Abstract
Objectives
The objectives of this study were: 1) to determine whether there is an association between C-reactive protein (CRP) levels and lower urinary tract symptoms (LUTS) as assessed by the American Urological Association Symptom Index (AUA-SI) among both men and women, 2) to determine the association of CRP levels with individual urologic symptoms comprising the AUA-SI among both men and women.
Methods
The Boston Area Community Health (BACH) Survey used a multistage stratified design to recruit a random sample of 5,502 adults age 30–79. Blood samples were obtained on 3,752 participants. Analyses were conducted on 1,898 men and 1,854 women with complete data on C-Reactive Protein (CRP) levels. Overall LUTS was defined as an AUA-SI≥8 (moderate to severe LUTS). Urologic symptoms comprising the AUA-SI were included in the analysis as reports of fairly often to almost always vs. non/rarely/a few times.
Results
A statistically significant association was observed between CRP levels and overall LUTS among both men and women. The pattern of associations between individual symptoms and CRP levels varied by gender. Nocturia and straining were associated with higher CRP levels among men, while incomplete emptying and weak stream were associated with higher CRP levels among women.
Conclusions
This study demonstrates an association between CRP levels and LUTS in both men and women. The dose-response relationship between increased CRP levels and increased odds of LUTS supports the hypothesized role of inflammatory processes in the etiology of LUTS.
Keywords: C-reactive protein, lower urinary tract symptoms, epidemiology
Introduction
Symptoms of storage, voiding, and post-voiding dysfunction, usually referred to as lower urinary tract symptoms (LUTS), are common in both aging women and men.1,2 LUTS can be caused by a number of pathologic conditions including benign prostatic hyperplasia (BPH) or overactive bladder.3 As inflammation is often present in prostate biopsy specimens, it has been hypothesized that chronic inflammation may be associated with BPH.4–7 However, few studies have investigated the association of LUTS and inflammatory markers. Data on men 60 years and older from the third National Health and Nutrition Examination Survey (NHANES III), have shown that men with C-reactive protein (CRP) levels above the detection limit were more likely to report three or four symptoms (nocturia, incomplete emptying, hesitancy, weak stream) than men with undetectable CRP levels.8 A recent report using longitudinal data from the Olmsted County Study (OCS) reported an association of CRP levels with worsening of storage (irritative) symptoms in men (frequency, urgency and nocturia) and peak urinary flow, but not with overall symptom scores, voiding (obstructive) symptoms (incomplete emptying, intermittency, weak stream, and straining), and prostate specific antigen levels.9 To our knowledge, the association between LUTS and inflammatory markers has not been examined previously in women, nor have potential covariates in men been adequately investigated.
Using data from the Boston Area Community health (BACH) survey, the purpose of this study was to investigate the association between CRP levels and LUTS in a population-representative sample of both men and women. The objectives of this study are: 1) to determine whether there is an association between CRP levels and overall LUTS assessed using the American Urological Association Symptom Index (AUA-SI) among both men and women, and 2) to determine the association of CRP levels and individual urologic symptoms comprising the AUA-SI among both men and women.
Materials and Methods
Overall Design
The BACH survey is a population-based epidemiologic survey of a broad range of urologic symptoms and risk factors in a randomly selected sample. Detailed methods have been described elsewhere.10 A multi-stage stratified design was used to recruit approximately equal numbers of subjects according to age, gender, and race/ethnicity (Black, Hispanic, and White). The BACH sample was recruited from April 2002 through June 2005. Interviews were completed with 63.3% of eligible subjects, resulting in a total sample of 5,502 adults (2,301 men, 3,201 women) after written informed consent was obtained. All protocols and informed consent procedures were approved by the New England Research Institutes' Institutional Review Board.
Data collection
Data were obtained during a 2-hour in-person interview, conducted by a trained (bilingual) phlebotomist/interviewer, in the subject's home. A venous blood sample (20 ml) was obtained and height, weight, hip and waist circumference were measured along with self-reported information on medical and reproductive history, major comorbidities, lifestyle and psychosocial factors, and symptoms of urogynecological conditions. Medication use in the past month was collected using a combination of drug inventory and self-report with a prompt by indication.
Lower Urinary Tract Symptoms (LUTS)
LUTS were assessed using the American Urological Symptom Index (AUA-SI), a clinically validated measure of urological symptoms in men.11,12 The scale is widely used in epidemiologic studies and clinical trials of LUTS, and has a validated and reliable US Spanish version.1,2,13–17 Although to our knowledge there are no validation studies of the AUA-SI in women, studies conducted among both healthy individuals and patients with voiding difficulties have shown that women often report LUTS and have AUA-SI scores similar to those of age-matched men.18,19 The AUA-SI was used both as a continuous variable and categorized into two groups as none or mild symptoms (AUA-SI<8) versus moderate or severe symptoms (AUA-SI≥8). Using a similar approach, individual symptoms were used both as continuous (coded 0–5 from never to almost always) and dichotomized as severe (fairly often/usually/almost always) vs. none or mild symptoms (no symptoms/rarely/a few times). Nocturia was defined as having to get up to urinate at night fairly often or more frequently, and/or having to get up to urinate more than once nightly.
C-Reactive Protein (CRP)
The concentration of CRP was determined using an immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics - Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN). In this assay, an antigen-antibody reaction occurs between CRP in the sample and an anti-CRP antibody that has been sensitized to latex particles, and agglutination results. This antigen-antibody complex causes an increase in light scattering, which is detected spectrophotometrically, with the magnitude of the change being proportional to the concentration of CRP in the sample. Assays were performed at the Children's Hospital Medical Center Research Laboratories, Boston, MA, with a reported a sensitivity of 0.03 mg/l. The coefficients of variation at concentrations of 0.91, 3.07 and 13.38 mg/L are 2.81, 1.61 and 1.1%, respectively.
Covariates
Self-reported race/ethnicity was defined as Black, Hispanic, or White. Body mass index (BMI) was categorized as <25.0, 25.0–29.9, and ≥30.0 kg/m2. Physical activity was measured using the Physical Activity Scale for the Elderly (PASE) and was categorized as low (<100), medium (100–250), and high (>250).20 Alcohol consumption was defined as alcoholic drinks including beer, wine and hard liquor consumed per day: 0, <1, 1–2.9, ≥3 drinks per day. Smoking was defined as never smokers (smoked <100 cigarettes lifetime and not currently smoking), former smokers (smoked ≥100 cigarettes lifetime and currently non-smoker), and current smoker (smoked ≥100 cigarettes and currently a smoker). The socioeconomic status (SES) index was calculated using a combination of education and household income.21 SES was categorized as low (lower 25% of the distribution of the SES index), middle (middle 50% of the distribution), and high (upper 25% of the distribution). The presence of comorbidities (heart disease, type 2 diabetes, hypertension) was defined as a yes response to “Have you ever been told by a health care provider that you have or had….”? Heart disease was defined by self-report of myocardial infarction, angina, congestive heart failure, coronary artery bypass, or angioplasty stent. Participants reporting five or more depressive symptoms (out of 8) using the abbreviated Center for Epidemiological Studies – Depression (CES-D) scale were considered to have clinically significant depression.22 Medication use included in the analysis include use of anti-inflammatory and other medications that could affect CRP levels (both prescription and over the counter),23 and prescription medications for LUTS (Table 1).
Table 1.
Anti-inflammatory and LUTS medications included in the analysis.
| LUTS medications |
|---|
| Medications for overactive bladder, urinary incontinece * |
| Oxybutinin |
| Tolterodine Tartrate |
| Detrol |
| Propantheline |
| Hyoscyamine Sulfate |
| Medications for painful bladder syndrome |
| Pentosan polysulfate sodium |
| Amitryptiline |
| Imipramine |
| Hydroxyzine |
| Medications for BPH ** |
| Doxasozin |
| Terazosin |
| Prazosin |
| Alfuzosin |
| Tamsulosin |
| Finasteride |
| Anti-inflammatory and other medications that could affect CRP levels |
|---|
| Blood form & coagulant/anti-platelet agent (clopidogrel, ticlopidine) |
| Cardiovascular/antilipemics/hmg-coa reductase inhibitors (statins) |
| Non-statin anti-cholesterol drugs |
| Beta-blockers |
| Calcium channel blockers |
| Select ACE inhibitors (captopril, ramipril, fosinopril) |
| Rosiglitazone, pioglitazone |
| Angiotensin II receptor agonists: |
| Losartan |
| Valsartan |
| Irbesartan |
| Olmesartan |
| Candesartan |
| Telmisartan |
| Aspirin |
| Naproxen |
| Ibuprofen |
No reported use of darifenacin, trospium, or solifenacin in BACH
No reported use of dutasteride in BACH
Statistical analysis
As the distribution of CRP levels was skewed, natural log transformations of CRP levels were used. Log(CRP) levels were analyzed as a continuous variable. Additionally, CRP levels were categorized into three groups: <1 mg/l (low cardiovascular risk), 1–3 mg/l (moderate cardiovascular risk), >3 mg/l (high cardiovascular risk). Multivariate logistic regression models were used to assess the association between CRP and LUTS and to adjust for potential confounders. Odds ratios (OR) and 95% confidence intervals (95%CI) were estimated to describe the magnitude of the association. Age, race/ethnicity, and anti-inflammatory and LUTS medication use were always included in the models by default. Other covariates were included in the model if they were potential confounders of the CRP and LUTS association. Analyses were repeated using the AUA-SI and individual symptoms as continuous dependent variables in multivariate linear regression analyses. Of the 2,301 male participants, blood samples were obtained for 1,899 (82.5%) men and 1,858 (58.0%) women. CRP levels were obtained for 1,898 men and 1,854 women. The proportion of participants with missing data was 0.4% (16 subjects) for the AUA-SI, 0.9% (33 subjects) for comorbid conditions and depressive symptoms, 1.0% (37 subjects) for lifestyle variables (physical activity, alcohol consumptions, cigarette smoking), and 4.7% (178 subjects) for the SES index. Overall, 6.7% participants had missing data on at least one of these variables. A multiple imputation technique was used to obtain plausible values for missing data.24 To be representative of the city of Boston, observations were weighted inversely proportional to their probability of selection.25 Weights were post-stratified to the Boston population according to the 2000 census. Analyses were conducted in version 9.1 of SAS (SAS Institute, Cary, NC, USA) and version 9.0.1 of SUDAAN (Research Triangle Institute, Research Triangle Park, NC, USA).
Results
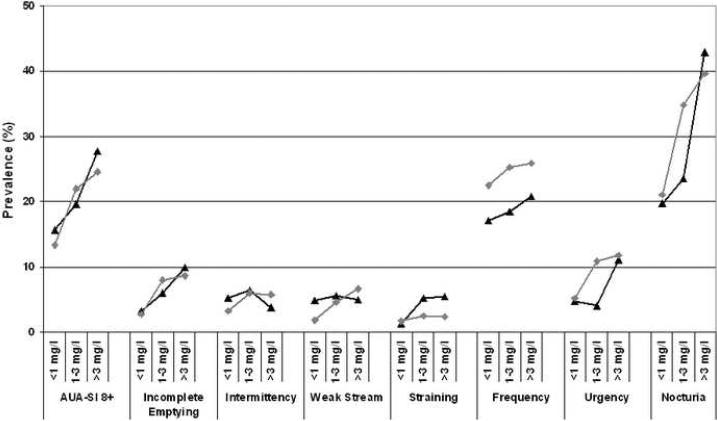
Characteristics of the analysis sample are presented in Table 2. Overall prevalence of obesity was over 30% and was higher in women (39%) compared to men (33.7%). Women had higher CRP levels compared to men (3.50 vs. 2.67 mg/l). This pattern is consistent with previous reports from population based studies.26,27 The increase in the prevalence of LUTS (overall and individual symptoms) by CRP levels and by gender is shown in Figure 2. Overall, there is an increase in prevalence of LUTS with increasing CRP with a comparable pattern in men and women.
Table 2.
Characteristics of the analysis sample overall and by gender. N (weighted percentages) except where indicated.
| Overall N=3,752 | Men N=1,898 | Women N=1,854 | ||
|---|---|---|---|---|
| Age | 30–39 | 956 (35.3) | 512 (37.2) | 444 (33.6) |
| 40–49 | 1036 (25.1) | 553 (25.8) | 484 (24.4) | |
| 50–59 | 910 (18.1) | 436 (17.8) | 474 (18.3) | |
| 60–69 | 563 (14.0) | 260 (12.2) | 303 (15.7) | |
| 70–79 | 287 (7.5) | 137 (7.0) | 150 (8.0) | |
| Race | White | 1413 (59.4) | 710 (61.9) | 703 (57.0) |
| Black | 1119 (27.5) | 537 (25.1) | 582 (29.8) | |
| Hispanic | 1220 (13.1) | 651 (13.0) | 569 (13.2) | |
| Socioeconomic | Low | 1621 (26.6) | 784 (23.9) | 837 (29.2) |
| Status | Middle | 1539 (47.5) | 787 (48.9) | 751 (46.3) |
| High | 593 (25.8) | 327 (27.2) | 266 (24.6) | |
| Body Mass Index (kg/m2) | <25.0 | 947 (30.7) | 495 (26.8) | 452 (34.3) |
| 25.0–29.9 | 1278 (32.8) | 740 (39.5) | 538 (26.7) | |
| ≥30.0 | 1528 (36.5) | 664 (33.7) | 864 (39.0) | |
| Physical | Low <100) | 1181 (25.5) | 534 (25.5) | 646 (25,4) |
| Activity (PASE) | Moderate (200–250) | 1819 (51.0) | 899 (48.2) | 920 (53.6) |
| High(>250) | 752 (24.5) | 465 (26.3) | 287 (21.0) | |
| Smoking | Never | 1760 (48.1) | 815 (45.6) | 1702 (50.2) |
| Former | 1022 (28.2) | 541 (28.9) | 776 (27.2) | |
| Current | 970 (23.7) | 542 (25.6) | 721 (22.6) | |
| Alcohol Consumption (drinks per day) | None | 1550 (34.1) | 636 (26.2) | 915 (41.3) |
| <1/day | 1410 (41.5) | 694 (40.2) | 716 (42.7) | |
| 1–2.9/day | 541 (18.6) | 362 (24.4) | 179 (13.2) | |
| ≥3/day | 250 (5.8) | 206 (9.2) | 44 (2.8) | |
| Heart disease | 370 (9.0) | 182 (9.6) | 188 (8.6) | |
| Type 2 diabetes | 395 (7.8) | 188 (8.1) | 208 (7.6) | |
| Hypertension | 1228 (27.3) | 587 (26.4) | 641 (28.1) | |
| Depression | 803 (17.5) | 317 (14.0) | 486 (20.7) | |
| Medication use | Anti-inflammatory | 2001 (57.5) | 884 (52.6) | 1117 (61.8) |
| LUTS medications | 195 (4.1) | 113 (4.4) | 82 (3.8) | |
| CRP mg/l | Mean (SE*) | 3.10 (0.20) | 2.67 (0.27) | 3.50 (0.22) |
| Median | 1.26 | 1.08 | 1.49 | |
| 25th, 75th percentiles | 0.53, 3.05 | 0.47, 2.51 | 0.60, 3.85 | |
| CRP | <1 mg/l | 1401 (42.1) | 843 (47.3) | 558 (37.3) |
| 1–3 mg/l | 1235 (32.4) | 655 (32.9) | 580 (32.0) | |
| >3 mg/l | 1116 (25.5) | 580 (19.8) | 716 (30.7) | |
| Log[CRP(mg/l)] | Mean (SE*) | 0.29 (0.04) | 0.11 (0.05) | 0.45 (0.05) |
| Median | 0.23 | 0.08 | 0.40 | |
| 25th, 75th percentiles | −0.64, 1.12 | −0.76, 0.92 | −0.51, 1.35 |
Standard error
Among men, a statistically significant association was observed between overall LUTS (AUA-SI≥8) and CRP levels (adjusted OR for log(CRP) levels was 1.21 (95%CI:1.04, 1.39) (Table 3). In unadjusted analyses, statistically significant associations were observed between elevated CRP levels and incomplete emptying, straining, urgency, and nocturia. The association of CRP levels with incomplete emptying and urgency was attenuated in multivariate analyses and was not statistically significant. In contrast, the association of CRP levels with straining (adjusted OR for log(CRP) levels of 1.71, 95%CI:1.22, 2.39) and nocturia (adjusted OR for log(CRP) levels of 1.36, 95%CI:1.15, 1.59) remained statistically significant. Similar to results in men, a statistically significant association was observed between overall LUTS (AUA-S≥8I) and CRP levels (adjusted OR for log(CRP) levels of 1.16, 95%CI:1.00, 1.34). Statistically significant association in multivariate analyses were observed between CRP levels and both incomplete emptying (adjusted OR for log(CRP) levels of 1.27, 95%CI:1.03, 1.58) and weak stream (adjusted OR for log(CRP) levels of 1.40, 95%CI:1.10, 1.77). In unadjusted analyses, statistically significant associations were observed for both urgency and nocturia with CRP levels. However, the magnitude of these associations were attenuated substantially and were statistically non-significant in multivariate analyses. No associations were observed between CRP levels and intermittency, straining, and frequency. Repeating analyses using the AUA-SI and individual symptom reports as continuous variables, the same pattern of associations was observed (data not shown).
Table 3.
Association of C-reactive proteine (CRP) levels and urologic symptoms assessed using odds ratios (OR) and 95% confidence intervals (95%CI) for continuous CRP levels (natural log transformation) and categorized as <1 mg/l (reference), 1–3 mg/l, and >3 mg/l. Statistically significant ORs are in bold.
| Men |
Women |
||||
|---|---|---|---|---|---|
| Dependent Variable | CRP | Unadjusted OR (95%CI) | Adjusted* OR (95%CI) | Unadjusted OR (95%CI) | Adjusted** OR (95%CI) |
| AUA-SI ≥8 | log(CRP) | 1.38 (1.20, 1.57) | 1.21 (1.04, 1.39) | 1.33 (1.16, 1.54) | 1.16 (1.00, 1.34) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 1.31 (0.85, 2.00) | 1.21 (0.76, 1.92) | 1.84 (1.24, 2.74) | 1.50 (0.99, 2.28) | |
| >3 mg/l | 2.05 (1.33, 3.16) | 1.43 (0.91, 2.24) | 2.13 (1.40, 3.25) | 1.45 (0.94, 2.26) | |
|
| |||||
| Incomplete | log(CRP) | 1.50 (1.04, 2.17) | 1.30 (0.91, 1.86) | 1.45 (1.20, 1.75) | 1.27 (1.03, 1.58) |
| Emptying | <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–3 mg/l | 1.89 (0.91, 3.94) | 1.56 (0.79, 3.07) | 3.10 (1.52, 6.32) | 2.42 (1.20, 4.88) | |
| >3 mg/l | 3.27 (1.06, 10.11) | 2.20 (0.78, 6.17) | 3.27 (3.37, 6.65) | 2.32 (1.16, 4.65) | |
|
| |||||
| Intermittency | log(CRP) | 1.11 (0.89, 1.39) | 0.93 (0.74, 1.17) | 1.35 (0.99, 1.84) | 1.20 (0.91, 1.57) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 1.25 (0.56, 2.77) | 1.07 (0.49, 2.35) | 1.85 (0.83, 4.14) | 1.70 (0.73, 3.98) | |
| >3 mg/l | 0.72 (0.30, 1.70) | 0.42 (0.18, 1.00) | 1.73 (0.81, 3.71) | 1.28 (0.61, 2.68) | |
|
| |||||
| Weak stream | log(CRP) | 1.09 (0.90, 1.33) | 1.00 (0.82, 1.22) | 1.53 (1.23, 1.90) | 1.40 (1.10, 1.77) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 1.18 (0.52, 2.71) | 1.13 (0.49, 2.58) | 2.57 (1.12, 5.89) | 1.94 (0.86, 4.36) | |
| >3 mg/l | 1.06 (0.52, 2.14) | 0.87 (0.42, 1.79) | 3.83 (1.66, 8.86) | 2.68 (1.17, 6.12) | |
|
| |||||
| Straining | log(CRP) | 1.78 (1.20, 2.64) | 1.71 (1.22, 2.39) | 1.10 (0.75, 1.61) | 0.94 (0.64, 1.37) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 4.29 (1.13, 16.37) | 3.52 (0.88, 14.13) | 1.47 (0.42, 5.14) | 1.21 (0.44, 3.33) | |
| >3 mg/l | 4.55 (1.43, 14.47) | 3.36 (0.98, 11.45) | 1.34 (0.44, 4.04) | 0.87 (0.28, 2.66) | |
|
| |||||
| Frequency | log(CRP) | 1.09 (0.93, 1.27) | 1.04 (0.89, 1.22) | 1.07 (0.92, 1.24) | 1.02 (0.88, 1.18) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 1.09 (0.72, 1.66) | 1.10 (0.73, 1.66) | 1.16 (0.76, 1.79) | 1.12 (0.72, 1.74) | |
| >3 mg/l | 1.28 (0.80, 2.05) | 1.18 (0.73, 1.90) | 1.20 (0.78, 1.86) | 1.06 (0.67, 1.66) | |
|
| |||||
| Urgency | log(CRP) | 1.44 (1.11, 1.87) | 1.21 (0.94, 1.56) | 1.35 (1.13, 1.62) | 1.16 (0.96, 1.42) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 0.85 (0.45, 1.54) | 0.74 (0.38, 1.43) | 2.21 (1.25, 3.92) | 1.66 (0.92, 3.00) | |
| >3 mg/l | 2.52 (1.32, 4.80) | 1.62 (0.83, 3.18) | 2.41 (1.32, 4.41) | 1.57 (0.85, 2.90) | |
|
| |||||
| Nocturia | log(CRP) | 1.54 (1.33, 1.78) | 1.36 (1.15, 1.59) | 1.35 (1.19, 1.52) | 1.07 (0.93, 1.23) |
| <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | |
| 1–3 mg/l | 1.25 (0.87, 1.80) | 1.09 (0.75, 1.6) | 2.00 (1.39, 2.87) | 1.39 (0.94, 2.06) | |
| >3 mg/l | 3.04 (1.95, 4.72) | 2.09 (1.30, 3.38) | 2.46 (1.65, 3.65) | 1.37 (0.89, 2.12) | |
Adjusted for age, race/ethnicity, SES, heart disease, type 2 diabetes, depression, anti-inflammatory and LUTS medication use
Adjusted for age race/ethnicity, physical activity, heart disease, depression, anti-inflammatory and LUTS medication use. In addition, ORs for nocturia adjusted for BMI.
The association of CRP levels with both the composite voiding and storage symptom scores was similar among men and women, and was attenuated in multivariate analyses (Table 4). Analyses were repeated using cutoff values of 4 and 5 for storage and voiding scores respectively. Statistically significant results were observed between the categorized voiding score and CRP levels among women only.
Table 4.
Association of CRP levels with voiding and storage symptom scores. Odds ratios (OR) or linear regression coefficients (β) and 95% confidence intervals. Statistically significant ORs or βs are in bold.
| Men |
Women |
||||
|---|---|---|---|---|---|
| Dependent variable | Independent variable | Unadjusted OR (95%CI) | Multivariate* adjusted OR (95%CI) | Unadjusted OR (95%CI) | Multivariate** adjusted OR (95%CI) |
| Voiding | Log(CRP) | 1.26 (1.04, 1.52) | 1.08 (0.89, 1.31) | 1.39 (1.17, 1.63) | 1.24 (1.04, 1.48) |
| score | CRP <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥5 vs <5 | 1–3 mg/l | 1.33 (0.74, 2.38) | 1.19 (0.65, 2.16) | 2.51 (1.50, 4.21) | 2.14 (1.26, 3.63) |
| >3 mg/l | 1.56 (0.86, 2.83) | 1.02 (0.55, 1.88) | 2.84 (1.60, 5.05) | 2.10 (1.18, 3.73) | |
|
| |||||
| Unadjusted β (95%CI) | Multivariate* adjusted β (95%CI) | Unadjusted β (95%CI) | Multivariate* adjusted β (95%CI) | ||
|
|
|||||
| Voiding | Log(CRP) | 0.36 (0.16, 0.55) | 0.20 (0.02, 0.39) | 0.30 (0.16, 0.44) | 0.22 (0.04, 0.41) |
| score | CRP <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 |
| continuous | 1–3 mg/l | 0.32 (−0.16, 0.79) | 0.21 (−0.24, 0.65) | 0.68 (0.32, 1.05) | 0.19 (−0.26, 0.64) |
| >3 mg/l | 0.78 (0.25, 1.31) | 0.37 (−0.10, 0.84) | 0.74 (0.34, 1.14) | 0.44 (−0.04, 0.93) | |
|
| |||||
| Unadjusted OR (95%CI) | Multivariate* adjusted OR (95%CI) | Unadjusted OR (95%CI) | Multivariate* adjusted OR (95%CI) | ||
|
|
|||||
| Storage | Log(CRP) | 1.24 (1.09, 1.41) | 1.11 (0.96, 1.27) | 1.22 (1.07, 1.38) | 1.13 (0.99, 1.28) |
| score | CRP <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥4 vs <4 | 1–3 mg/l | 0.96 (0.67, 1.38) | 0.90 (0.61, 1.31) | 1.19 (0.82, 1.75) | 1.07 (0.72, 1.58) |
| >3 mg/l | 1.66 (1.14, 2.43) | 1.24 (0.84, 1.83) | 1.71 (1.17, 2.49) | 1.40 (0.94, 2.08) | |
|
| |||||
| Unadjusted β (95%CI) | Multivariate* adjusted β (95%CI) | Unadjusted β (95%CI) | Multivariate* adjusted β (95%CI) | ||
|
|
|||||
| Storage | Log(CRP) | 0.34 (0.20, 0.49) | 0.15 (0.01, 0.30) | 0.33 (0.16, 0.49) | 0.16 (0.01, 0.32) |
| score | CRP <1 mg/l | 1.00 | 1.00 | 1.00 | 1.00 |
| continuous | 1–3 mg/l | 0.23 (−0.18, 0.64) | 0.13 (−0.25, 0.50) | 0.56 (0.07, 1.04) | 0.11 (−0.27, 0.48) |
| >3 mg/l | 0.93 (0.49, 1.38) | 0.40 (0.00, 0.80) | 0.82 (0.35, 1.30) | 0.45 (−0.03, 0.92) | |
Adjusted for age, race/ethnicity, SES, heart disease, type 2 diabetes, depression, anti-inflammatory and LUTS medication use
Adjusted for age race/ethnicity, physical activity, heart disease, depression, anti-inflammatory and LUTS medication use.
Results for overall LUTS (AUA-SI≥8) by the three race/ethnic groups, (White, Black, Hispanic) show consistent results among White (adjusted OR for log(CRP) of 1.23, 95%CI:1.07, 1.41) and Black participants (adjusted OR for log(CRP) of 1.25, 95%CI:1.02, 1.53) after adjusting for age, gender, heart disease, diabetes, depression, and LUTS and anti-inflammatory medication use. However, this association was not observed among Hispanics (adjusted OR for log(CRP) of 0.97, 95%CI:0.78, 1.21), when adjusting for potential confounders.
Comment
Results from the BACH study demonstrate a similar prevalence of LUTS in both men and women, consistent with previous reports. Unique to this study is a significant association between serum levels of CRP and LUTS in both men and women. A dose-response relationship was observed between CRP levels and LUTS. Increased levels of CRP were associated with increased odds of moderate to severe LUTS (AUA-SI≥8), a result that was consistent by gender and in both unadjusted and adjusted multivariate models. This finding provides strong confirmation of the observed association, relatively monotonic in both genders, between CRP levels and overall LUTS. These results were consistent by race/ethnic groups among White and Black participants, but were attenuated among Hispanics potentially due to a slightly lower prevalence of LUTS and younger age distribution. The pattern of association of individual urologic symptoms with CRP levels however, varied by gender. In men, a positive association was observed between CRP levels and both nocturia and straining, while in women, CRP levels were associated with incomplete emptying and weak stream.
Few studies have examined the potential role of chronic inflammation in the etiology of LUTS. Chronic inflammation has been hypothesized to play a role in the pathogenesis and progression of BPH.5 Evidence of chronic inflammation in BPH has been reported previously;4,6 however, the association between CRP levels and LUTS was investigated in only one study previously using data from NHANES III.8 Results of this study conducted in 2,337 men 60 years and older show a statistically nonsignificant increase in the odds of reporting three or four symptoms (OR=1.47, 95%CI:0.87, 2.50) among men with detectable CRP levels. However, this study has a number of limitations: only 4 of the 7 symptoms comprising the AUA-SI were available for analysis, the sample was restricted to men over 60 years old, and the more variable general CRP test instead of high-sensitivity CRP test was used. A recent report from the OCS investigating the association of CRP levels with longitudinal change in urologic measures among 2,447 men age 40–79 reported an association of high CRP levels with declining peak urinary flow rates and worsening storage symptoms, but not with worsening AUA-SI, voiding score, or prostate specific antigen levels.9 The finding from the BACH study of a significant relationship between overall AUA-SI and CRP is in marked contrast to the OCS report.
Results from the present study show a statistically significant and robust association between CRP and overall LUTS (AUA-SI≥8) with adjusted ORs of 1.20 (95%CI:1.03, 1.39) per log(CRP) levels among men, and 1.16 (95%CI:1.00, 1.35) per log(CRP) levels among women. This similar quantitative relationship between overall LUTS as measured by the AUA-SI in men and women is noteworthy, in that traditionally, LUTS have been ascribed to prostate problems in men, and bladder symptoms in women. However, the mechanisms of LUTS have been poorly understood in both men and women, and perhaps this observed relationship between these common symptoms and a systemic inflammatory marker is a clue to a novel, as yet undefined universal mechanism of LUTS in both men and women. Alternatively, as the patterns of associations of individual symptoms with CRP levels are somewhat different for men and women, perhaps the similar quantitative association of overall LUTS with CRP in both genders is coincidental.
The pattern of associations of individual symptoms with CRP levels observed was different for men and women with nocturia and straining associated with CRP levels in men, while incomplete emptying and weak stream are associated with CRP levels in women. When examining the relationship of CRP levels with the pattern of individual symptoms, men and women's CRP values center more towards voiding symptoms, while the storage components tended to washout with adjustment (urgency (male and female), nocturia (female)). Paradoxically, women's CRP was more associated with voiding than storage symptoms, while men's CRP was significantly associated with both a single storage and voiding symptom (straining and nocturia respectively). The centering of female LUTS to voiding symptoms may represent inflammatory changes in the bladder, detrusor, bladder outlet or the nervous system serving one or more of these locations.
The potential role of inflammatory factors as a causal factor in LUTS among men has an intrinsic appeal, given the recent focus on inflammation in the prostate as a potential initiating event for prostate growth and urinary symptoms in men.28 It is possible that the influence of inflammatory factors on LUTS may relate in part to an effect on bladder function, resulting in both storage and voiding symptoms. In this scenario, voiding symptoms may result from reduced bladder function, rather than increased outlet resistance.29
It is interesting to hypothesize that these female related associations may represent loss of bladder function secondary to bladder inflammation at the detrusor or bladder outlet locations. The combination of voiding and storage symptoms noted in males suggests an additional location of inflammatory effects other than the bladder as proposed in females. The obvious suspect would be the prostate. If inflammatory infiltrates were involved in prostate tissue, then afferent neural feedback may well result in male storage symptoms. Such inflammatory cell infiltrates were actually noted in the REDUCE trial at baseline biopsy, where more severe inflammation was associated with higher AUA-SI scores.7
Strengths of the BACH study include a community-based random sample across a wide age range (30–79), inclusion of large numbers of minority participants representative of Black and Hispanic populations, and a wide range of covariates including sociodemographic, lifestyle, and health variables, which can be adjusted for in the analysis. Although history of comorbid conditions was assessed by self-report with the potential for reporting and/or recall bias, previous research has demonstrated the reliability and validity of self-report for heart disease, diabetes, and hypertension.30 Although the effect of LUTS and anti inflammatory medications are controlled for in the present analysis, the effect of the treatment of comorbid conditions, which could have further attenuated CRP levels, are not accounted for in this analysis. A full analysis of the potential influence of medication use in general on LUTS is beyond the scope of this paper. The BACH study was limited geographically to the Boston area. However, comparison of sociodemographic and health-related variables from BACH with other large regional (Boston Behavioral Risk Factor Surveillance System) and national (National Health Interview Survey) surveys have shown that BACH estimates are comparable to national trends on key health related variables. Moreover, estimates of the strength of the association between CRP levels and LUTS that were statistically significant in bivariate analyses all attenuated to some degree in multivariate analyses, though the relationship with overall LUTS and a number of individual symptoms remained statistically significant after adjusting for potential confounders. Although BACH measured demographics, medications and comorbidities in detail, residual confounding in this observational study cannot be excluded as the explanation for these associations.
Conclusions
In summary, the results of this study demonstrate an association between CRP levels and LUTS in both men and women. The pattern of associations between specific symptoms and CRP levels varied by gender. Nocturia and straining were associated with CRP levels among men, while incomplete emptying and weak stream were associated with CRP levels among women. The dose-response relationship between increased CRP levels and increased odds of LUTS supports the hypothesized role of inflammatory processes in the etiology of LUTS.
Figure 1.
Prevalence of lower urinary tract symptoms (AUA-SI≥8) and individual symptoms (occurring fairly often to almost always) by CRP levels (<1 mg/l, 1–3 mg/l, >3 mg/l) among men and women.

Acknowledgments
Funding: The BACH survey is supported by DK 56842 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. Additional funding was provided from Pfizer, Inc. for analyses presented in this paper. Varant Kupelian, Raymond Rosen, Carol L. Link, and John B. McKinlay are employees of NERI, who received funding from Pfizer in connection with the development of the manuscript. The Corresponding Author retains the right to provide a copy of the final manuscript to the NIH upon acceptance for publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs FD, Fourcade R, Kiemeney L, Lee C. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409–14. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 2.Kupelian V, Wei JT, O'Leary MP, Kusek JW, Litman HJ, Link CL, McKinlay JB. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 4.Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, Sciarra A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164–75. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 5.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006;16:25–9. [PubMed] [Google Scholar]
- 6.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–81. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 7.Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The Relationship between Prostate Inflammation and Lower Urinary Tract Symptoms: Examination of Baseline Data from the REDUCE Trial. Eur Urol. 2008;54:1379–84. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohrmann S, De Marzo AM, Smit E, Giovannucci E, Platz EA. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III) Prostate. 2005;62:27–33. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 9.St Sauver JL, Sarma AV, Jacobson DJ, McGree ME, Lieber MM, Girman CJ, Nehra A, Jacobsen SJ. Association Between C-Reactive prostein Levels and Longitudinal Changes in Urologic Measures. J Urol. 2008;179:S30. [Google Scholar]
- 10.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389–96. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry MJ, Fowler FJ, Jr., O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol. 1992;148::1558–63. doi: 10.1016/s0022-5347(17)36967-7. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 12.Barry MJ, Fowler FJ, Jr., O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 13.Badia X, Garcia-Losa M, Dal-Re R, Carballido J, Serra M. Validation of a harmonized Spanish version of the IPSS: evidence of equivalence with the original American scale. International Prostate Symptom Score. Urology. 1998;52:614–20. doi: 10.1016/s0090-4295(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 14.Gades NM, Jacobson DJ, Girman CJ, Roberts RO, Lieber MM, Jacobsen SJ. Prevalence of conditions potentially associated with lower urinary tract symptoms in men. BJU Int. 2005;95:549–53. doi: 10.1111/j.1464-410X.2005.05337.x. [DOI] [PubMed] [Google Scholar]
- 15.Joseph MA, Harlow SD, Wei JT, Sarma AV, Dunn RL, Taylor JM, James SA, Cooney KA, Doerr KM, Montie JE, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. 2003;157:906–14. doi: 10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 16.Seim A, Hoyo C, Ostbye T, Vatten L. The prevalence and correlates of urinary tract symptoms in Norwegian men: the HUNT study. BJU Int. 2005;96:88–92. doi: 10.1111/j.1464-410X.2005.05573.x. [DOI] [PubMed] [Google Scholar]
- 17.Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the Health Professionals Follow-up Study. Urology. 2002;59:245–50. doi: 10.1016/s0090-4295(01)01506-0. [DOI] [PubMed] [Google Scholar]
- 18.Chai TC, Belville WD, McGuire EJ, Nyquist L. Specificity of the American Urological Association voiding symptom index: comparison of unselected and selected samples of both sexes. J Urol. 1993;150:1710–3. doi: 10.1016/s0022-5347(17)35874-3. [DOI] [PubMed] [Google Scholar]
- 19.Lepor H, Machi G. Comparison of AUA symptom index in unselected males and females between fifty-five and seventy-nine years of age. Urology. 1993;42:36–40. doi: 10.1016/0090-4295(93)90332-5. discussion 40-1. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–27. [PMC free article] [PubMed] [Google Scholar]
- 22.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 23.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24:33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 24.Schafer J. Analysis of incomplete multivariate data. Chapman and Hall; London: 1997. [Google Scholar]
- 25.Cochran W. Sampling Techniques. John Wiley and Sons; New York, NY: 1977. [Google Scholar]
- 26.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr., Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–9. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 27.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr., Herrington DM. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–8. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–16. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Pinggera GM, Mitterberger M, Steiner E, Pallwein L, Frauscher F, Aigner F, Bartsch G, Strasser H. Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. BJU Int. 2008;102:470–4. doi: 10.1111/j.1464-410X.2008.07587.x. [DOI] [PubMed] [Google Scholar]
- 30.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]