Maizel J. V. Evidence for ambiguity in the posttranslational cleavage of poliovirus proteins. Virology. 1970 Jul;41(3):408–418. doi: 10.1016/0042-6822(70)90161-3. [DOI] [PubMed] [Google Scholar]
- DALES S. ASSOCIATION BETWEEN THE SPINDLE APPARATUS AND REOVIRUS. Proc Natl Acad Sci U S A. 1963 Aug;50:268–275. doi: 10.1073/pnas.50.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R. E., Bray D. Actin in growing nerve cells. Nat New Biol. 1971 Nov 24;234(47):115–118. doi: 10.1038/newbio234115a0. [DOI] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E. Virus-specific antigens in hamster cells transformed by Rous sarcoma virus. J Virol. 1970 Jan;5(1):14–21. doi: 10.1128/jvi.5.1.14-21.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Rokutanda H., Rokutanda M. Virus specific RNA in cells transformed by RNA tumour viruses. Nat New Biol. 1971 Apr 21;230(16):229–232. doi: 10.1038/newbio230229a0. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niall H. D., Sauer R., Allen D. W. The N-terminal amino acid sequence of two avian leukosis group specific antigens. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1804–1809. doi: 10.1073/pnas.67.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., SHATKIN A. J., LEVINTOW L. ASSOCIATION OF NEWLY FORMED VIRAL PROTEIN WITH SPECIFIC POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1963 Oct;50:686–694. doi: 10.1073/pnas.50.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N. P., Sebring E. D. Sequential formation of vaccinia virus proteins and viral deoxyribonucleic acid replication. J Virol. 1967 Feb;1(1):16–23. doi: 10.1128/jvi.1.1.16-23.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Vecchio G., Attardi D., Green M. Immunological studies on viral polypeptide synthesis in cells replicating murine sarcoma-leukemia virus. J Virol. 1972 Sep;10(3):447–455. doi: 10.1128/jvi.10.3.447-455.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine A. F., Bader J. P. Production of virus by mammalian cells tranformed by Rous sarcoma and murine sarcoma viruses. J Virol. 1968 Mar;2(3):224–237. doi: 10.1128/jvi.2.3.224-237.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]


