Abstract
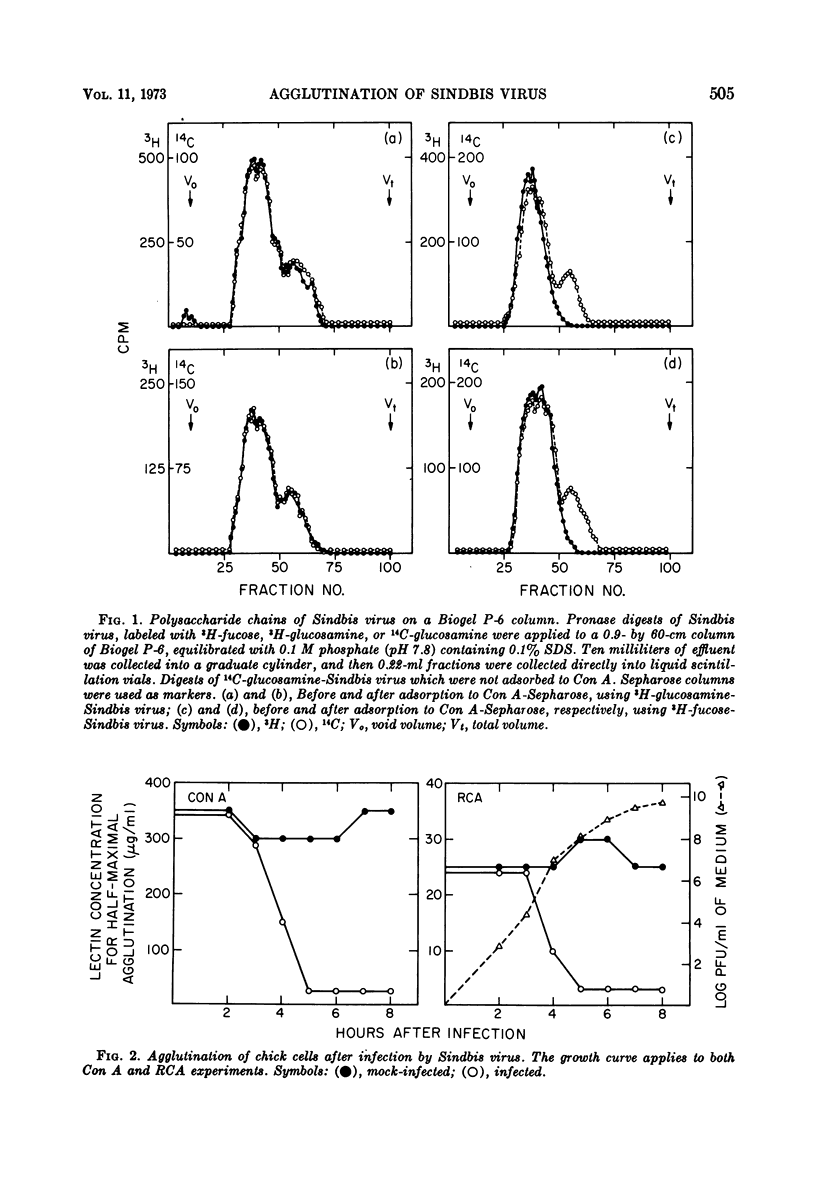
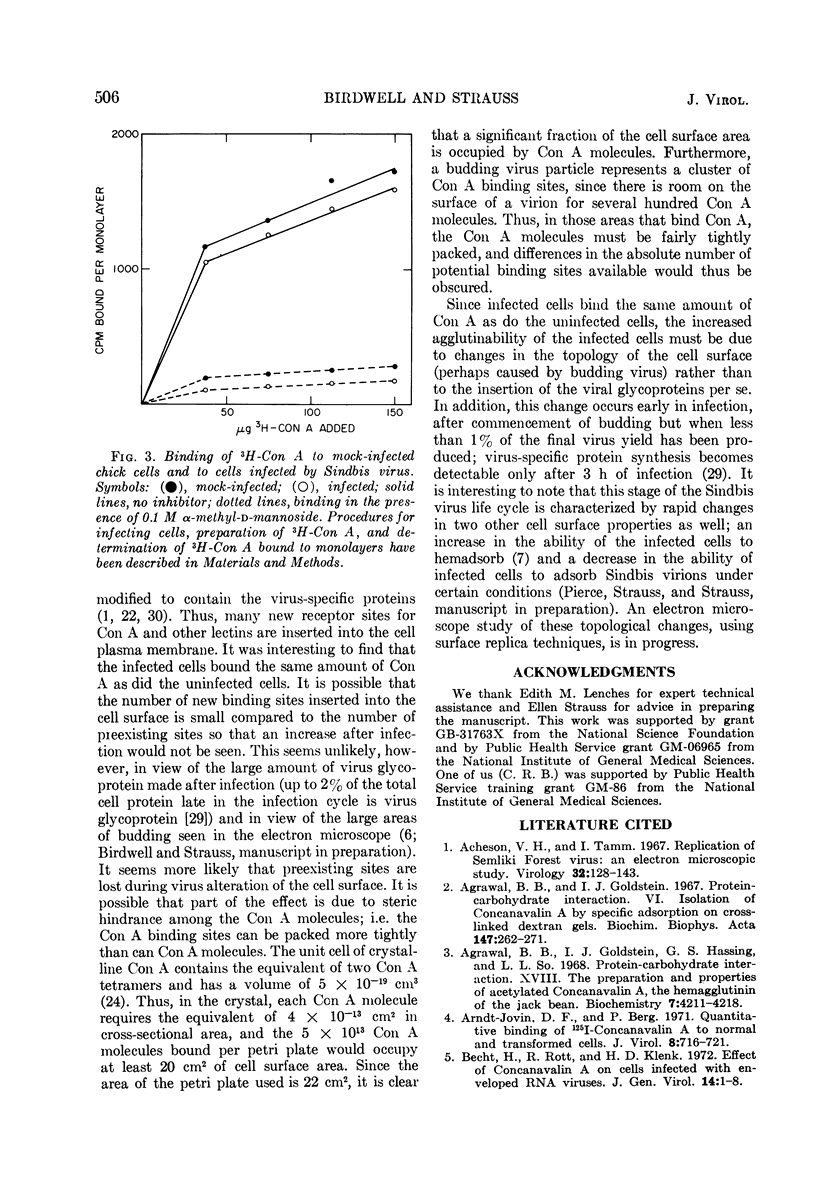
We have examined the agglutination of Sindbis virus and of chick and hamster cells infected with Sindbis virus by two of the plant lectins, concanavalin A and Ricinus communis agglutinin. Both lectins agglutinate the virus by binding to the polysaccharide chains of the envelope glycoproteins. Both chick and hamster cells exhibit increased agglutination by the lectins after infection by Sindbis virus. In the case of chick cells infected with Sindbis virus, this increase in agglutinability occurs between 3 and 5 h after infection. Infected and mock-infected cells bind the same amount of 3H-labeled concanavalin A, which suggests that the increase in agglutination after infection is due to rearrangements at the cell surface rather than to insertion of new lectin binding sites per se.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Agrawal B. B., Goldstein I. J., Hassing G. S., So L. L. Protein--carbohydrate interaction. 18. The preparation and properties of acetylated concanavalin A, the hemagglutinin of the jack bean. Biochemistry. 1968 Dec;7(12):4211–4218. doi: 10.1021/bi00852a011. [DOI] [PubMed] [Google Scholar]
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Berg P. Quantitative binding of 125 I-concanavalin A to normal and transformed cells. J Virol. 1971 Nov;8(5):716–721. doi: 10.1128/jvi.8.5.716-721.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht H., Rott R., Klenk H. D. Effect of Concanavalin A on cells infected with enveloped RNA viruses. J Gen Virol. 1972 Jan;14(1):1–8. doi: 10.1099/0022-1317-14-1-1. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Temperature-sensitive mutants of Sindbis virus: biochemical correlates of complementation. J Virol. 1967 Oct;1(5):956–962. doi: 10.1128/jvi.1.5.956-962.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Livingston D. C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat New Biol. 1971 Aug 4;232(31):155–156. doi: 10.1038/newbio232155a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Ellwood D. C., Appleyard G., Stanley J. L. Agglutination of an arbovirus by concanavalin A. Nat New Biol. 1971 Sep 8;233(36):50–51. doi: 10.1038/newbio233050a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Poste G., Reeve P. Agglutination of normal cells by plant lectins following infection with nononcogenic viruses. Nat New Biol. 1972 May 24;237(73):113–114. doi: 10.1038/newbio237113a0. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Reeke G. N., Jr, Becker J. W., Lipscomb W. N., Edelman G. M. Structure of soncanavalin A at 4 A resolution. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1853–1857. doi: 10.1073/pnas.68.8.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]


