Abstract
Alterations in the renin angiotensin aldosterone system (RAAS) contribute to the underlying pathophysiology of insulin resistance in humans; however, individual differences in the treatment response of insulin resistance to RAAS blockade persist. Thus, understanding inter-individual differences in the relationship between the RAAS and insulin resistance may provide insights into improved personalized treatments and improved outcomes. The effects of the systemic RAAS on blood pressure regulation and glucose metabolism have been studied extensively; however, recent discoveries on the influence of local tissue RAAS in the skeletal muscle, heart, vasculature, adipocytes, and pancreas have led to an improved understanding of how activated tissue RAAS influences the development of insulin resistance and diabetes in humans. Angiotensin II (ANGII) is the predominant RAAS component contributing to insulin resistance; however, other players such as aldosterone, renin, and ACE2 are also involved. This review examines the role of local ANGII activity on insulin resistance development in skeletal muscle, adipocytes, and pancreas, followed by a discussion of the other RAAS components implicated in insulin resistance, including ACE2, Ang1-7, renin, and aldosterone.
Keywords: Renin angiotensin aldosterone system, Angiotensin II, Insulin resistance, Aldosterone, Insulin signaling, Angiotensin converting enzyme, Angiotensin 1-7, Diabetes mellitus, Glucose metabolism, Skeletal muscle, Adipocytes, Pancreas, Insulin secretion, Inflammation, Genetics, Polymorphisms, Angiotensin II receptor blocker, Angiotensin converting enzyme inhibitor, Insulin sensitivity, Human studies, Animal models, Renin inhibitor, Obesity, Hypertension, Clinical trials
Introduction
Insulin resistance is a well-known risk factor for type 2 diabetes, cardiovascular disease and the metabolic syndrome [1, 2]; leading causes of morbidity and mortality worldwide. Alterations in glucose homeostasis, including dysregulation of 1) pancreatic insulin secretion, 2) insulin stimulated glucose uptake in skeletal muscle and adipocytes, and 3) hepatic glucose production are hallmarks of insulin resistance, and current prevention and treatment strategies aim to address this pathophysiology [3]. Unfortunately, the prevalence of insulin resistance is increasing; this is particularly relevant in hypertensive and obese individuals who are at high cardiovascular risk [4–6]. Complicating this issue is the fact that current medications used to treat comorbidities associated with insulin resistance (i.e. hypertension) may adversely affect insulin sensitivity in some individuals [7, 8]. Thus, understanding the specific mechanisms contributing to development of insulin resistance on an individual level is essential for designing more effective and personalized prevention and treatment strategies.
The renin angiotensin aldosterone system (RAAS) contributes to the underlying pathophysiology of insulin resistance; however, the optimum approaches to target the RAAS for personalized prevention and treatment of insulin resistance are still under debate. Blockade of the RAAS has been shown to prevent insulin resistance and type 2 diabetes in some [9••, 10••, 11], but not all, studies [12, 13], suggesting that inter-individual differences contribute to the effects of the RAAS on the development of insulin resistance. In vitro and in vivo studies highlight the complex nature and multiple mechanisms by which the RAAS influences insulin resistance, thus providing avenues for further investigation in humans. This review will discuss the pertinent studies linking the RAAS to insulin resistance in humans, and will highlight possible mechanisms for inter-individual differences in the relationship between RAAS and insulin resistance. A discussion on how these inter-individual differences may influence the development of more effective treatment strategies is also included.
Overview of the Renin Angiotensin Aldosterone System
The RAAS is a dynamic physiologic system with a key role in regulating blood pressure and fluid and electrolyte balance. Renin, a proteolytic enzyme produced in the juxtaglomerular cells of the kidney, is released into the circulation in response to vasodilation or low sodium diet [14]. Renin metabolizes angiotensin (AGT) into angiotensin I (ANGI). Subsequently, the angiotensin converting enzyme (ACE) converts ANGI to angiotensin II (ANGII), the predominant peptide of the RAAS, whose actions are directly linked to many aspects of cardio-metabolic pathophysiology [15]. ANGII exerts physiologic effects through both the angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R) in the vasculature, kidney, skeletal muscle, heart, adipocytes, pancreas, and adrenal tissues [16–18]. AT1R mediated ANGII effects include: 1) vasoconstriction of the vascular smooth muscle cells of the arteriole, 2) sodium retention in the renal proximal tubules, and 3) aldosterone release from the adrenal zonaglomerulosa [14]. AT2R-mediated ANGII effects generally oppose those effects mediated by AT1R, and include: 1) vasodilation of arterioles, 2) anti-inflammatory effects in vascular smooth muscle cells, and 3) anti-proliferative effects in the myocardium [19].
The effects of the systemic RAAS on blood pressure regulation, glucose metabolism and inflammation have been studied extensively; however, recent discoveries on the influence of local tissue RAAS in the skeletal muscle, heart, vasculature, adipocytes, and pancreas have led to an improved understanding of how activated tissue RAAS influences the development of insulin resistance and diabetes in humans [20]. ANGII seems to be the predominant RAAS player contributing to insulin resistance. This review examines the role of local ANGII activity on insulin resistance development in skeletal muscle, adipocytes, and pancreas, followed by a discussion of the other RAAS components implicated in insulin resistance, including ACE2, Ang1-7, renin, and aldosterone.
RAAS Blockade Insulin Sensitivity and Type 2 Diabetes Mellitus: Large-Scale Human Trials
Clinical trials suggest a role for the RAAS in the development of insulin resistance and type 2 diabetes mellitus (DM) in humans. Trials comparing the effects of angiotensin-converting-enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) treatment versus other anti-hypertensive medications (hydrochlorothiazide, β-blockers, calcium channel blockers) consistently demonstrate that RAAS blockade results in significant improvements in insulin sensitivity compared to other anti-hypertensive agents [21–23], raising the possibility that RAAS is involved in the pathophysiology of insulin resistance, and thus the development of type 2 diabetes. Two large-scale clinical trials evaluated the effect of RAAS blockade on the development of diabetes, with progression to diabetes as the primary outcome. The Nateglinide and Valsartan in Impaired Glucose Tolerance Outcome Research (NAVIGATOR) study compared the effect of valsartan (160 mg daily) versus placebo for a median of 5 years on the development of diabetes in 9,306 individuals with impaired glucose tolerance [9••]. The ARB treated group had a lower incidence of diabetes (hazard ratio 0.86, p < 0.0001) and lower fasting glucose (mean difference 0.03 mM/L, p < 0.01) and post-load glucose (mean difference 0.17 mM/L, p < 0.0001) values as compared with the placebo treated group [9••]. The Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) study included 5,269 individuals, of whom half were randomized to ramipril with or without rosiglitazone for a median of 3 years. Individuals on ramipril had significantly lower plasma glucose levels in response to a glucose load when compared with the placebo control (mean difference 5.4 mg/dL, p = 0.01); however, rates of diabetes development did not differ significantly between groups (hazard ration 0.91, p = 0.15) [24••]. The lack of consistency between these two large trials is consistent with prior studies evaluating the effects of ACEI or ARB on the development of diabetes in clinical trials where diabetes risk was not the primary endpoint. Some of these studies demonstrated significantly decreased type 2 diabetes risk [25–27], while others showed no significant effect [28]. However, sub-analyses of these larger trials demonstrated that the ACEI or ARB treatment did improve glucose levels and risk for diabetes in higher risk populations, and when compared with other anti-hypertensive treatments [25, 29]. Further, meta-analysis of these studies demonstrated a 22–30 % decrease risk in diabetes development [11].
These large-scale human trials demonstrate heterogeneity in the relationship between the RAAS and development of DM, with some studies demonstrating significant benefit of ACEI or ARB therapy. It is likely outcomes are dependent on the type of population studied, concurrent medication use, and dosage and duration of study drug, as well as inter-individual variability in the relationship between RAAS and insulin resistance. Thus, the evidence suggests that some, but not all, individuals will benefit from prevention and treatment strategies targeting the RAAS. Understanding the physiology underlying the relationship between the RAAS and insulin resistance and being able to identify who is at risk for developing specific pathophysiology will guide more effective personalized prevention and treatment.
Physiologic Underpinnings of RAAS and IR: Angiotensin II
Recent work in the area of RAAS and insulin resistance has uncovered the importance of both systemic and local ANGII in dysregulated insulin secretion, adipogenesis, and microvascular blood flow to muscle (Fig. 1).
Fig 1.
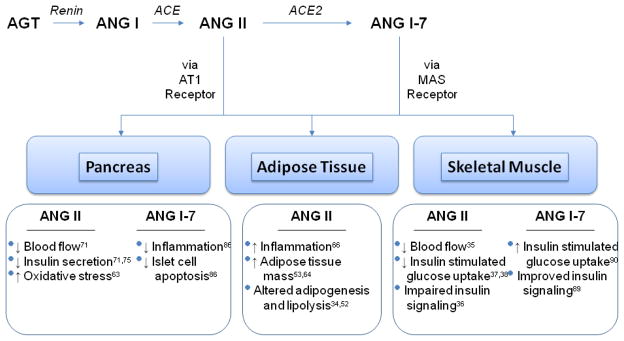
Effects of ANG II and ANG1-7 in pancreas, adipose tissue, and skeletal muscle
ANGII Action in Skeletal Muscle
Angiotensin II is present in both skeletal myocytes and the vascular tissue of arterioles that supply blood to these myocytes [17]. The effects of ANGII on skeletal muscle glucose uptake is related to 1) alterations in skeletal muscle blood flow, as well as 2) non-hemodynamic effects of ANGII on insulin signaling and insulin stimulated glut-4 glucose uptake [17, 30–32]. A discussion of the role of ANGII in both mechanisms follows.
Hemodynamic Effects of ANGII on Skeletal Muscle Glucose Metabolism
There is a well-documented negative relationship between ANGII and microvascular blood flow in skeletal muscle tissue that contributes to the development of insulin resistance [16]. Animal studies demonstrate that ANGII reduces (and ARB improves) the effects of insulin on both microvascular blood volume and glucose extraction [33]. Recently, several human studies confirmed this relationship, demonstrating that ANGII decreases microvascular blood flow to the skeletal muscle and these effects are reversed with ARB administration [34•, 35]. Further, Saunder et al. [35] demonstrated that acute oral administration of candesartan (32 mg) resulted in improvements in skeletal muscle microvascular blood volume and flow, as measured by contrast enhanced ultrasound in healthy individuals. Interestingly, improvements in whole body glucose uptake were not demonstrated in this study; this may be related to the short duration of candesartan treatment or the small sample size of eight healthy subjects.
Non-Hemodynamic Effects of ANGII on Skeletal Muscle Glucose Metabolism
ANGII, acting via AT1R, directly and negatively influences glut-4 translocation, insulin stimulated glucose uptake and the intracellular insulin signaling pathway in skeletal muscle [30, 31, 36].For example, chronic ANGII infusion (100 ng/kg/min for 12 days) led to decreased glut-4 translocation, altered insulin stimulated glucose uptake, and increased oxidative stress in rat skeletal muscle [31]. Further, incubation in ANGII inhibited insulin signaling by decreasing phosporylation of AKT Ser473 and glycogen synthase kinase 3 beta Ser(9) in isolated rat soleus muscles [36]. In other studies, interrupting the RAAS with ACEI or ARB treatment led to improvements in whole body insulin sensitivity and skeletal muscle glucose transport in hypertensive and diabetic rats [37, 38]. Further, incubating L6 myotube cells in ANGII results in altered insulin stimulated IRS1 phosphorylation, decreased AKT activation, and decreased Glut-4 translocation to the cell membrane; all alterations were remedied with ARB treatment [39, 40]. In vivo studies demonstrated that the influence of ANGII on insulin signaling and glucose uptake in skeletal muscle cell is independent of the effects of ANGII on blood flow to the muscle [17, 36].
A recent study assessed prospectively the effect of an ARB (valsartan), as compared with placebo, on glucose homeostasis in 79 humans with impaired glucose tolerance. ARB treatment for 26 weeks improved both whole body glucose uptake and insulin secretion [10••]. Thus, these findings are consistent with the preclinical studies and suggest a beneficial effect of RAAS inhibition on glucose homeostasis. The failure of earlier studies to demonstrate a beneficial effect of ACEI administration on glucose parameters in individuals with obesity [41] or hypertension (HTN) and type 2 diabetes [42] may be related to the smaller number of individuals, shorter treatment periods, the use of ACEI versus ARB treatment, and to inter-individual differences.
Our understanding of the relationship between RAAS and glucose homeostasis is further complicated by early studies examining the effects of an acute ANGII infusion on insulin stimulated glucose uptake, assessed by the euglycemic insulin clamp technique. ANGII had either 1) no effect on insulin sensitivity [43] or 2) significantly improved insulin sensitivity [44–46]. It may be that the specific mechanisms mediating the acute effects of AngII on glucose homeostasis differ from those involving chronic RAAS inhibition, possibly involving acute hemodynamic changes. Further, there may be inter-individual differences in response to ANGII. Collectively, these studies suggest a beneficial effect of chronic RAAS blockade on insulin sensitivity and insulin secretion, though the lack of consistent results across all studies raises the possibility that there are inter-individual differences that modify responses.
Adipose Tissue
The relationship between ANGII, adipogenesis, and insulin resistance has been studied extensively and has been the topic of many recent reviews [14, 47]. Thus, we will discuss only the most recent, pertinent studies and refer readers to these reviews for additional details. The possibility that a local RAS directly affects adipogenesis, obesity, and insulin resistance in humans was introduced after Karlsson et al. [48] demonstrated that angiotensinogen and ACE were present in adipose tissue of obese humans, raising the possibility that ANGII was made locally and could exert local effects on insulin resistance.
Subsequently, many human studies have shown that both the local and systemic renin-angiotensin system (RAS) are activated in obese individuals [49,50–52] and are associated with insulin resistance. These observational studies make it difficult to determine whether obesity is causing the activated RAS or vice-versa. However, mice with deletions in RAS genes and Dahl salt sensitive rats (a model of low renin and ANGII levels) are protected from diet-induced obesity [53, 54], suggesting the RAS may have a role in causing obesity and its cardio-metabolic effects, including insulin resistance. Further, insulin resistance is improved with both RAS blockade [55] and weight loss [56] in humans.
The presence of AT1R in adipocytes is also well-established, and recently it was discovered that dysfunctional AT1R leads to altered adipogenesis [57]. In humans, many studies demonstrate a role for adipose specific ANGII in adipocyte differentiation; although conflicting results also exist. For example, ANGII was found to increase adipocyte size and induce adipocyte differentiation in isolated adipocytes from human visceral adipose tissue in a few [58, 59], but not all, studies [60]. Thus, one of the effects of the local adipose RAS on insulin resistance is believed to be through increased adipocyte differentiation leading to obesity and insulin resistance [58, 61, 62, 63••, 64, 65]. Goossens et al. [61] demonstrated that 26 wks of valsartan treatment reduced subcutaneous adipose tissue size, decreased markers of adipogenesis (PPAR ) and inflammatory markers (CD68, CD163), and improved adipocyte function in obese humans. Further, decreases in inflammatory markers and improvement in measurements of insulin resistance were observed in obese individuals after valsartan treatment [66].
The effects of ANGII on adipose tissue appear to be complex and may have opposing actions on glucose homeostasis. The beneficial effects of ANGII need confirmation, but if true, would suggest that ANGII can have both beneficial and adverse effects on adipose tissue regulation of glucose homeostasis.
Pancreas
The existence of a local pancreatic RAS in humans is known. Angiotensinogen, (pro)renin, ANGII, and AT1R have all been characterized in human pancreatic islet and/or beta cells [20, 67, 68]. Further, ANG1-7 is also present in the pancreas and may act to counterbalance the negative effects of ANGII in this tissue [69]. Numerous studies demonstrate that increased pancreatic RAS activity leads to alterations in glucose metabolism in in-vitro models, animals, and humans. As discussed below, mechanisms contributing to this association have been clarified, and include direct RAS effects on 1) pancreatic blood flow, 2) insulin secretion, and 3) inflammation and pancreatic fibrosis [20, 70].
ANGII is the predominant peptide in the pancreatic RAS [67], and its role in decreasing pancreatic blood flow is well-characterized. Direct ANGII infusion to the pancreas led to reduced whole pancreatic and islet blood flow in rats; effects that were reversed with ARB administration [71]. Similar results were demonstrated by Huang et al. [72], reporting that both captopril and irbesartan improved pancreatic blood flow in female rats. Further work is necessary to evaluate the role of RAAS on pancreatic blood flow in humans.
Recent studies in both animal models and humans provide strong support for a direct role of ANGII on insulin secretion. ANGII administration decreased insulin secretion in isolated mouse pancreatic beta cells in a dose dependent manner [73]. Further, exposure to an ARB improved early phase insulin secretion in the same cells [73]. Similarly, in animal models, ANGII infusion decreased insulin secretion [74] and ARB administration improved insulin secretion in response to western diet and glucose supplementation [63••, 75••]. Interestingly, although 6 weeks of olmesartan improved insulin secretion and increased GLP-1 signaling in the obese Otsuka Long-Evans Tojushima Fatty (OLETF) rat model, a model of metabolic syndrome, changes in insulin signaling proteins were not observed in the skeletal muscle, suggesting that ARB-related improvements in glucose metabolism are independent of changes in insulin signaling [75••].
In humans, 26 weeks of treatment with the ARB valsartan led to improvements in both phase 1 and phase 2 insulin secretion when compared with placebo controls [10••]. Similar improvements in insulin secretion were found in other clinical studies employing longer durations of ARB treatment (> 2 months) and in combination with HCTZ [76]. These improvements were not observed in short term (6 weeks) administration of ARB [77], which suggests that the duration of treatment, as well as the use of other anti-hypertensive medications, are important factors to consider when evaluating the effect of RAAS blockade on insulin secretion.
Non-ANGII Factors
ACE2
Heretofore, we have discussed the negative relationship between the ACE/ANGII/AT1R axis and insulin resistance demonstrating that increases in ANGII activity lead to alterations in: 1) insulin signaling and glucose uptake, 2) insulin secretion, and 3) inflammation which portend the development of insulin resistance. Further, blockade of this axis via AT1R receptor blockade or ACEI addresses these alterations and improves insulin resistance, providing insight on best treatment options in individuals with increased RAS activation. Recent discoveries have led to the realization that intervening at a second RAS pathway, the ACE2/Ang1-7/MAS axis, may compliment blockade of ACE/ANGII/AT1R [78–80] (Fig. 1). Discovered in 2000, ACE2 is a homologue of ACE and acts by cleaving ANGII to make Ang1-7 [69, 81, 82]. ACE2 is ubiquitously present in heart, kidney, brain, lung, adipose, and pancreatic tissue [81, 83, 84] and acts upon MAS receptors leading to actions opposing ANGII: vasodilation via activation of nitric oxide and bradykinin, and decreased fibrosis [79, 85, 86•]. It is important to note that since ANG1-7 is a byproduct of ANGII degradation by ACE2, it is unclear whether the improved vascular effects seen are related to decreases in ANGII or increases in ANG1-7; however, it is likely a combination of both, and a ratio of the two peptides may be a better determinant of overall activity [82, 87]. Studies examining the direct influence of Ang1-7 infusion and ACE2 deletion have addressed this issue and defined the effects of ACE2/ANG1-7/MAS axis on insulin resistance.
ANG1-7 improves glucose metabolism and insulin resistance both in vitro and in vivo. Chronic administration of ANG1-7 in fructose fed rats led to improved whole body insulin sensitivity, as measured by OGTT, and also led to improved intra-cellular insulin signaling when compared with rats receiving a sham infusion [88]. Subsequent investigation revealed that ANG1-7 administration attenuated the inhibitory effects of ANGII infusion on insulin-stimulated glucose uptake in isolated skeletal muscle from female lean Zucker rats, and that this effect was mediated through increases in Akt Ser473 phosphorylation [89]. Loss of ACE2 also leads to altered glucose metabolism, and this appears to be due to a loss of the opposing actions of ANG1-7 on ANGII’s actions. Loss of ACE2 in mice fed a high calorie diet or given ANGII led to decreased insulin sensitivity (measured via intra-peritoneal glucose tolerance test) compared with wild type mice [90]. In the ACE2 knockout mice, AT1R blockade with losartan did not improve these alterations; however, administration of Ang1-7 with or without AT1R blockade did result in improved glucose tolerance suggesting a significant role of Ang1-7 in the homeostasis of glucose metabolism in environments of altered RAS activity [90]. Supporting the role of the ACE2/ANG1-7/MAS axis in the development of insulin resistance, both ACE2 partial knockout animals and MAS receptor knockout mice exhibited impaired glucose intolerance and elevated fasting glucoses [79, 82]. Further, in diabetic db/db mice, ACE2 therapy provided directly to the pancreas led to improvements in fasting glucose and glucose tolerance [86•].
Understanding the interplay between ANGII and ACE2 in the development of insulin resistance has led to an investigation of the effects of treatment of either the ACE/ANGII/AT1R or ACE2/Ang1-7/MAS receptor axis abnormalities. ACE2/ApoE knockout mice demonstrated increased atherosclerotic plaques that are further increased in the setting of a low salt diet (an environment of ANGII activation), linking the altered interplay of ANGII/ACE2 with worsening cardio-metabolic disease [84]. Further, in the Akita diabetic mouse model, loss of ACE2 led to greater diastolic dysfunction, increased oxidative stress, and greater impairments in flow mediated dilation; again linking loss of ACE2 with complications of insulin resistance and DM [91]. Interestingly, both increased atherosclerosis and cardiac dysfunction were improved with ACEI or ARB administration alone.
Human investigation of the ACE2/ANGII balance is an under-investigated field. Serum ACE2 activity has been shown to be elevated in individuals with T1DM and microalbuminuria, suggesting a possible compensatory mechanism may be at play in this population [92]. Further, urinary ACE2 levels are higher in kidney transplant patients with diabetes mellitus when compared with patients without diabetes [93]. Further investigation into the role of ACE2/ANGII balance in human insulin resistance is warranted.
Aldosterone
A relationship between mineralocorticoid receptor activation and decreased insulin sensitivity has been demonstrated through different animal models and human studies and has been the focus of many recent reviews. Readers are referred to these reviews for a more extensive discussion of the topic [94–96]. In this review, we will discuss the recent, pertinent studies evaluating the relationship between aldosterone and insulin resistance in humans. Human studies suggest that aldosterone has an effect on insulin resistance independent from its relationship with ANGII or renin, since higher levels of insulin resistance are found in individuals with primary hyperaldosteronism; a condition where ANGII levels and renin levels are low [97–102]. Further, higher serum aldosterone levels have been associated with an increased risk for the metabolic syndrome; a clustering of metabolic risk factors where insulin resistance is a defining characteristic. Prospective analysis demonstrates that plasma aldosterone levels predicted the development of insulin resistance in a large Japanese cohort followed over 10 years [103]. Blockade of the mineralocorticoid receptor (spironolactone), but not AT1R blockade (irbesartan), improved chlorthalidone-induced insulin resistance in hypertensive individuals, suggesting a causative relationship between activated mineralocorticoid receptor, increased aldosterone, and greater levels of insulin resistance [104].
In vitro and in vivo studies suggest that an underlying mechanism contributing to the relationship between aldosterone and insulin resistance is related to the inhibitory effects of aldosterone on insulin signaling and insulin-stimulated glucose uptake via glut-4 translocation in adipocytes, skeletal muscle, and vascular smooth muscle cells [105–107]. Further, mineralocorticoid receptor activation is pro-inflammatory, promotes adipocyte differentiation, alters adipokine expression and affects vascular function—all actions that could influence glucose homeostasis. Furthering the link between obesity and excess RAAS activity are studies demonstrating that adipocytes produce aldosterone, as well as a factor that stimulates production by the liver of an aldosterone secretagogue [108•, 109]. Activation of the mineralocorticoid receptor by aldosterone or cortisol clearly contributes to the development of insulin resistance, and these effects are independent of the effects of ANGII on insulin resistance. More research is necessary to determine the effects of RAAS blockade on insulin resistance in different populations of varying cardio-metabolic risk, to determine if one population would achieve greater benefit than another, or whether RAAS blockade at multiple points (i.e. ARB and mineralocorticoid receptor blockade) would provide greater benefit than either alone.
Renin
Blockade of renin has also been shown to improve measurements of insulin sensitivity in human and animal models. In individuals with the metabolic syndrome and hypertension, aliskiren (300 mg per day for 12 weeks) resulted in improved insulin sensitivity (measured via euglycemic hyperinsulinemic clamp) compared to losartan [110]. Similar results were found in animal models, where aliskiren improved hyperglycemia, dyslipidemia, and vascular function in rats fed a high fructose diet [111, 112]. Closer examination of the mechanism underlying the relationship between renin inhibition and improved insulin sensitivity found that aliskiren treatment led to improvements in systemic insulin resistance and improved insulin signaling and glucose uptake in skeletal muscle in a rat model of RAAS activation (TG(mRen2)27) [113]. These improvements were associated with decreases in levels of ANGII, aldosterone, AT1R, oxidative stress, and fibrosis, making it difficult to determine whether the effects of renin inhibition on insulin sensitivity are through the direct effects of renin blockade, or through the effects of renin blockade on decreasing downstream components of the RAAS. Cross-sectional human studies also demonstrated a link between elevated plasma renin activity and increased risk for insulin resistance and the metabolic syndrome in humans [101, 114]; however, as in the studies above, determining whether these results are due to the effect of renin alone or renin’s effects on subsequent components of the RAAS is unknown.
Genetics
Human genetic studies examining the relationship between RAAS candidate gene variants and insulin resistance provides some insight into the observed inter-individual differences between altered RAAS physiology and insulin resistance discussed thus far. Variants within two RAAS candidate genes, ACE and AGT, consistently demonstrate a relationship with insulin resistance [115, 116•], suggesting that a subgroup of individuals are at increased risk for insulin resistance secondary to alterations in the RAAS. Identifying these individuals and providing personalized, RAAS specific treatment may result in more consistent outcomes than those observed in the human clinical trials listed above, where the effect of RAAS blockade on insulin resistance was examined in populations without regard to an individual’s genotype. Studies providing the most promising insights into the relationships between RAAS genes, RAAS pathophysiology and insulin resistance are described below. Of note, large genome wide association studies examining the relationship between variants in the entire genome and insulin resistance found no significant relationship between variants in RAAS genes and risk for insulin resistance [117–119]. However, many variants of the RAAS genes are not included on current genome-wide association study (GWAS) platforms. Additionally, the effects of these genetic variants may not have a sufficiently large effect size to be identified by GWAS studies that require correction for thousands of comparisons. Thus, candidate gene studies are likely the best design for evaluating the effects of RAAS gene on insulin resistance in humans, as the following studies demonstrate.
ACE Gene
One variant in the ACE gene, a 287 bpAlu repeat element deemed the ACE insertion/deletion polymorphism, has been associated with both cardiovascular disease and altered glucose metabolism in multiple human studies [116•, 120, 121]. The deleted variant (D) is associated with increased ACE activity in animals and humans [122–124]. In healthy Caucasian individuals of European descent, individuals homozygous for the deletion have been shown to have increased plasma glucose levels 2 hours after oral glucose challenge [116•], decreased insulin sensitivity measured via euglycemic hyperinsulinemic clamp [116], and increased fasting glucose levels [125, 126]. Further, individuals homozygous for the deletion variant of Chinese descent were also found to have increased plasma glucose levels [127]. Of note, other studies have found conflicting results, where individuals homozygous for the ACE deletion were found to have improved insulin sensitivity [128, 129]; however, these latter studies consisted of extremely small samples sizes of obese and hypertensive individuals, which may have contributed to the disparate findings. Importantly, two meta-analyses have pooled the results of all human studies examining the effects of the ACE I/D polymorphism on risk for type 2 diabetes mellitus [130, 131]. The results of these analyses, with each study consisting of greater than 15,000 individuals, demonstrated a strong effect of the D polymorphism with increased risk for type 2 diabetes, confirming a role for this polymorphism in identifying those with increased risk for altered glucose metabolism. Other meta-analyses pooling the results of human studies evaluating the D allele with insulin resistance are warranted.
AGT Gene
The angiotensinogen gene (AGT) encodes pre-angiotensinogen in the liver, which is then cleaved into angiotensin I and converted to angiotensin II by ACE. Variants in this gene are associated with increased plasma angiotensinogen levels, hypertension, and increased risk for cardiac hypertrophy [132–134]. In particular, the M235T (M268T according to newer nomenclature) polymorphism, a missense coding variant of the gene, is associated with cardio-metabolic phenotypes in humans. Guo and colleagues [115] first demonstrated an association between the minor allele of this variant and insulin sensitivity. Subsequent studies found associations with this variant and insulin sensitivity [135, 136]. These conflicting results are likely the result of the heterogeneous populations that were studied, differing definitions of insulin sensitivity, and small sample sizes. Analyzing variants of genes in a more homogeneous subset of individuals (i.e., an intermediate phenotype), pooling the results of these studies using meta-analysis, or evaluating the effects of other genes that regulate the expression of the AGT gene, ACE gene, or the RAAS may provide clarification on the role of genes and the RAAS on the development of insulin resistance.
Conclusion
The studies described in this review clearly delineate a relationship between the RAAS and insulin resistance in humans. Since the randomized control trials that evaluated the outcomes of RAAS blockade on insulin resistance and type 2 diabetes often have conflicting results, it is important for us to understand the basic pathophysiologic mechanisms underlying the relationship between the RAAS and insulin resistance. This review demonstrates that increased ANGII activity in skeletal muscle, adipose tissue, and the pancreas contribute to altered glucose metabolism leading to insulin resistance. Recent studies suggest countering these effects through the ACE2 pathway may provide some benefit. Further, genetic studies demonstrate that some individuals are predisposed to an increased risk for altered RAAS activity and insulin resistance. Additional studies are warranted to evaluate the interaction between these genetic risk factors and other environmental and behavioral risk factors known to influence the RAAS and vascular function, including sodium diet, smoking, and exercise. Identifying those individuals at greatest risk for altered RAAS and insulin resistance and targeting them with personalized treatment with RAAS blockade would likely result in more consistent results in evaluating the relationship between RAAS blockade and altered glucose metabolism. This is important as we continue to evaluate the most effective treatment strategies for individuals at greatest risk. The RAAS clearly influences glucose metabolism in some, but not all individuals. Understanding this relationship is important when prescribing anti-hypertensive medications in those at risk for insulin resistance.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Patricia C Underwood, Email: punderwood1@partners.org, Harvard Medical School, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women’s Hospital, 221 Longwood Ave. Boston, MA 02115, Phone: 617-525-7361, Fax: 617-732-5764.
Gail K. Adler, Email: gadler@partners.org, Harvard Medical School, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women’s Hospital, 221 Longwood Ave. Boston, MA 02115, Phone number: 617-732-8742, Fax number: 617-732-5764.
References
Published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasso FC, Carbonara O, Nasti R, et al. Glucose metabolism and coronary heart disease in patients with normal glucose tolerance. JAMA. 2004;291:1857–63. doi: 10.1001/jama.291.15.1857. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Davila EP, Florez H, Fleming LE, et al. Prevalence of the metabolic syndrome among U.S. workers. Diabetes Care. 33:2390–5. doi: 10.2337/dc10-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES. Prevalence of the metabolic syndrome in US populations. Endocrinol Metab Clin North Am. 2004;33:333–50. doi: 10.1016/j.ecl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31:1898–904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ofili EO, Oparil S, Giles T, et al. Moderate versus intensive treatment of hypertension using amlodipine/valsartan and with the addition of hydrochlorothiazide for patients uncontrolled on angiotensin receptor blocker monotherapy: results in racial/ethnic subgroups. J Am Soc Hypertens. 2011;5:249–58. doi: 10.1016/j.jash.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell CY, Blumenthal RS. Pharmacogenetics of antihypertensive response. Hypertension. 2012;59:1094–6. doi: 10.1161/HYPERTENSIONAHA.112.192559. [DOI] [PubMed] [Google Scholar]
- 9••.McMurray JJ, Holman RR, Haffner SM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–90. doi: 10.1056/NEJMoa1001121. The NAVIGATOR study, one of the first prospective randomized control trials powered to evaluate the effect of RAAS blockade (valsartan) on incidence of diabetes as a primary endpoint. Individuals receiving valsartan had a 14% reduction in the incidence of diabetes compared to the placebo control group. [DOI] [PubMed] [Google Scholar]
- 10••.van der Zijl NJ, Moors CC, Goossens GH, Hermans MM, Blaak EE, Diamant M. Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial. Diabetes Care. 2011;34:845–51. doi: 10.2337/dc10-2224. This is the first randomized control trial in humans that evaluated the effect of an ARB versus placebo on beta cell function. Chronic valsartan treatment, compared with placebo control, improved glucose stimulated insulin secretionduring an oral glucose tolerance test as well as insulin sensitivity assessed by euglycemic clamp, suggesting that ARB blockade has an effect not only on glucose uptake, but also pancreatic insulin secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abuissa H, Jones PG, Marso SP, O'Keefe JH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–6. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 12.Perlstein TS, Henry RR, Mather KJ, et al. Effect of angiotensin receptor blockade on insulin sensitivity and endothelial function in abdominally obese hypertensive patients with impaired fasting glucose. Clin Sci (Lond) 2012;122:193–202. doi: 10.1042/CS20110284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lteif AA, Chisholm RL, Gilbert K, Considine RV, Mather KJ. Effects of losartan on whole body, skeletal muscle and vascular insulin responses in obesity/insulin resistance without hypertension. Diabetes Obes Metab. 2012;14:254–61. doi: 10.1111/j.1463-1326.2011.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus Y, Shefer G, Stern N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–30. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muniyappa R, Yavuz S. Metabolic actions of angiotensin II and insulin: A microvascular endothelial balancing act. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksen EJ, Prasannarong M. The role of the renin-angiotensin system in the development of insulin resistance in skeletal muscle. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Yatabe J, Yoneda M, Yatabe MS, et al. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011;152:1582–8. doi: 10.1210/en.2010-1070. [DOI] [PubMed] [Google Scholar]
- 19.Qi Y, Li H, Shenoy V, et al. Moderate cardiac-selective overexpression of angiotensin II type 2 receptor protects cardiac functions from ischaemic injury. Exp Physiol. 2012;97:89–101. doi: 10.1113/expphysiol.2011.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung PS. Mechanisms of protective effects induced by blockade of the renin-angiotensin system: novel role of the pancreatic islet angiotensin-generating system in Type 2 diabetes. Diabet Med. 2007;24:110–6. doi: 10.1111/j.1464-5491.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Dell'Oro R, et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21:1761–9. doi: 10.1097/00004872-200309000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Olsen MH, Wachtell K, Neland K, et al. Losartan but not atenolol reduce carotid artery hypertrophy in essential hypertension. A LIFE substudy. Blood Press. 2005;14:177–83. doi: 10.1080/08037050510034185. [DOI] [PubMed] [Google Scholar]
- 23.Jin HM, Pan Y. Angiotensin type-1 receptor blockade with losartan increases insulin sensitivity and improves glucose homeostasis in subjects with type 2 diabetes and nephropathy. Nephrol Dial Transplant. 2007;22:1943–9. doi: 10.1093/ndt/gfm049. [DOI] [PubMed] [Google Scholar]
- 24••.Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–62. doi: 10.1056/NEJMoa065061. The DREAM trial is one of the first prospective randomized control trials powered to evaluate the effect of RAAS blockade (ramipril) on incidence of diabetes as a primary endpoint. There was no significant difference in the incidence of diabetes in those given ramipril vs. placebo; however, the ramipril group did have a higher regression to normoglycemia compared with controls. [DOI] [PubMed] [Google Scholar]
- 25.Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751–6. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 26.Trial AOaCftACRGTAaL-LTtPHA. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 27.Kjeldsen SE, Dahlöf B, Devereux RB, et al. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–8. doi: 10.1001/jama.288.12.1491. [DOI] [PubMed] [Google Scholar]
- 28.Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 29.Lindholm LH, Ibsen H, Borch-Johnsen K, et al. Risk of new-onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens. 2002;20:1879–86. doi: 10.1097/00004872-200209000-00035. [DOI] [PubMed] [Google Scholar]
- 30.Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci U S A. 1996;93:12490–5. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogihara T, Asano T, Ando K, et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002;40:872–9. doi: 10.1161/01.hyp.0000040262.48405.a8. [DOI] [PubMed] [Google Scholar]
- 32.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93:569–82. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai W, Wang W, Liu J, et al. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55:523–30. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Goossens GH, Blaak EE, Saris WH, van Baak MA. Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab. 2004;89:2690–6. doi: 10.1210/jc.2003-032053. A physiologic human study that evaluated the effects of ANGII infusion on lipolysis in skeletal muscle and adipose tissue, demonstrating that ANGII inhibits lipolysis (which may increase insulin resistance) in these tissues in both obese and healthy individuals. A limitation of this study is that measurements of insulin resistance were not measured to determine whether they correlated with decreases in lipolysis. [DOI] [PubMed] [Google Scholar]
- 35.Sauder MA, Liu J, Jahn LA, Fowler DE, Chai W, Liu Z. Candesartan acutely recruits skeletal and cardiac muscle microvasculature in healthy humans. J Clin Endocrinol Metab. 2012;97:E1208–12. doi: 10.1210/jc.2011-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond-Stanic MK, Henriksen EJ. Direct inhibition by angiotensin II of insulin-dependent glucose transport activity in mammalian skeletal muscle involves a ROS-dependent mechanism. Arch Physiol Biochem. 2010;116:88–95. doi: 10.3109/13813451003758703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob S, Henriksen EJ, Fogt DL, Dietze GJ. Effects of trandolapril and verapamil on glucose transport in insulin-resistant rat skeletal muscle. Metabolism. 1996;45:535–41. doi: 10.1016/s0026-0495(96)90021-9. [DOI] [PubMed] [Google Scholar]
- 38.Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension. 2001;38:884–90. doi: 10.1161/hy1101.092970. [DOI] [PubMed] [Google Scholar]
- 39.Wei Y, Sowers JR, Nistala R, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–46. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 40.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:E345–51. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 41.Goossens GH, Blaak EE, Schiffers PM, Saris WH, van Baak MA. Effect of short-term ACE inhibitor treatment on peripheral insulin sensitivity in obese insulin-resistant subjects. Diabetologia. 2006;49:3009–16. doi: 10.1007/s00125-006-0458-2. [DOI] [PubMed] [Google Scholar]
- 42.Seghieri G, Yin W, Boni C, et al. Effect of chronic ACE inhibition on glucose tolerance and insulin sensitivity in hypertensive type 2 diabetic patients. Diabet Med. 1992;9:732–8. doi: 10.1111/j.1464-5491.1992.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 43.Townsend RR, DiPette DJ. Pressor doses of angiotensin II increase insulin-mediated glucose uptake in normotensive men. Am J Physiol. 1993;265:E362–6. doi: 10.1152/ajpendo.1993.265.3.E362. [DOI] [PubMed] [Google Scholar]
- 44.Fliser D, Arnold U, Kohl B, Hartung R, Ritz E. Angiotensin II enhances insulin sensitivity in healthy volunteers under euglycemic conditions. J Hypertens. 1993;11:983–8. doi: 10.1097/00004872-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Widgren BR, Urbanavicius V, Wikstrand J, Attvall S, Persson B. Low-dose angiotensin II increases glucose disposal rate during euglycemic hyperinsulinemia. Am J Hypertens. 1993;6:892–5. doi: 10.1093/ajh/6.10.892. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan TA, Thawani H, Kades W, et al. Angiotensin II increases glucose utilization during acute hyperinsulinemia via a hemodynamic mechanism. J Clin Invest. 1993;92:720–6. doi: 10.1172/JCI116642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goossens GH. The Renin-Angiotensin system in the pathophysiology of type 2 diabetes. Obes Facts. 2012;5:611–24. doi: 10.1159/000342776. [DOI] [PubMed] [Google Scholar]
- 48.Karlsson C, Lindell K, Ottosson M, Sjöström L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–9. doi: 10.1210/jcem.83.11.5276. [DOI] [PubMed] [Google Scholar]
- 49.Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–5. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaidya A, Forman JP, Underwood PC, et al. The influence of body mass index and renin-angiotensin-aldosterone system activity on the relationship between 25-hydroxyvitamin D and adiponectin in Caucasian men. Eur J Endocrinol. 2011;164:995–1002. doi: 10.1530/EJE-11-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasue S, Masuzaki H, Okada S, et al. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: impact of nutritional status and adipocyte hypertrophy. Am J Hypertens. 2010;23:425–31. doi: 10.1038/ajh.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goossens GH, Blaak EE, Arner P, Saris WH, van Baak MA. Angiotensin II: a hormone that affects lipid metabolism in adipose tissue. Int J Obes (Lond) 2007;31:382–4. doi: 10.1038/sj.ijo.0803388. [DOI] [PubMed] [Google Scholar]
- 53.Yvan-Charvet L, Even P, Bloch-Faure M, et al. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–9. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 54.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension. 2012;60:404–10. doi: 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Zijl NJ, Serné EH, Goossens GH, et al. Valsartan-induced improvement in insulin sensitivity is not paralleled by changes in microvascular function in individuals with impaired glucose metabolism. J Hypertens. 2011;29:1955–62. doi: 10.1097/HJH.0b013e32834a7667. [DOI] [PubMed] [Google Scholar]
- 56.Engeli S, Böhnke J, Gorzelniak K, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–62. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 57.Putnam K, Batifoulier-Yiannikouris F, Bharadwaj KG, et al. Deficiency of Angiotensin type 1a receptors in adipocytes reduces differentiation and promotes hypertrophy of adipocytes in lean mice. Endocrinology. 2012;153:4677–86. doi: 10.1210/en.2012-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MH, Song HK, Ko GJ, et al. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008;74:890–900. doi: 10.1038/ki.2008.313. [DOI] [PubMed] [Google Scholar]
- 59.Sarzani R, Marcucci P, Salvi F, et al. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int J Obes (Lond) 2008;32:259–67. doi: 10.1038/sj.ijo.0803724. [DOI] [PubMed] [Google Scholar]
- 60.Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 61.Goossens GH, Moors CC, van der Zijl NJ, et al. Valsartan improves adipose tissue function in humans with impaired glucose metabolism: a randomized placebo-controlled double-blind trial. PLoS One. 2012;7:e39930. doi: 10.1371/journal.pone.0039930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 2006;55:367–74. doi: 10.2337/diabetes.55.02.06.db05-1022. [DOI] [PubMed] [Google Scholar]
- 63••.Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–21. doi: 10.1161/HYPERTENSIONAHA.109.148049. An elegant study demonstrating that Valsartan blocks western diet induced increases in inflammation, insulin levels, and glucose stimulated insulin secretion in a mouse model of the metabolic syndrome. Results of this study demonstrate that the RAAS influencesthe relationship between high fat diet and a worsening metabolic profile and suggests that blocking the RAAS in individuals eating a high fat diet, a common problem in today's society, may be beneficial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muñoz C, Giani JF, Dominici FP, Turyn D, Toblli JE. Long-term treatment with an angiotensin II receptor blocker decreases adipocyte size and improves insulin signaling in obese Zucker rats. J Hypertens. 2009;27:2409–20. doi: 10.1097/HJH.0b013e3283310e1b. [DOI] [PubMed] [Google Scholar]
- 65.Fujisaka S, Usui I, Kanatani Y, et al. Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice. Endocrinology. 2011;152:1789–99. doi: 10.1210/en.2010-1312. [DOI] [PubMed] [Google Scholar]
- 66.Pscherer S, Heemann U, Frank H. Effect of Renin-Angiotensin system blockade on insulin resistance and inflammatory parameters in patients with impaired glucose tolerance. Diabetes Care. 2010;33:914–9. doi: 10.2337/dc09-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chappell MC, Millsted A, Diz DI, Brosnihan KB, Ferrario CM. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens. 1991;9:751–9. doi: 10.1097/00004872-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Chappell MC, Jacobsen DW, Tallant EA. Characterization of angiotensin II receptor subtypes in pancreatic acinar AR42J cells. Peptides. 1995;16:741–7. doi: 10.1016/0196-9781(95)00044-k. [DOI] [PubMed] [Google Scholar]
- 69.Batlle D, Jose Soler M, Ye M. ACE2 and diabetes: ACE of ACEs? Diabetes. 2010;59:2994–6. doi: 10.2337/db10-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan L, Li X, Xu GL, Qi CJ. Effects of renin-angiotensin system blockade on islet function in diabetic rats. J Endocrinol Invest. 2010;33:13–9. doi: 10.1007/BF03346544. [DOI] [PubMed] [Google Scholar]
- 71.Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–33. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 72.Huang Z, Jansson L, Sjöholm A. Vasoactive drugs enhance pancreatic islet blood flow, augment insulin secretion and improve glucose tolerance in female rats. Clin Sci (Lond) 2007;112:69–76. doi: 10.1042/CS20060176. [DOI] [PubMed] [Google Scholar]
- 73.Lau T, Carlsson PO, Leung PS. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47:240–8. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- 74.Juan CC, Chien Y, Wu LY, et al. Angiotensin II enhances insulin sensitivity in vitro and in vivo. Endocrinology. 2005;146:2246–54. doi: 10.1210/en.2004-1136. [DOI] [PubMed] [Google Scholar]
- 75••.Rodriguez R, Viscarra JA, Minas JN, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology. 2012;153:1684–95. doi: 10.1210/en.2011-1885. A novel study demonstrating the effects of ARB treatment (olmesartan) on pancreatic insulin secreation and GLP-1 signalling in a rat model of the metabolic syndrome-OLETF rats. Interestingly, olmesartan resulted in improvements in glucose stimulated pancreatic insulin secretion, decreases in pancreatic AT1R activation, and increases in GLP-1 signaling without changes in insulin signaling in the skeletal muscle. This is the first study to suggest that the relationship between RAAS blockade and improvements in insulin resistance may be mediated by changes in GLP-1 signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sowers JR, Raij L, Jialal I, et al. Angiotensin receptor blocker/diuretic combination preserves insulin responses in obese hypertensives. J Hypertens. 2010;28:1761–9. doi: 10.1097/HJH.0b013e32833af380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bokhari S, Israelian Z, Schmidt J, Brinton E, Meyer C. Effects of angiotensin II type 1 receptor blockade on beta-cell function in humans. Diabetes Care. 2007;30:181. doi: 10.2337/dc06-1745. [DOI] [PubMed] [Google Scholar]
- 78.Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens Res. 2009;32:533–6. doi: 10.1038/hr.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santos SH, Fernandes LR, Mario EG, et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008;57:340–7. doi: 10.2337/db07-0953. [DOI] [PubMed] [Google Scholar]
- 80.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, Angiotensin-(1-7) and Mas: new players of the Renin Angiotensin System. J Endocrinol. 2012 doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 81.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 82.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193–202. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gembardt F, Sterner-Kock A, Imboden H, et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–7. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tikellis C, Pickering RJ, Tsorotes D, et al. Activation of the Renin-Angiotensin system mediates the effects of dietary salt intake on atherogenesis in the apolipoprotein E knockout mouse. Hypertension. 2012;60:98–105. doi: 10.1161/HYPERTENSIONAHA.112.191767. [DOI] [PubMed] [Google Scholar]
- 85.Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli De Carvalho MH. Potentiation of bradykinin by angiotensin-(1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 2001;37:703–9. doi: 10.1161/01.hyp.37.2.703. [DOI] [PubMed] [Google Scholar]
- 86•.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–8. doi: 10.2337/db09-0782. This is one of the first animal studies to show the beneficial effects of ACE2 on pancreatic function and glycemia. Human ACE2 delivered directly to the pancreas resulted in improved fasting glucose, reduced beta cell apoptosis, and improved beta cell proliferation in 8 week old db/db mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–63. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giani JF, Mayer MA, Muñoz MC, et al. Chronic infusion of angiotensin-(1-7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am J Physiol Endocrinol Metab. 2009;296:E262–71. doi: 10.1152/ajpendo.90678.2008. [DOI] [PubMed] [Google Scholar]
- 89.Prasannarong M, Santos FR, Henriksen EJ. ANG-(1-7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem Biophys Res Commun. 2012;426:369–73. doi: 10.1016/j.bbrc.2012.08.093. [DOI] [PubMed] [Google Scholar]
- 90.Takeda M, Yamamoto K, Takemura Y, et al. Loss of ACE 2 Exaggerates High-Calorie Diet-Induced Insulin Resistance by Reduction of GLUT4 in Mice. Diabetes. 2012 doi: 10.2337/db12-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel VB, Bodiga S, Basu R, et al. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res. 2012;110:1322–35. doi: 10.1161/CIRCRESAHA.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soro-Paavonen A, Gordin D, Forsblom C, et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens. 2012;30:375–83. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 93.Xiao F, Hiremath S, Knoll G, et al. Increased urinary angiotensin-converting enzyme 2 in renal transplant patients with diabetes. PLoS One. 2012;7:e37649. doi: 10.1371/journal.pone.0037649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 13:163–72. doi: 10.1007/s11906-011-0182-2. [DOI] [PubMed] [Google Scholar]
- 95.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 2010;95:1986–90. doi: 10.1210/jc.2009-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32:734–9. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Somlóová Z, Widimský J, Rosa J, et al. The prevalence of metabolic syndrome and its components in two main types of primary aldosteronism. J Hum Hypertens. 2010;24:625–30. doi: 10.1038/jhh.2010.65. [DOI] [PubMed] [Google Scholar]
- 98.Fallo F, Della Mea P, Sonino N, et al. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens. 2007;20:855–61. doi: 10.1016/j.amjhyper.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 99.Catena C, Lapenna R, Baroselli S, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–63. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 100.Ingelsson E, Pencina MJ, Tofler GH, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–92. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 101.Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–45. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 102.Hannemann A, Meisinger C, Bidlingmaier M, et al. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol. 2011;164:751–8. doi: 10.1530/EJE-10-1074. [DOI] [PubMed] [Google Scholar]
- 103.Kumagai E, Adachi H, Jacobs DR, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58:1043–8. doi: 10.1161/HYPERTENSIONAHA.111.180521. [DOI] [PubMed] [Google Scholar]
- 104.Raheja P, Price A, Wang Z, et al. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension. 2012;60:319–25. doi: 10.1161/HYPERTENSIONAHA.112.194787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Selvaraj J, Sathish S, Mayilvanan C, Balasubramanian K. Excess aldosterone-induced changes in insulin signaling molecules and glucose oxidation in gastrocnemius muscle of adult male rat. Mol Cell Biochem. 2012 doi: 10.1007/s11010-012-1452-2. [DOI] [PubMed] [Google Scholar]
- 106.Wada T, Ohshima S, Fujisawa E, Koya D, Tsuneki H, Sasaoka T. Aldosterone inhibits insulin-induced glucose uptake by degradation of insulin receptor substrate (IRS) 1 and IRS2 via a reactive oxygen species-mediated pathway in 3T3-L1 adipocytes. Endocrinology. 2009;150:1662–9. doi: 10.1210/en.2008-1018. [DOI] [PubMed] [Google Scholar]
- 107.Luther JM, Luo P, Kreger MT, et al. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia. 2011;54:2152–63. doi: 10.1007/s00125-011-2158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108•.Briones AM, Nguyen Dinh Cat A, Callera GE, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–78. doi: 10.1161/HYPERTENSIONAHA.111.190223. The first study to demonstrate that aldosterone is produced locally in adipocytes, providing a physiologic link between the association of higher aldosterone levels and insulin resistance in obese individuals. [DOI] [PubMed] [Google Scholar]
- 109.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–5. doi: 10.1016/s0952-3278(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 110.Fogari R, Zoppi A, Mugellini A, Lazzari P, Derosa G. Different effects of aliskiren and losartan on fibrinolysis and insulin sensitivity in hypertensive patients with metabolic syndrome. Horm Metab Res. 2010;42:892–6. doi: 10.1055/s-0030-1263123. [DOI] [PubMed] [Google Scholar]
- 111.Chou CL, Lai YH, Lin TY, Lee TJ, Fang TC. Aliskiren prevents and ameliorates metabolic syndrome in fructose-fed rats. Arch Med Sci. 2011;7:882–8. doi: 10.5114/aoms.2011.25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chou CL, Pang CY, Lee TJ, Fang TC. Direct renin inhibitor prevents and ameliorates insulin resistance, aortic endothelial dysfunction and vascular remodeling in fructose-fed hypertensive rats. Hypertens Res. 2012 doi: 10.1038/hr.2012.124. [DOI] [PubMed] [Google Scholar]
- 113.Lastra G, Habibi J, Whaley-Connell AT, et al. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology. 2009;150:2561–8. doi: 10.1210/en.2008-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen W, Srinivasan SR, Berenson GS. Plasma renin activity and insulin resistance in African American and white children: the Bogalusa Heart Study. Am J Hypertens. 2001;14:212–7. doi: 10.1016/s0895-7061(00)01274-7. [DOI] [PubMed] [Google Scholar]
- 115.Guo X, Cheng S, Taylor KD, et al. Hypertension genes are genetic markers for insulin sensitivity and resistance. Hypertension. 2005;45:799–803. doi: 10.1161/01.HYP.0000154786.17416.ea. [DOI] [PubMed] [Google Scholar]
- 116•.Bonnet F, Patel S, Laville M, et al. Influence of the ACE gene insertion/deletion polymorphism on insulin sensitivity and impaired glucose tolerance in healthy subjects. Diabetes Care. 2008;31:789–94. doi: 10.2337/dc07-1788. The largest human genetics study (N = 1,286) that demonstrates a strong positive association between ACE gene insertion/deletion polymorphism and increased insulin resistance in humans. [DOI] [PubMed] [Google Scholar]
- 117.Chen G, Bentley A, Adeyemo A, et al. Genome-wide association study identifies novel loci association with fasting insulin and insulin resistance in African Americans. Hum Mol Genet. 2012;21:4530–6. doi: 10.1093/hmg/dds282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strawbridge RJ, Dupuis J, Prokopenko I, et al. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624–34. doi: 10.2337/db11-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawai T, Ohishi M, Onishi M, et al. Influence of Renin Angiotensin System Gene Polymorphisms on Visit-to-Visit Blood Pressure Variability in Hypertensive Patients. Am J Hypertens. 2012 doi: 10.1038/ajh.2012.118. [DOI] [PubMed] [Google Scholar]
- 121.Chen YH, Liu JM, Hsu RJ, et al. Angiotensin converting enzyme DD genotype is associated with acute coronary syndrome severity and sudden cardiac death in Taiwan: a case-control emergency room study. BMC Cardiovasc Disord. 2012;12:6. doi: 10.1186/1471-2261-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Danser AH, Schalekamp MA, Bax WA, et al. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–8. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 124.Bloem LJ, Manatunga AK, Pratt JH. Racial difference in the relationship of an angiotensin I-converting enzyme gene polymorphism to serum angiotensin I-converting enzyme activity. Hypertension. 1996;27:62–6. doi: 10.1161/01.hyp.27.1.62. [DOI] [PubMed] [Google Scholar]
- 125.Huang XH, Rantalaiho V, Wirta O, et al. Relationship of the angiotensin-converting enzyme gene polymorphism to glucose intolerance, insulin resistance, and hypertension in NIDDM. Hum Genet. 1998;102:372–8. doi: 10.1007/s004390050707. [DOI] [PubMed] [Google Scholar]
- 126.Ohishi M, Rakugi H, Miki T, et al. Deletion polymorphism of angiotensin-converting enzyme gene is associated with postprandial hyperglycaemia in individuals undergoing general check-up. Clin Exp Pharmacol Physiol. 2000;27:483–7. doi: 10.1046/j.1440-1681.2000.03278.x. [DOI] [PubMed] [Google Scholar]
- 127.Lee YJ, Tsai JC. ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care. 2002;25:1002–8. doi: 10.2337/diacare.25.6.1002. [DOI] [PubMed] [Google Scholar]
- 128.Katsuya T, Horiuchi M, Chen YD, et al. Relations between deletion polymorphism of the angiotensin-converting enzyme gene and insulin resistance, glucose intolerance, hyperinsulinemia, and dyslipidemia. Arterioscler Thromb Vasc Biol. 1995;15:779–82. doi: 10.1161/01.atv.15.6.779. [DOI] [PubMed] [Google Scholar]
- 129.Panahloo A, Andrès C, Mohamed-Ali V, et al. The insertion allele of the ACE gene I/D polymorphism. A candidate gene for insulin resistance? Circulation. 1995;92:3390–3. doi: 10.1161/01.cir.92.12.3390. [DOI] [PubMed] [Google Scholar]
- 130.Zhou JB, Yang JK, Lu JK, An YH. Angiotensin-converting enzyme gene polymorphism is associated with type 2 diabetes: a meta-analysis. Mol Biol Rep. 2010;37:67–73. doi: 10.1007/s11033-009-9648-6. [DOI] [PubMed] [Google Scholar]
- 131.Niu W, Qi Y, Gao P, Zhu D. Angiotensin converting enzyme D allele is associated with an increased risk of type 2 diabetes: evidence from a meta-analysis. Endocr J. 2010;57:431–8. doi: 10.1507/endocrj.k09e-360. [DOI] [PubMed] [Google Scholar]
- 132.Kosachunhanun N, Hunt SC, Hopkins PN, et al. Genetic determinants of nonmodulating hypertension. Hypertension. 2003;42:901–8. doi: 10.1161/01.HYP.0000095615.83724.82. [DOI] [PubMed] [Google Scholar]
- 133.Niemiec P, Zak I, Wita K. The M235T polymorphism of the AGT gene modifies the risk of coronary artery disease associated with the presence of hypercholesterolemia. Eur J Epidemiol. 2008;23:349–54. doi: 10.1007/s10654-008-9241-7. [DOI] [PubMed] [Google Scholar]
- 134.Watkins WS, Hunt SC, Williams GH, et al. Genotype-phenotype analysis of angiotensinogen polymorphisms and essential hypertension: the importance of haplotypes. J Hypertens. 2010;28:65–75. doi: 10.1097/HJH.0b013e328332031a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pollex RL, Hanley AJ, Zinman B, Harris SB, Khan HM, Hegele RA. Metabolic syndrome in aboriginal Canadians: prevalence and genetic associations. Atherosclerosis. 2006;184:121–9. doi: 10.1016/j.atherosclerosis.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 136.Underwood PC, Sun B, Williams JS, et al. The association of the angiotensinogen gene with insulin sensitivity in humans: a tagging single nucleotide polymorphism and haplotype approach. Metabolism. 2011;60:1150–7. doi: 10.1016/j.metabol.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


