Abstract
Background
Psychological therapies have been developed for parents of children and adolescents with a chronic illness. Such therapies include parent only or parent and child/adolescent, and are designed to treat parent behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and/or family functioning. No comprehensive, meta-analytic reviews have been published in this area.
Objectives
To evaluate the effectiveness of psychological therapies that include coping strategies for parents of children/adolescents with chronic illnesses (painful conditions, cancer, diabetes mellitus, asthma, traumatic brain injury, inflammatory bowel diseases, skin diseases or gynaecological disorders). The therapy will aim to improve parent behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and family functioning.
Search methods
We searched CENTRAL, MEDLINE, EMBASE and PsyclNFO for randomised controlled trials (RCTs) of psychological interventions that included parents of children and adolescents with a chronic illness. The initial search was from inception of these databases to June 2011 and we conducted a follow-up search from June 2011 to March 2012. We identified additional studies from the reference list of retrieved papers and from discussion with investigators.
Selection criteria
Included studies were RCTs of psychological interventions that delivered treatment to parents of children and adolescents (under 19 years of age) with a chronic illness compared to active control, wait list control or treatment as usual. We excluded studies if the parent component was a coaching intervention, the aim of the intervention was health prevention/promotion, the comparator was a pharmacological treatment, the child/adolescent had an illness not listed above or the study included children with more than one type of chronic illness. Further to this, we excluded studies when the sample size of either comparator group was fewer than 10 at post-treatment.
Data collection and analysis
We included 35 RCTs involving a total of 2723 primary trial participants. Two review authors extracted data from 26 studies. We analysed data using two categories. First, we analysed data by each medical condition across all treatment classes at two time points (immediately post-treatment and the first available follow-up). Second, we analysed data by each treatment class (cognitive behavioural therapy (CBT), family therapy (FT), problem solving therapy (PST) and multisystemic therapy (MST)) across all medical conditions at two time points (immediately post-treatment and the first available follow-up). We assessed treatment effectiveness on six possible outcomes: parent behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and family functioning.
Main results
Across all treatment types, psychological therapies that included parents significantly improved child symptoms for painful conditions immediately post-treatment. Across all medical conditions, cognitive behavioural therapy (CBT) significantly improved child symptoms and problem solving therapy significantly improved parent behaviour and parent mental health immediately post-treatment. There were no other effects at post-treatment or follow-up. The risk of bias of included studies is described.
Authors' conclusions
There is no evidence on the effectiveness of psychological therapies that include parents in most outcome domains of functioning, for a large number of common chronic illnesses in children. There is good evidence for the effectiveness of including parents in psychological therapies that reduce pain in children with painful conditions. There is also good evidence for the effectiveness of CBT that includes parents for improving the primary symptom complaints when available data were included from chronic illness conditions. Finally, there is good evidence for the effectiveness of problem solving therapy delivered to parents on improving parent problem solving skills and parent mental health. All effects are immediately post-treatment. There are no significant findings for any treatment effects in any condition at follow-up.
Plain language summary
Psychological therapy for parents of children with a longstanding or life-threatening physical illness
Parenting a child with a longstanding or life-threatening illness is very difficult, and can have a negative impact on many aspects of the parent's life. Parents of these children often have difficulty balancing caring for their child with other responsibilities such as work, social life, finance and other household tasks. As a result they may experience more stress, worries, sad feelings, family arguments and troubling child behaviour. Parents also have a major influence on their child's well-being and adjustment, and play an important role in how their child adapts to living with an illness. Treatments for parents of children with a longstanding illness aim to improve parent distress, parenting behaviours, family conflict, child distress, child disability and the child's medical symptoms.
Thirty-five studies were found in the search, but only 26 of these had data that could be used in the analyses. We found studies for six child illnesses (painful conditions, cancer, diabetes, asthma, traumatic brain injury and eczema) and four types of psychological therapies (cognitive behavioural therapy, family therapy, problem solving therapy and multisystemic therapy). We looked at the effects of the treatments on parent distress, parenting behaviours, family conflict, child distress, child disability and symptoms of the child's illness immediately after the treatment and at the first available follow-up time point after the treatment had ended. We analysed the data in two ways; first we grouped the studies by each individual illness and then we grouped the studies by each individual psychological therapy.
Psychological therapies can help reduce pain in children with painful conditions. Where there were results available from studies of different chronic illnesses, we found that cognitive behavioural therapy can improve the child's medical symptoms. Problem solving therapy can improve parent's distress and their ability to solve problems. More studies of psychological treatments for parents of children with a longstanding illness are needed.
Background
Description of the condition
Chronic illness affects the lives of many children and their families. The prevalence of illness and disability differs by geographical and economical context. In the USA, Canada, Northern Europe, UK and Australia chronic activity-limiting conditions are reported to be frequent, with painful illness, allergy, asthma and obesity being common (McDougall 2004). The changing demographic of childhood illness in economically wealthy countries has prompted a re-analysis of the role of paediatric medicine, as chronic illness becomes more prevalent than acute (e.g. Halfon 2010; Van Cleave 2010). Other parts of the world present different clinical challenges. In Africa, for example, life expectancy is 54 years and shorter in sub-Saharan Africa where almost half the population are children and the most prevalent chronic conditions are related to communicable diseases, in particular HIV-related disease, malaria and tuberculosis (WHO 2011).
The existing published literature shows a bias towards the medical management of chronic illness related to environment or lifestyle. Chronic pain in childhood is known to have widespread negative outcomes for children and parents (Palermo 2000). Psychological intervention reviews have also been undertaken on the impact of sickle cell disease (Anie 2012), recurrent abdominal pain/irritable bowel syndrome (Huertas-Ceballos 2008), type 1 diabetes (McBroom 2009), traumatic brain injury in children (Soo 2007) and asthma (Yorke 2009).
The impact of childhood chronic illness on other family members, including parents, has been of growing interest for two reasons. First, it is now recognised that parents who have significant emotional distress of their own and poor family functioning can indirectly affect child outcomes (Logan 2005; Palermo 2007). Second, it is now recognised that parents can have a positive effect on child adjustment to chronic illness (Logan 2005).
Description of the intervention
Addressing the mental health problems of parents, and enabling parents to be agents of change in the management of their child's chronic illness, have recently been promoted as viable treatment approaches (Jordan 2007; Palermo 2009b). Studies have focused on the education of parents about the specific condition or treatment (e.g. Savage 2011), whilst others concern lay- or nurse-mediated social support (e.g. Lewin 2010). In psychological science, specific treatment approaches have been developed that focus on reducing the emotional distress expressed by parents, or on altering parenting behaviours to promote better child outcomes, whether this be decreasing emotional distress, or improving physical symptoms or behaviour.
Psychological interventions of interest are defined as any psychotherapeutic treatment specifically designed to change parent cognition or behaviour, or both, with the intention of improving child outcomes. Psychological interventions are varied in their approaches and there is still debate surrounding which treatment is most effective at improving mental health and behaviour in parents and children with chronic illnesses. Such interventions include cognitive behavioural therapy (CBT) which has been found to be effective with children with painful conditions (e.g. Eccleston 2009a; Palermo 2009a). Problem solving therapy (PST) has also been used with parents and children with various chronic illnesses (D'Zurilla 1995; Sahler 2002). Other treatments have emerged from a family systems approach that focuses explicitly on the family as a unit of intervention (Ellis 2005; Wysocki 2000) such as multisystemic therapy (MST) or family therapy (FT).
How the intervention might work
There are a variety of interventions described as psychological. Cognitive and cognitive behavioural therapies dominate, but therapies with a psychodynamic or systemic tradition are also represented. Family and couple therapies have also been developed. All psychological interventions include a rationale for therapy. Common is education around illness and behaviour. Establishing the therapy and the therapist as credible is an important general stage (Nock 2001). Next, a therapeutic relationship is established that will enable a confidential, non-blaming investigation of behaviour. Then, depending on the illness and behavioural presentation, specific components may include anxiety management, exposure for phobic targets, problem solving skills, cognitive therapy for depression and relationship management. Finally, most treatments will include a maintenance component that focuses on robust behavioural change within a normal home environment outside the clinic, over time. Such components can be seen in parent interventions using different therapies to improve parental functioning, child behaviour and mental health.
Cognitive behavioural interventions specifically are based on a number of foundational assumptions. First, behaviour is socially and historically contingent (Skinner 1953). Second, cognition is an emergent property of behavioural context (James 1980). Third, behaviour is regulated by cognitive goals (Bandura 1989). Fourth, emotions influence both behaviour and cognition (Ashby 1999; Gilliom 2002). Fifth, most behaviour is deployed outside of conscious awareness or control (Bargh 2008). Finally, some attempts to control cognition and behaviour can have paradoxical negative effects on desired outcomes (Wegner 1994).
Other interventions such as PST (D'Zurilla 1995) provide a specific framework that includes positive problem orientation towards an issue. Cognitive-behavioural strategies are used in PST and include the following steps: Identify the problem, Define your options, Evaluate your options, Act, and finally See if it worked. PST has previously been effective with depression, anxiety and stress-related syndromes (D'Zurilla 1999).
Family and systemic therapies specifically focus on a contextual and relational view of the aetiology and maintenance of behaviour. In particular, the target of health behaviour change is typically related to family functioning, or in the cognitive representation of the family, rather than on individual attitudes, beliefs or behaviour. Typically, family or systems therapy approaches will include multiple family members and outcomes are often expressed on behalf of the family or dyad (two individuals regarded as a pair).
Why it is important to do this review
The prevalence of childhood chronic illness has more than doubled in the last 20 years (Perrin 2007). Parents provide a major influence in children's lives, influence that can have both a positive or negative effect on child outcomes. Psychological interventions are being developed that focus on helping parents to help both themselves and their children. Establishing the evidence at this stage of development will provide comment on current best practice, and serve to guide new treatment development.
Objectives
To evaluate the effectiveness of psychological parent interventions on reducing the distress associated with parenting a child with a chronic illness.
To evaluate the effectiveness of psychological parent interventions on reducing the primary symptom or behavioural expression of illness for the child.
To assess primary outcomes and adverse events of different parent interventions in the 14 different conditions (see ‘Types of participants’).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared parental psychological interventions with attention control, other active treatment or waiting list control were considered for this review. The parent intervention had to be primarily psychological in nature. Studies that met the inclusion criteria consisted of the following:
RCT, published in full in a peer-reviewed journal;
primary aim of the trial was an evaluation of a psychological intervention;
involved parents of children who have an illness for three months or more (Van der Lee 2007);
involved parents of children adjusting to a diagnosis of cancer;
had a n of 10 or more in both the treatment and control arm at end of treatment or follow-up.
Types of participants
Parents of children who have endured a chronic illness for three months or more. Parents were regarded as the primary caregiver of a child or adolescent under the age of 19 years. Parents were defined, for the purposes of this review as any adult who adopts the responsibility for the role of parenting the child (this could include biological parent, guardian, other adult family member). There was no lower age limit for the children, however, by the definition of ‘chronic illness’, the child must be three months or more. The children must also be experiencing one (or more) of the following physical illnesses:
headache;
recurrent abdominal pain;
back pain;
idiopathic pain conditions;
complex regional pain syndrome (CRPS);
rheumatological conditions (e.g. arthritis and fibromyalgia);
sickle cell disease;
cancer;
diabetes mellitus;
asthma;
traumatic brain injury;
inflammatory bowel diseases;
skin diseases (e.g. eczema);
gynaecological disorders (e.g. chronic dysmenorrhoea and endometriosis).
Chronic illnesses were selected from the National Survey of Children with Special Health Care Needs 2009 to 2010 (Data Resource Center 2010). It was impractical to include all chronic illnesses on this list, therefore, we selected the most common. However, three illnesses (cancer, inflammatory bowel diseases and gynaecological disorders) were not included in the Current Health Conditions and Functional Difficulties but were added for the purposes of this review. Cancer has a high incidence level and it was predicted that in 2007, there were 10,400 children with cancer in the US alone under the age of 14 (Linabery 2007). Studies that investigate interventions with parents of children who have ‘survived’ an illness such as childhood survivors of cancer were also eligible for inclusion. Inflammatory bowel diseases and gynaecological disorders are also common conditions in childhood and adolescence and were included because they are thought to be prevalent but under-represented in the academic literature.
Types of interventions
Studies were included if the interventions were primarily psychological, and had credible, recognisable psychological/psychotherapeutic content, and were specifically for, or included parents. Psychological interventions were defined as any psychotherapeutic treatment specifically designed to change parent cognition or behaviour, or both, and had the intention of improving parent or child outcomes. However, studies in which parents acted as ‘coaches’ were excluded from this review. The intervention had to aim to provide treatment to the parent rather than teach them to deliver an intervention to their child. Similarly, we also excluded health promotion therapies such as intervening with the parent to cease smoking to improve their child's asthma. We have excluded studies that combine psychological interventions with pharmacological interventions or are qualitative in nature as it is difficult to combine qualitative and quantitative data.
Types of outcome measures
Primarily, parent outcomes were the target of our review. However, if the study also reported child outcomes as stated below, we also analysed and reported these data. We analysed data at post-treatment and the first available follow-up period, where reported.
Primary outcomes, depending on specific treatment, were: parent behaviour, parent mental health, child behaviour/disability, child mental health, child primary symptom, family function and adverse events.
We made a judgement when studies reported multiple measures within one of the six outcome domains without defining their primary or secondary outcome measure. The rules of this judgement were to select the most generic, reliable and most frequently used measure within the field, and most appropriate for the given outcome category. When both parents and children reported on a measure, we extracted the self report item unless the non-self report measure was a more generic measure. For family functioning measures, we extracted parent data over child data as the review is focused on whether interventions can help parents of children with a chronic illness.
Search methods for identification of studies
We searched electronic databases and reference lists to identify studies matching the criteria. In addition, we also contacted experts and study authors for additional studies.
Electronic searches
We searched four databases for studies from inception to June (week 4) 2011 and again in March (week 1) 2012:
The Cochrane Central Register of Controlled Trials (CENTRAL, beginning 1968);
MEDLINE via Ovid (beginning 1946);
EMBASE via Ovid (beginning 1974);
PsyclNFO via Ovid (beginning 1806).
We adapted the search strategies from the MEDLINE search (see Appendix 1) and they are included in Appendix 2. There was no language restriction imposed and no unpublished literature or grey material was included. The search strategy included four categories of words: psychological interventions, parents, children/adolescents and chronic illnesses (as stated above), and was refined by a methodological filter used to identify RCTs according to Cochrane guidance (Higgins 2011).
Searching other resources
We performed a reference list and citation search of each selected study which identified further studies meeting the inclusion criteria. We then repeated this stage for such studies. We also checked meta-analyses and systematic reviews that met the inclusion criteria for appropriate studies and included them if they met the inclusion criteria. We also contacted authors of selected studies and experts in the field for further studies that had not already been identified from the search.
Data collection and analysis
Selection of studies
EF performed the searches of each database and collated results. Two review authors (EF, EL) then sifted through potential studies and identified those eligible to be included with CE acting as arbiter. No blinding of study authors' names, institutions or journals occurred during this process. We resolved any disagreements by discussion between all review authors.
We made selection of abstracts using the following criteria.
- Participants
- Parents must be referred to in the title or abstract of each study
- The parent must be the primary caregiver of the child
- Children must have one or more of the chronic illnesses listed above
- Children must be in the age range three months to 19 years
- There must be 10 or more participants in each condition at the end of the treatment assessment
- Intervention
- The intervention must be primarily psychological in at least one condition
- Must be of RCT in design
- One or more parents must be treated by the intervention
- The parents and/or child must be measured at baseline and at a point in time during or after the intervention
- Comparison groups
- Attention control group
- Active treatment group
- Treatment as usual group: this would consist of usual doctors' appointments and treatment without added psychological therapy
- Wait list control
Numerical outcomes presented
We then obtained the selected studies meeting the criteria in full and EF and EL read and assessed them independently.
Data extraction and management
Two review authors (EF and EL) carried out data extraction from studies that were identified by all review authors as appropriate for inclusion. The data extraction sheet was adapted from Eccleston 2009a and Eccleston 2009b. It included references, the diagnosis of the child's chronic illness, aspects of the intervention or therapy, characteristics of the treatment team, the setting of the intervention and outcome measures.
Assessment of risk of bias in included studies
We assessed risk of bias using the recommended Cochrane guidance (Higgins 2011). Of the five suggested risk of bias categories, we judged studies on random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). We excluded the option of ‘blinding participants and personnel’ because we deemed it redundant as neither therapists nor patients can be blinded to whether they deliver or receive treatment.
Decisions about random sequence generation were based on whether authors gave a convincing method of randomisation. Allocation concealment judgements were based on whether sufficient methods were employed for random allocation to take place. Participants being stratified by age or gender did not count as bias but are noted in the tables. We judged risk of blinding of outcome assessment on whether the measures were administered and collected by an assessor who was blind to the treatment allocation. We judged high risk of attrition bias when no description of attrition was reported. We made an unclear decision when there was an adequate decision given but authors did not report whether there were significant differences between completers and non-completers. We concluded low risk of bias when authors gave both a description of attrition and stated that there were no significant differences between completers and non-completers. Third, we judged selective reporting bias in two parts. First, we judged studies on whether data were fully reported in the study or if authors later responded to data requests. Second, we rated each study on a three-point scale for concordance (two points = full concordance, one point = partial concordance, zero points = no concordance). We rated studies for concordance between study aims and measures (i.e. if aims corresponded to measures stated in methods section) and between measures and results (i.e. if all measures were reported in results, and no additional measures were added to results that were not stated in the methods section).
Assessment of quality in included studies
We assessed quality of studies using the method advocated by Yates 2005. Two authors (EF, EL) rated study quality for each study and disagreements were settled by discussion between all authors. The rating scale consists of two sections which creates an overall quality of study score of 35. The first section measures treatment quality (0 to 9) which assesses the treatment rationale, duration of treatment, manualisation, therapist training and engagement of patients. The second section measures the quality of the study design and methods used (0 to 26). This section measures the inclusion/exclusion criteria, rates of attrition, description of patient sample, steps taken to minimise bias (randomisation, allocation bias, measurement bias and treatment expectations), justification of outcomes and whether they are reliable and valid, follow-up, adequate statistical analyses (power, sufficient sample size, planned data analysis, statistical reporting and intention-to-treat analysis) and finally choice of control group. The studies are then categorised as ‘ high quality’ or ‘ low quality’ of being biased. The boundary between high and low quality was defined as the mid-point (quality of study high quality ≥ 18, low quality ≤ 17, treatment quality high quality ≥ 5, low quality ≤ 4, quality of study design and methods used high quality ≥ 15, low quality ≤ 14).
Measures of treatment effect
We investigated four classes of psychological therapies: cognitive behavioural therapy (CBT), family therapy (FT), problem solving therapy (PST) and multisystemic therapy (MST). CBT is based on theories of behavioural analysis (Bergin 1975), cognitive theory (Beck 1979) and social learning theory (Bandura 1977). CBT therefore includes a range of strategies with the goals of modifying social/environmental and behavioural factors that may exacerbate or cause symptoms, and modifying maladaptive thoughts, feelings and behaviours to reduce symptoms and prevent relapse. FT is based on family systems theory (Haley 1976; Minuchin 1974), which emphasises the role of the family context in an individual's emotional functioning. FT interventions typically focus on altering patterns of interactions between family members, and include structural family therapy (Minuchin 1974), strategic family therapy (Haley 1976) and behavioural systems family therapy (Robin 1989). PST is based on the D'Zurilla 1982 social problem solving model, which defines problem solving in terms of an individual's ability to recognise problems and use cognitive and behavioural skills to solve them. PST includes didactic instruction in problem solving skills, followed by in-session modelling, behavioural rehearsal and performance feedback, as well as homework assignments (D'Zurilla 2007). Finally, MST is an intensive family and community-based intervention based on the Bronfenbrenner 1979 social ecological model and family systems theory (Haley 1976; Minuchin 1974). MST therefore targets the patient, their family and broader systems such as the patient's school, work or medical team as needed. MST incorporates a wide range of evidence-based intervention techniques based on the individual needs of the patient and family (Henggeler 2003), including cognitive-behaviour approaches, parent training and family therapies.
We extracted data immediately post-treatment (i.e. immediately after the treatment programme had finished). Where data were available, we also analysed studies at follow-up, which is classed as the first available time point after post-treatment. We categorised outcomes into one of six outcome domains: parent behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and family functioning. Where studies had more than one comparator group, we chose the ‘active control group’ over ‘standard treatment’ or ‘wait list control’ groups.
There are four therapies (CBT, FT, PST and MST), eight conditions (asthma, cancer, diabetes, gynaecological disorders, inflammatory bowel syndrome, painful conditions (these were grouped together due to the homogeneous nature of the trials), skin diseases and traumatic brain injury), two time points (post-treatment and follow-up) and six possible outcomes (parent behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and family functioning). There are six categories by which we analysed data.
For each condition, across all types of psychological therapy, what is the effectiveness for the six outcomes immediately post-treatment?
For each condition, across all types of psychological therapy, what is the effectiveness for the six outcomes at follow-up?
For each psychological therapy, across all conditions, what is the effectiveness for the six outcomes immediately post-treatment?
For each psychological therapy, across all conditions, what is the effectiveness for the six outcomes at follow-up?
The interaction between the condition and the psychological therapy effectiveness.
Investigation of characteristics of particularly effective treatments.
Analyses are presented for each of the six outcomes, however, due to the heterogeneous nature of the conditions and studies, this was not always possible. We pooled data using standardised mean difference and random-effect models as studies did not consistently use the same scales when measuring the same outcomes. Cohen's d effect sizes can be interpreted as follows: 0.2 = small, 0.5 = medium, 0.8 = large (Cohen 1992). Where possible, we combined data in a metaanalysis and, following Cochrane guidance (Higgins 2011), presented data in the form of numbers needed to treat and numbers needed to harm.
Dealing with missing data
We contacted authors of studies when data were not reported fully in publications. However, when authors could not send data to the review authors or were non-responsive to emails, we excluded data.
Assessment of heterogeneity
Subgroup analysis explored the possible sources of heterogeneity (see Results).
Assessment of reporting biases
Biases are reported within the results section of the review following Cochrane guidance on bias reporting (Higgins 2011). When possible, we attempted to use a failsafe N to control for publication bias.
Subgroup analysis and investigation of heterogeneity
When there were multi-arm trials or trials that compared more than one active treatment, we used the primary active treatment and compared with the least biased comparator (typically standard care or treatment as usual). Analyses of the following subgroups are presented where data permitted:
parent-only interventions versus family-based interventions;
intervention effects within specific illnesses;
intervention effects across specific types of psychological interventions.
We also explored heterogeneity through subgroup analysis (see ‘Results’).
Results
Description of studies
See: ‘Characteristics of included studies’ and ‘Characteristics of excluded studies’.
Results of the search
We extracted a total of 114 papers to identify whether they met the full inclusion criteria; 107 papers were found in the initial search, and a further seven studies were identified later in an updated search before publication. Of these 114 papers, 99 were found from the search of databases, six papers from the citation search, four papers from reference searches and five papers from authors of included studies. We deemed 35 studies (45 papers) to meet the inclusion criteria for the review, whilst 61 studies (69 papers) were excluded (Aleman 1992; Anderson 1999; Betancourt 2004; Braga 2005; Bruzzese 2008; Burke 1997; Burke 2001; Cakan 2007; Canino 2008; Carey 2008; Chernoff 2002; Chiang 2009; Ellis 2007; Ellis 2008; Evans 1999; Field 1998; Forsander 1995; Forsander 2003; Garbutt 2010; Gerber 2010; Giallo 2008; Glang 2007; Gustafsson 1986; Harris 2001; Haus 1976; Hernandez 1998; Hommel 2012; Hovell 1994; Humphreys 2000; Ireys 1996; Ireys 2001; Jay 1990; Johnson 1987; Kamps 2008; Kaslow 2000; Kazak 1996; Kazak 2005; Ketchen 2006; Klinnert 2005; Klinnert 2007; Kroner-Herwig 1998; Kupfer 2010; Lasecki 2008; Logan 1997; Mendez 1997; Nelson 2011; Perez 1999; Rasoli 2008; Sanders 1989; Sanders 1996; Satin 1989; Scholten 2011; Sieberg 2011; Staab 2002; Sullivan-Bolyai 2010; Szczepanski 2010; Wade 2010; Walders 2006; Walker 1996; Warner 2011; Wysocki 1997).
Included studies
Of the 35 studies (45 papers) included in this review, 31 had two comparator arms and four studies had three comparator arms. Of the 31 studies that had two arms, 15 studies used active controls where patients had to actively engage in another type of treatment (e.g. education) whilst 19 used wait list or “treatment as usual controls”. The total number of participants at the end of treatment was 2723 (mean = 80 per study). The total number of participants entering treatment was 3214 (mean = 95 per study). Therefore, the completion rate for all studies was 85%, making the attrition percentage 15%. The proportion of completers across studies ranged from 59% to 100%.
We categorised the studies by the primary illness of the children. There were 12 painful condition studies (Allen 1998; Barakat 2010; Barry 1997; Connelly 2006; Duarte 2006; Hicks 2006; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Levy 2010; Palermo 2009; Robins 2005; Sanders 1994). Six studies with the primary illness of cancer met the inclusion criteria (Askins 2009; Hoekstra-Weebers 1998; Kazak 2004; Sahler 2002; Sahler 2005; Stehl 2009), nine diabetes studies (Ambrosino 2008; Ellis 2004; Ellis 2005; Grey 2011; Laffel 2003; Lehmkuhl 2010; Olivares 1997; Wysocki 1999; Wysocki 2006), four asthma studies (Celano 2012; Lask 1979; Ng 2008; Seid 2010), three traumatic brain injury studies (Wade 2006; Wade 2006b; Wade 2011) and one atopic eczema study (Niebel 2000). However, no studies met the inclusion criteria for inflammatory bowel disease or gynaecological disorders.
Similarly, we also categorised studies by the type of psychological therapy delivered. There were 19 studies that delivered CBT (Allen 1998; Ambrosino 2008; Barakat 2010; Barry 1997; Connelly 2006; Duarte 2006; Grey 2011; Hicks 2006; Hoekstra-Weebers 1998; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Laffel 2003; Levy 2010; Niebel 2000; Olivares 1997; Palermo 2009; Robins 2005; Sanders 1994; Stehl 2009), seven studies that delivered FT (Celano 2012; Kazak 2004; Lask 1979; Lehmkuhl 2010; Ng 2008; Wysocki 1999; Wysocki 2006), seven studies that delivered PST (Askins 2009; Sahler 2002; Sahler 2005; Seid 2010; Wade 2006; Wade 2006b; Wade 2011) and two studies that delivered MST (Ellis 2004; Ellis 2005).
We were unable to extract quantitative data from nine of the 35 studies (Barry 1997; Celano 2012; Duarte 2006; Grey 2011; Kazak 2004; Lask 1979; Lehmkuhl 2010; Olivares 1997; Robins 2005). These studies did not present means or standard deviations, or combined data with another study already included in the review (Grey 2011). Therefore 26 studies (36 papers, 2253 participants at end of treatment) presented data that were included in at least one analysis (Allen 1998; Ambrosino 2008; Askins 2009; Barakat 2010; Connelly 2006; Ellis 2004; Ellis 2005; Hicks 2006; Hoekstra-Weebers 1998; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Laffel 2003; Levy 2010; Ng 2008; Niebel 2000; Palermo 2009; Sahler 2002; Sahler 2005; Sanders 1994; Seid 2010; Stehl 2009; Wade 2006; Wade 2006b; Wade 2011; Wysocki 1999; Wysocki 2006).
The proportion of therapy received by parent and child varied between studies. The majority of studies gave equal attention to both parent and child (22 studies). In seven studies only the parent received therapy, four of which studies were delivering treatment to parents whose children had been diagnosed with cancer. Four further studies spent the majority of treatment time with the child. The final two studies did not specify how much therapy the parent and child received. Twenty-eight studies treated patients in-person with the therapist, and seven studies used online programmes to deliver part or all of the therapy to patients. Twenty-five studies carried out therapy with individuals or with individual families, whilst eight studies used a group format. One further study used a combination of group and individual work. One study did not specify how treatment was carried out. A summary of the characteristics of therapy, and a narrative summary of treatment content, are presented in Table 1 and Table 2 respectively.
1. Therapy characteristics of included studies.
| Study | Patient group | Therapy type | Duration oftherapy (child/parent) | Proportion of therapy (child parent) | Mode of delivery, group/individual | Therapy delivered by | Therapist training |
|---|---|---|---|---|---|---|---|
| Allen 1998 | Painful condition (headache) | CBT | 4 hours/not reported | Not reported | In-person, individual | Authors | Not reported |
| Ambrosino 2008 | Diabetes | CBT | 9 hours/9 hours | 50:50 | In-person, group | Mental health professionals | Not reported |
| Askins 2009 | Cancer | PST | 0/8 hours | 0:100 | In-person, individual | Therapists with graduate training in Clinical Psychology | Specialised training in PSST |
| Barakat 2010 | Painful condition (sickle cell disease) | CBT | 6 hours/6 hours | 50:50 | In-person, individual families | Clinical Psychology doctoral students | Not reported |
| Barry 1997 | Painful condition (headache) | CBT | 3 hours/3 hours | 50:50 | In-person, group | Mental health professionals | Not reported |
| Celano 2012 | Asthma | FT | 4 to 6 sessions/4 to 6 sessions | 50:50 | In-person, individual families | Mental and health are professionals | Not reported |
| Connelly 2006 | Painful condition (headache) | CBT | 4 hours/1 hour | 80:20 | Computer, phone calls, individual | CD-ROM and research staff | Not reported |
| Duarte 2006 | Painful condition (recurrent abdominal pain) | CBT | 3 hours, 20 minutes/3 hours, 20 minutes | 50:50 | In-person, not reported | General health professionals | Not reported |
| Ellis 2004 | Diabetes | MST | 48 sessions/48 sessions | 50:50 | In-person, Individual Families | Mental health professionals | Course on MST |
| Ellis 2005 | Diabetes | MST | 46 sessions/46 sessions | 50:50 | In-person + phone calls, individual families | Mental health professionals | Course on MST |
| Grey 2011 | Diabetes | CBT | 0 hours/9 hours | 0:100 | In-person, group | Mental health professionals | Not reported |
| Hicks 2006 | Painful condition (headache + recurrent abdominal pain) | CBT | Not reported/not reported | Not reported | Online + phone calls, individual | Internet + researcher | Not reported |
| Hoekstra-Weebers 1998 | Cancer | CBT | 0/12 hours | 0:100 | In-person, individual | Master's student in Psychology | Not reported |
| Kashikar-Zuck 2005 | Painful condition (fibromyalgia) | CBT | 6 sessions/3 sessions | 66:33 | In-person + phone calls, individual | Pre-doctoral Psychology Intern and post-doctoral Psychology Fellow | Trained by PI |
| Kashikar-Zuck 2012 | Painful conditions (fibromyalgia) | CBT | 6 hours/2 hours 15 minutes | 73:27 | In-person, individual | Post-doctoral Psychology Fellow | 6 to 8 hours CBT training by PI |
| Kazak 2004 | Cancer | FT | 5 hours/5 hours | 50:50 | In-person, group | Nurses, Social Workers, Clinical Psychologists, graduate and postdoctoral Psychology Trainees | 12-hour training |
| Laffel 2003 | Diabetes | CBT | 4 sessions/4 sessions | 50:50 | In-person, individual families | Research assistant | Not reported |
| Lask 1979 | Asthma | FT | 6 hours/6 hours | 50:50 | In-person, individual families | Mental health professional | Not reported |
| Lehmkuhl 2010 | Diabetes | CBT | 9 to 12 hours/9 to 12 hours | 50:50 | Phone calls, | Clinical Psychologists and pre-doctoral Psychology Interns | Not reported |
| Levy 2010 | Painful condition (recurrent abdominal pain) | CBT | 4 hours/4 hours | 50:50 | In-person, individual families | Therapists | Not reported |
| Ng 2008 | Asthma | FT | 22 hours/22 hours | 50:50 | In-person, group | Not reported | Not reported |
| Niebel 2000 | Atopic eczema | CBT | 0/22 hours | 0:100 | In-person + video, both | Mental health professional | Not reported |
| Olivares 1997 | Diabetes | CBT | 0/9 hours, 20 minutes | 0:100 | In-person, group | Not reported | Not reported |
| Palermo 2009 | Painful condition (mixed pain conditions) | CBT | 4 hours/4 hours | 50:50 | Online, individual families | Online + Psychology Fellow | 1 year of experience delivering face-to-face CBT to children with chronic pain |
| Robins 2005 | Painful condition (recurrent abdominal pain) | CBT | 3 hours, 20 minutes/2 hours | 63:37 | In-person, group | Pre-doctoral Psychology Intern and post-doctoral Psychology Fellow | Not reported |
| Sahler 2002; | Cancer | PST | 0/8 hours | 0:100 | In-person, individual | Mental health Drofessional and Psychology graduate student | 3-day workshop |
| Sahler 2005 | Cancer | PST | 0/8 hours | 0:100 | In-person, individual | Not reported | Not reported |
| Sanders 1994 | Painful condition (recurrent abdominal pain) | CBT | 5 hours/5 hours | 50:50 | In-person, individual | Clinical Psychologists | Not reported |
| Seid 2010; | Asthma | PST | 11 hours/11 hours | 50:50 | In-person, individual families | Master's level Health Educator | 2-week training |
| Stehl 2009; | Cancer | CBT | 2 hours, 15 minutes/2 hours, 15 minutes | 50:50 | In-person + CD-ROM, group | Psychology Fellows, Psychology Intern, Master's level Psychologist, doctoral-level Nurse | 18 hours training |
| Wade 2006 | TBI | PST | 10 hours, 20 minutes/10 hours, 20 minutes | 50:50 | In-person, individual families | Clinical Psychology graduate student | 2 months training |
| Wade 2006b | TBI | PST | 14 modules/14 modules | 50:50 | Online + video conferencing, individual | Clinical Psychology graduate student | 2 months training |
| Wade 2011 | TBI | PST | 16 modules/16 modules | 50:50 | Online + video conferencing, individual | Staff Psychologist, Clinical Psychology graduate students | Multi-day training |
| Wysocki 1999 | Diabetes | FT | 10 sessions/10 sessions | 50:50 | In-person, individual families | Clinical Psychologists | 150 hours training |
| Wysocki 2006 | Diabetes | FT | 12 sessions/12 sessions | 50:50 | In-person, individual families | Clinical Psychologist, Social Worker | Trained in BFST-D |
BFST-D: Behavioural Family Systems Therapy for Diabetes; CBT: cognitive behavioural therapy; FT: family therapy; MST: multisystemic therapy; PI: principal investigator; PSST: problem solving skills training; PST: problem solving therapy; TBI: traumatic brain injury
Excluded studies
Sixty-one studies did not meet the inclusion criteria for this study. Thirty-one studies had insufficient psychotherapeutic content, such as instruction, education, parents trained as ‘coaches’ for their children or health prevention interventions (Aleman 1992; Anderson 1999; Braga 2005; Burke 1997; Burke 2001; Chernoff 2002; Chiang 2009; Evans 1999; Field 1998; Garbutt 2010; Giallo 2008; Glang 2007; Hovell 1994; Humphreys 2000; Ireys 1996; Ireys 2001; Johnson 1987; Kaslow 2000; Kazak 1996; Ketchen 2006; Klinnert 2005; Klinnert 2007; Kupfer 2010; Logan 1997; Mendez 1997; Nelson 2011; Perez 1999; Staab 2002; Sullivan-Bolyai 2010; Szczepanski 2010; Walders 2006). Sixteen studies had an aim that was irrelevant to the aim of the review such as fidelity studies, mixed illnesses or the intervention focusing on the parents communication with professionals (Bruzzese 2008; Cakan 2007; Canino 2008; Carey 2008; Ellis 2007; Ellis 2008; Forsander 1995; Gerber 2010; Harris 2001; Hommel 2012; Jay 1990; Rasoli 2008; Scholten 2011; Wade 2010; Walker 1996; Wysocki 1997). Thirteen studies had an insufficient number of participants (n < 10) at post-treatment in or one more arms of treatment (Forsander 2003; Gustafsson 1986; Haus 1976; Hernandez 1998; Kamps 2008; Kazak 2005; Kroner-Herwig 1998; Lasecki 2008; Sanders 1989; Sanders 1996; Satin 1989; Sieberg 2011; Warner 2011) and one paper recruited participants prospectively (Betancourt 2004). These judgements were often difficult to make and led to extended discussion between review authors.
Risk of bias in included studies
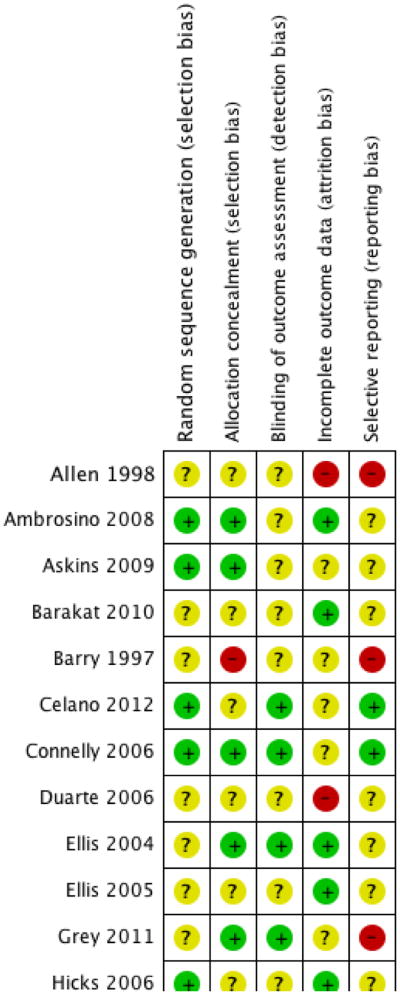
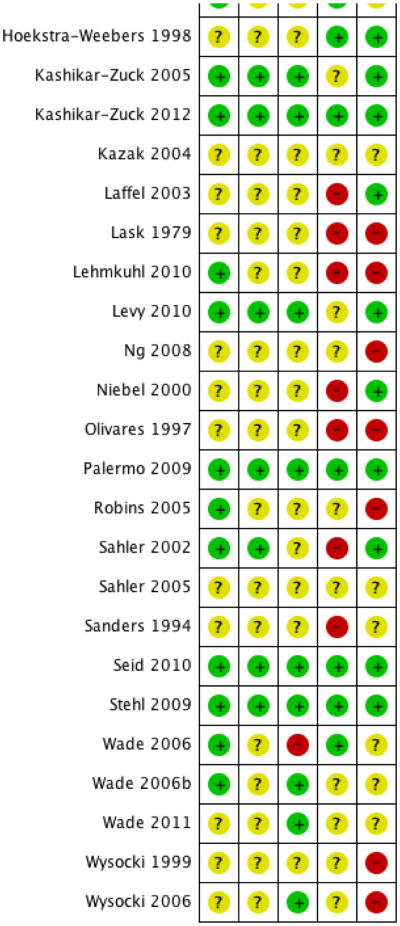
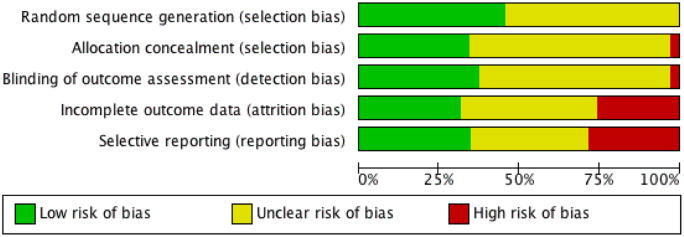
We used five ‘Risk of bias’ categories: random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias) (Figure 1; Figure 2). Sixteen studies described a convincing method of randomisation and we judged them to have a low risk of bias, a further 19 studies did not provide an adequate description and we judged them to be unclear. We rated no studies as high risk of bias for random allocation. There were 12 studies that described a convincing method of allocation and we judged them to have a low risk of allocation bias, a further 22 studies did not provide an adequate description and we judged them to be unclear. We rated ne study as high risk of allocation bias. Thirteen studies reported outcome assessors that were blinded to treatment allocation and we judged them to have a low risk of bias, a further 21 studies did not provide an adequate description and we judged them to be unclear, and we judged one study to have a high risk of outcome bias. Eleven studies reported attrition and found no significant differences between completers and non-completers, so we judged them to have a low risk of bias. Five studies reported attrition but did not report differences between completers and non-completers and so we judged them to be unclear and nine studies did not give an adequate description of attrition and so we judged them to be of high risk. Data could be fully extracted in 12 studies and were fully concordant between aims, measures and results and we judged them to have low risk of selective reporting bias. A further 13 studies were unclear, meaning data could not be extracted or aims, measures and results were only partially concordant. We found 10 studies to have high risk of selective reporting bias because data could not be extracted and they were only partially concordant.
Figure 1.
‘Risk of bias’ summary: review authors' judgements about each risk of bias item for each included study.
Figure 2.
‘Risk of bias’ graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Assessment of quality in included studies
For the 35 studies that met the inclusion criteria, the mean overall quality of the study was 21.49 (standard deviation (SD) = 6.09, range seven to 32). This score is made up of the treatment quality score (M = 6.74, SD 2.06, range one to nine) and the quality of design and methods (M = 14.74, SD = 4.52, range three to 23). The ‘Risk of bias’ figures show the overall quality total, treatment quality and quality of design and methods. We performed a Spearman's correlation to investigate whether the total study quality, treatment quality, design quality or n at the end of treatment were correlated to the year of study. Year of publication was significantly and positively associated with total study quality (rho = 0.581, P < 0.001), design quality of the study (rho = 0.525, P < 0.01) and treatment quality of the study (rho = 0.566, P < 0.01). Treatment quality was significantly associated with design quality (rho = 0.665, P < 0.001). End of treatment n was not significantly associated with year of publication, treatment quality or design quality (rho = 0.169, P > 0.05; rho = 0.066, P > 0.05; rho = 0.136, P > 0.05), respectively.
When assessing all 45 analyses reported at post-treatment and follow-up, 15 showed low heterogeneity (I2 = < 25%), 16 showed moderate heterogeneity (I2 = > 25% to < 50%) and 14 showed high heterogeneity (I2 = > 50%).
Effects of interventions
We analysed data in two categories. In the first, outcomes for each individual condition across all psychological therapies are analysed at post-treatment and follow-up. For the second, outcomes for each psychological therapy across all conditions at post-treatment and follow-up are presented. No analyses could be presented for gynaecological disorders or inflammatory bowel syndrome due to lack of studies meeting the inclusion criteria, and no adverse events were reported in any study reviewed.
Individual conditions across all psychological therapies
Painful conditions at post-treatment
We entered two studies of children with chronic pain, containing a total of 92 participants, into an analysis of parent behaviour. The overall effect of all psychological therapies on parent behaviour was not significant (Z = 0.80, P > 0.05) (Analysis 1.1). We entered six studies of children with chronic pain, containing a total of 429 participants, into an analysis of child behaviour/disability. The overall effect of all psychological therapies on child behaviour/disability was not significant (Z = 1.39, P > 0.05) (Analysis 1.2). We entered four studies of children with chronic pain, containing a total of 356 participants, into an analysis of child mental health. The overall effect of all psychological therapies on child mental health was not significant (Z = 0.14, P > 0.05) (Analysis 1.3). We entered eight studies of children with chronic pain, containing a total of 512 participants, into an analysis of child symptoms. The overall effect of all psychological therapies on child symptoms was significant (Z = 2.23, P < 0.05) with a small effect size of standardised mean difference (SMD) -0.29 (95% confidence interval (CI) -0.55 to -0.03) (Analysis 1.4; Figure 3). There was only one study of children with chronic pain that could be entered into an analysis of family functioning, therefore no conclusion could be drawn.
Figure 3 (Analysis 1.4).
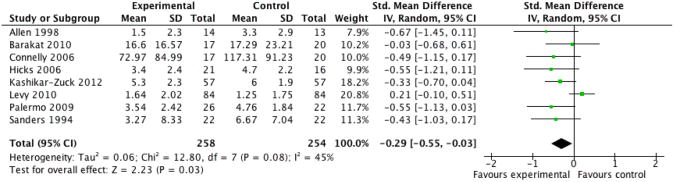
Forest plot of comparison: 1 Painful Conditions Post-treatment, outcome: 1.4 Child Symptoms.
No studies presented extractable data on parent mental health.
Painful conditions at follow-up
There was only one study of children with chronic pain that could be entered into an analysis of parent behaviour at follow-up, therefore no conclusions could be drawn. We entered three studies of children with chronic pain, containing a total of 289 participants, into an analysis of child behaviour/disability at follow-up. The overall effect of all psychological therapies on child behaviour/disability at follow-up was not significant (Z = 0.29, P > 0.05) (Analysis 2.1). We entered two studies of children with chronic pain, containing a total of 255 participants, into an analysis of child mental health at follow-up. The overall effect of all psychological therapies on child mental health at follow-up was not significant (Z = 0.28, P > 0.05) (Analysis 2.2). We entered six studies of children with chronic pain, containing a total of 391 participants, into an analysis of child symptoms at follow-up. The overall effect of all psychological therapies on child symptoms at follow-up was not significant (Z = 1.64, P > 0.05) (Analysis 2.3). There was only one study of children with chronic pain that could be entered into an analysis of family functioning at follow-up, therefore no conclusions could be drawn.
No studies presented extractable data on parent mental health.
Cancer at post-treatment
We entered four studies of children with cancer, containing a total of 629 participants, into an analysis of parent behaviour. The overall effect of all psychological therapies on parent behaviour was not significant (Z = 1.28, P > 0.05) (Analysis 3.1). We entered five studies of children with cancer, containing a total of 706 participants, into an analysis of parent mental health. The overall effect of all psychological therapies on parent mental health was not significant (Z = 1.36, P > 0.05) (Analysis 3.2).
No studies presented extractable data on child behaviour/disability, child mental health, child symptoms or family functioning.
Cancer at follow-up
We entered four studies of children with cancer, containing a total of 597 participants, into an analysis of parent behaviour at follow-up. The overall effect of all psychological therapies on parent behaviour at follow-up was not significant (Z = 0.54, P > 0.05) (Analysis 4.1). We entered four studies of children with cancer, containing a total of 598 participants, into an analysis of parent mental health at follow-up. The overall effect of all psychological therapies on parent mental health at follow-up was not significant (Z = 1.20, P > 0.05) (Analysis 4.2).
No studies presented extractable data on child behaviour/disability, child mental health, child symptoms or family functioning.
Diabetes at post-treatment
There was only one study of children with diabetes that could be entered into analyses of parent mental health, therefore no conclusions could be drawn. We entered two studies of children with diabetes, containing a total of 198 participants, into an analysis of child mental health. The overall effect of all psychological therapies on child mental health was not significant (Z = 0.28, P > 0.05) (Analysis 5.1). There was only one study of children with diabetes that could be entered into analyses of child behaviour/disability, therefore no conclusions could be drawn. We entered six studies of children with diabetes, containing a total of 455 participants, into an analysis of child symptoms. The overall effect of all psychological therapies on child symptoms was not significant (Z = 1.70, P > 0.05) (Analysis 5.2). We entered four studies of children with diabetes, containing a total of 306 participants, into an analysis of family functioning. The overall effect of all psychological therapies on family functioning was not significant (Z = 0.09, P > 0.05) (Analysis 5.3).
No studies presented extractable data on parent behaviour.
Diabetes at follow-up
There was only one study of children with diabetes that could be entered into an analysis of parent mental health at follow-up, therefore no conclusion could be drawn. We entered three studies of children with diabetes, containing a total of 239 participants, into an analysis of child symptoms at follow-up. The overall effect of all psychological therapies on child symptoms at follow-up was not significant (Z = 1.58, P > 0.05) (Analysis 6.1).
No studies presented extractable data on parent behaviour, child behaviour/disability, child mental health or family functioning.
Asthma at post-treatment
There was only one study of children with asthma that could be entered into analyses on parent behaviour, therefore no conclusions could be drawn. We entered two studies of children with asthma, containing a total of 74 participants, into an analysis of parent mental health. The overall effect of all psychological therapies on parent mental health was not significant (Z = 0.86, P > 0.05) (Analysis 7.1). There was only one study of children with diabetes that could be entered into analyses of child behaviour/disability, therefore no conclusions could be drawn. We entered three studies of children with asthma, containing a total of 170 participants, into an analysis of child symptoms. The overall effect of all psychological therapies on child symptoms was not significant (Z = 1.51, P > 0.05) (Analysis 7.2).
No studies presented extractable data on child mental health or family functioning.
Asthma at follow-up
We entered two studies of children with asthma, containing a total of 132 participants, into an analysis of child symptoms at follow-up. The overall effect of all psychological therapies on child symptoms at follow-up was not significant (Z = 0.55, P > 0.05) (Analysis 8.1).
No studies presented extractable data on parent behaviour, parent mental health, child behaviour/disability, child mental health or family functioning.
Traumatic brain injury at post-treatment
We entered two studies of children with traumatic brain injury, containing a total of 72 participants, into an analysis of parent mental health. The overall effect of all psychological therapies on parent mental health was not significant (Z = 1.49, P > 0.05) (Analysis 9.1). We entered two studies of children with traumatic brain injury, containing a total of 72 participants, into an analysis of child behaviour/disability. The overall effect of all psychological therapies on child behaviour/disability was not significant (Z = 0.65, P > 0.05) (Analysis 9.2). We entered two studies of children with traumatic brain injury, containing a total of 67 participants, into an analysis of family functioning. The overall effect of all psychological therapies on family functioning was not significant (Z = 0.33, P > 0.05) (Analysis 9.3).
No studies presented extractable data on parent behaviour, child mental health or child symptoms.
Traumatic brain injury at follow-up
No studies presented extractable data on parent behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms or family functioning.
Skin diseases at post-treatment
There was only one study of children with skin diseases that could be entered into an analysis of parent behaviour, parent mental health, child behaviour and child symptoms at post-treatment, therefore no conclusions could be drawn.
No studies presented extractable data on child mental health or family functioning.
Individual psychological therapies across all conditions
Cognitive behavioural therapy at post-treatment
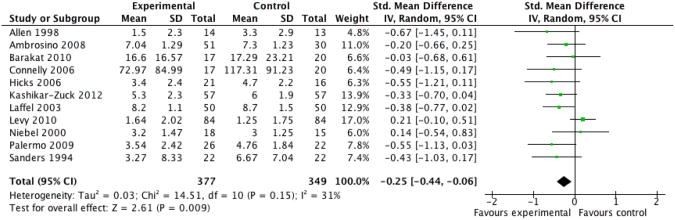
We entered four studies, containing a total of 166 participants, into an analysis of the effects of cognitive behavioural therapy (CBT) across all conditions on parent behaviour. The overall effect of CBT on parent behaviour was not significant (Z = 0.08, P > 0.05) (Analysis 10.1). We entered four studies, containing a total of 224 participants, into an analysis of the effects of CBT on parent mental health. The overall effect of CBT on parent mental health was not significant (Z = 1.05, P > 0.05) (Analysis 10.2). We entered seven studies, containing a total of 459 participants, into an analysis of the effects of CBT on child behaviour/disability. The overall effect of CBT on child behaviour/disability was not significant (Z = 0.84, P > 0.05) (Analysis 10.3). We entered five studies, containing a total of 439 participants, into an analysis of the effects of CBT on child mental health. The overall effect of CBT on child mental health was not significant (Z = 0.21, P > 0.05) (Analysis 10.4). We entered 11 studies, containing a total of 726 participants, into an analysis of the effects of CBT on child symptoms. The overall effect of CBT on child symptoms was significant (Z = 2.61, P < 0.05) with a small effect size of SMD -0.25 (95% CI -0.44 to -0.06) (Analysis 10.5; Figure 4). We entered three studies, containing a total of 211 participants, into an analysis of the effects of CBT on family functioning. The overall effect of CBT on family functioning was not significant (Z = 0.40, P > 0.05) (Analysis 10.6).
Figure 4 (Analysis 10.5).
Forest plot of comparison: 10 Cognitive Behavioural Therapy Post-treatment, outcome: 10.5 Child Symptoms.
Cognitive behavioural therapy at follow-up
We entered two studies, containing a total of 85 participants, into an analysis of the effects of CBT across all conditions on parent behaviour at follow-up. The overall effect of CBT on parent behaviour at follow-up was not significant (Z = 0.56, P > 0.05) (Analysis 11.1). We entered two studies, containing a total of 115 participants, into an analysis of the effects of CBT on parent mental health at follow-up. The overall effect of CBT on parent mental health at follow-up was not significant (Z = 1.26, P > 0.05) (Analysis 11.2). We entered three studies, containing a total of 289 participants, into an analysis of the effects of CBT on child behaviour/disability at follow-up. The overall effect of CBT on child behaviour/disability at follow-up was not significant (Z = 0.29, P > 0.05) (Analysis 11.3). We entered two studies, containing a total of 257 participants, into an analysis of the effects of CBT on child mental health at follow-up. The overall effect of CBT on child mental health at follow-up was not significant (Z = 0.27, P > 0.05) (Analysis 11.4). We entered seven studies, containing a total of 472 participants, into an analysis of the effects of CBT on child symptoms at follow-up. The overall effect of CBT on child symptoms at follow-up was not significant (Z = 1.78, P > 0.05) (Analysis 11.5). We entered two studies, containing a total of 107 participants, into an analysis of the effects of CBT on family functioning at follow-up. The overall effect of CBT on family functioning at follow-up was not significant (Z = 0.61, P > 0.05) (Analysis 11.6).
Family therapy at post-treatment
There was only one study that could be entered into an analysis on the effects of family therapy (FT) across all conditions on parent behaviour, therefore no conclusions could be drawn. We entered two studies, containing a total of 74 participants, into an analysis of the effects of FT on parent mental health. The overall effect of FT on parent mental health was not significant (Z = 0.86, P > 0.05) (Analysis 12.1). We entered two studies, containing a total of 107 participants, into an analysis of the effects of FT on child behaviour/disability. The overall effect of FT on child behaviour/disability was not significant (Z = 1.44, P > 0.05) (Analysis 12.2). We entered four studies, containing a total of 202 participants, into an analysis of the effects of FT on child symptoms. The overall effect of FT on child symptoms was not significant (Z = 0.94, P > 0.05) (Analysis 12.3). We entered two studies, containing a total of 132 participants, into an analysis of the effects of FT on family functioning. The overall effect of FT on functioning was not significant (Z = 0.45, P > 0.05) (Analysis 12.4).
No studies presented extractable data on child mental health.
Family therapy at follow-up
There was only one study that could be entered into an analysis on the effects of FT across all conditions on parent mental health at follow-up, therefore no conclusions could be drawn. We entered two studies, containing a total of 96 participants, into an analysis of the effects of FT on child symptoms at follow-up. The overall effect of FT on child symptoms was not significant (Z = 0.12, P > 0.05) (Analysis 13.1).
No studies presented extractable data on parent behaviour, child behaviour/disability, child mental health or family functioning.
Problem solving therapy at post-treatment
We entered three studies, containing a total of 588 participants, into an analysis of the effects of problem solving therapy (PST) across all conditions on parent behaviour. The overall effect of PST on parent behaviour was significant (Z = 2.64, P < 0.05) with a small effect size of SMD -0.22 (95% CI -0.38 to -0.06) (Analysis 14.1; Figure 5). We entered five studies, containing a total of 660 participants, into an analysis of the effects of PST on parent mental health. The overall effect of PST on parent mental health was significant (Z = 2.14, P < 0.05) with a small effect size of SMD -0.27 (95% CI -0.53 to -0.02) (Analysis 14.2; Figure 6). We entered two studies, containing a total of 72 participants, into an analysis of the effects of PST on child behaviour/disability. The overall effect of PST on child behaviour/disability was not significant (Z = 0.65, P > 0.05) (Analysis 14.3). There was only one study that could be entered into an analysis on the effects of PST on child symptoms, therefore no conclusions could be drawn. We entered two studies, containing a total of 67 participants, into an analysis of the effects of PST on family functioning. The overall effect of PST on family functioning was not significant (Z = 0.33, P > 0.05) (Analysis 14.4).
Figure 5 (Analysis 14.1).
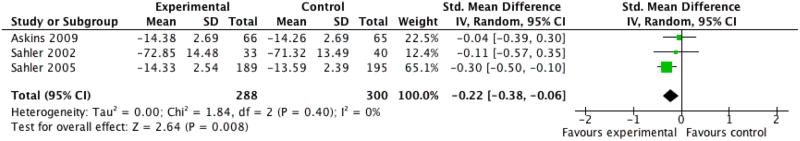
Forest plot of comparison: 14 Problem Solving Therapy Post-treatment, outcome: 14.1 Parent Behaviour.
Figure 6 (Analysis 14.2).
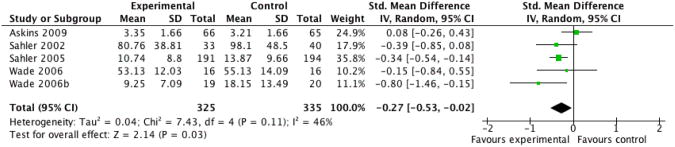
Forest plot of comparison: 14 Problem Solving Therapy Post-treatment, outcome: 14.2 Parent Mental Health.
No studies presented extractable data on child mental health.
Problem solving therapy at follow-up
We entered three studies, containing a total of 556 participants, into an analysis of the effects of PST on parent behaviour at follow-up. The overall effect of all psychological therapies on parent behaviour at follow-up was not significant (Z = 0.77, P > 0.05) (Analysis 15.1). We entered three studies, containing a total of 557 participants, into an analysis of the effects of PST on parent mental health at follow-up. The overall effect of all psychological therapies on parent mental health at follow-up was not significant (Z = 1.02, P > 0.05) (Analysis 15.2). There was only one study that could be entered into an analysis on the effects of PST on child symptoms at follow-up, therefore no conclusions could be drawn.
No studies presented extractable data on child behaviour/disability, child mental health or family functioning.
Multisystemic therapy at post-treatment
There was only one study that could be entered into an analysis on the effects of multisystemic therapy (MST) across all conditions on child mental health, therefore no conclusions could be drawn. We entered two studies, containing a total of 142 participants, into an analysis of the effects of MST on child symptoms. The overall effect of MST on child symptoms was not significant (Z = 1.81, P > 0.05) (Analysis 16.1).
No studies presented extractable data on parent behaviour, parent mental health, child behaviour/disability or family functioning.
Multisystemic therapy at follow-up
There was only one study that could be entered into an analysis on the effects of MST across all conditions on child symptoms at follow-up, therefore no conclusions could be drawn.
No studies presented extractable data on parent behaviour, parent mental health, child behaviour/disability, child mental health or family functioning.
Discussion
There were three objectives of this review. First, results show that only problem solving therapy (PST) interventions that include parents of children with chronic conditions are effective in reducing the distress (improving mental health and behaviour) associated with parenting a child with a chronic illness. Second, cognitive behavioural therapy (CBT) is effective at reducing the primary symptom of a child experiencing chronic illness, in particular chronic pain. Third, we were unable to assess adverse events of interventions for the 14 chronic conditions.
Evidence base
Parents are commonly included in the psychological treatment of children with chronic illness. Many psychological treatments do more than simply include parents, rather they actively focus on them, aiming to help parents improve their own coping, their ability to improve their child's coping, or both. We included 35 randomised controlled trials (RCTs) involving a total of 2723 primary trial participants. Over a third of the studies (n = 12) included in this review investigated conditions in which pain was the primary complaint. A further nine investigated diabetes, six examined cancer patients, four examined children with asthma, three trials investigated children with a traumatic brain injury (TBI) and one trial investigated eczema. There were no RCTs that met the inclusion criteria for gynaecological disorders or inflammatory bowel disease. The majority of studies could be classified within one of four broad treatment approaches: CBT, family therapy (FT), PST and multisystemic therapy (MST). The largest evidence base is of 19 studies in CBT, 18 of which had data that were available for extraction. We are currently able to draw few conclusions about PST, which had seven studies available of which six were included in our analyses. We are unable to draw any conclusions about FT and MST. FT had seven studies available, three of which were included in our analyses, and MST had two studies available, both of which were included in our analyses. Other psychotherapeutic approaches with parents and families have been discussed (e.g. Shapiro 2003) but we could find no studies or evaluations.
Summary of main results
There were a number of analyses which could not be run due to missing data, either because no study measured the selected outcome or because we were unable to extract the data from the study. This reflects the status of this developing field that has not yet met a consensus of agreed scales and questionnaires to measure relevant outcomes.
Combined psychological therapies for each illness condition
First, we analysed data by each medical condition across all treatment classes, giving 72 possible analyses. There were no effects for follow-up data, leaving 36 possible analyses (Table 3). For 22 of the 36 analyses, there were insufficient data to attempt a meta-analysis and so the findings are unknown (i.e. one or no studies available within a given outcome domain). Six analyses should be interpreted with caution because the total number of studies entering the meta-analysis was two. However, we have included these six analyses in this review for transparency; all six had no effect. Of the remaining eight analyses, there was one significant finding. Psychological therapies with a focus on parents were found to significantly improve child symptom reporting for painful conditions. There were no other effects of parent-focused treatment in any other condition for any other outcome that could be analysed.
Individual psychological therapies for combined illness conditions
Second, we analysed data by each treatment class across all medical conditions, giving 48 possible analyses. There were no effects for follow-up data, leaving 24 possible analyses (Table 3). For nine of the 24 analyses, there were insufficient data to attempt a meta-analysis and so the findings are unknown (i.e. one or no studies available within a given outcome domain). Six analyses were inconclusive because the total number of studies entering the meta-analysis was two. However, we have included these six analyses in this review for transparency; all six had no effect. Of the remaining nine analyses, there were three significant findings. CBT had a significant effect on child symptom reporting, and PST had a positive effect on parent behaviour and on parent mental health outcomes.
We did not present data in the form of numbers needed to treat because of the limited number of effects identified; therefore, presenting continuous data in a categorical format would not have been useful. None of the significant effects were strong and these results could be strengthened or overturned with additional trials; therefore we did not calculate a failsafe N. Furthermore, it was not possible to conduct subgroup analyses regarding comparisons of parent-only interventions versus family-based interventions, intervention effects within specific illnesses, and intervention effects across specific types of psychological interventions due to the small number of trials.
Quality of the evidence
The overall study quality was adequate. However, the field continues to be hampered by the common practice of short and limited descriptions of treatment content, the insufficient reporting of results and a reliance on small samples.
Analysis of this evidence presented a number of challenges.
First, multiple measurement tools within a given domain are often employed in individual studies, and there is little agreement as to the preferred measurement tool across studies. In some cases measurement is relatively homogenous (e.g. pain intensity) whereas in others there is greater variety (e.g. family functioning scales in diabetes). These trials do not routinely a priori identify the primary outcome, and there is unusual variety of outcome reporting. For example, one study discussed parent judgement of child outcome when the more valid measure, but non-significant finding, of child report was available (Levy 2010). A posteriori selection of outcome measures is a significant problem in this field. As per our protocol we were uninfluenced by the primary reporting of measures and focused on the best measure available in each domain. This field needs to take account of reporting biases and establish standards to improve the reporting of a priori decisions regarding measurement.
Second, we attempted to review evidence of trials with a dominant parent intervention component. This meant we were inevitably going to combine trials with varying amounts of parenting content. Although we planned subgroup analyses, the data were not of sufficient quantity and quality to enable such an investigation. For some analyses we combined studies that were designed specifically with parents as the sole focus, and in others they were part of a combined treatment. Further, the philosophy of some treatments (e.g. MST) was antithetical to our strategy of determining an individual as a treatment target, however, we included them in this study. It should be noted that significant findings in this review emerged when there was homogeneity of approach, homogeneity of outcome measurement and a larger n.
Third, it should be noted that we had some difficulties in data retrieval due to incomplete and partial data reporting. Data were sometimes reported graphically, and ns, means and/or standard deviations were often missing. We wrote to all 31 first authors an average of two emails. Complete outcome data (i.e. sample size, means, standard deviations) were available from the published paper in 13 trials (Barakat 2010; Connelly 2006; Hoekstra-Weebers 1998; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Laffel 2003; Ng 2008; Palermo 2009; Seid 2010; Stehl 2009; Wade 2006; Wade 2006b; Wade 2011). Seven authors provided data in response to our requests (Ambrosino 2008; Askins 2009; Celano 2012; Levy 2010; Niebel 2000; Sahler 2002; Sahler 2005). Other authors were unable or unwilling to provide additional data or did not respond. The non-production of data is a problem in science (Data's shameful neglect 2009), and has been particularly discussed in psychology (Wicherts 2006; Wicherts 2011). We support the general move toward central registries for all trial data.
Fourth, piecemeal and repeat publication was found in five cases where multiple manuscripts were published from the same trial. In particular, one study (Ellis 2005) was reported six times in five different journals while another trial (Wysocki 1999) was reported five times in four different journals, with variable citation of previous publications in later publications. Such practices are unhelpful, create confusion and increase unnecessary labour (American Psychological Association 2011). Many journals now have policies regarding publication of multiple manuscripts from the same trial, including a detailed description of previous publications from that trial and a statement regarding the unique contribution of the present manuscript (e.g. Drotar 2010).
Finally, replication by other research teams independent to the therapy progenitors is uncommon. For example, Ellis and colleagues are the only group who have evaluated MST in young people with diabetes (Ellis 2004; Ellis 2005). Similarly, PST for children and adolescents with TBI has not been evaluated by any research team outside of Wade and colleagues (Wade 2006; Wade 2006b; Wade 2011). Finally, some therapy approaches have been used exclusively within an illness group. Most notably, CBT was the only intervention evaluated for children with chronic pain.
Potential biases in the review process
This review was limited to the analysis of 14 conditions. Other studies in other conditions may be instructive. As is common practice within the Cochrane Pain, Palliative and Supportive Care Group (PaPaS), we did not searched grey literature. It is always possible that trials of parent-focused interventions were undertaken but unreported in peer-reviewed publications. We consider it unlikely that any such trials exist in the grey literature but this should be acknowledged. Only RCTs were included in this review. However, therapists were not blind to the therapy being delivered. Bias is most likely due to small sample sizes. Unpublished studies are always possible but unlikely given that there appear to be few barriers to publishing small, negative or poor quality studies. Bias in the field may be due largely to the lack of available studies.
Agreements and disagreements with other studies or reviews
Agreements and disagreements with other reviews: combined psychological therapies for each illness condition
Only a handful of reviews have also investigated psychological interventions for parents of children with a chronic illness. Our results are consistent with a previous meta-analysis regarding the effectiveness of CBT in reducing child symptoms in young people with chronic pain (Eccleston 2009a). Our results were somewhat consistent with a meta-analysis of psychological paediatric oncology interventions, which showed no effects on child behaviour or child mental health but positive effects for parent mental health and parent behaviour (Pai 2006). Our results are not consistent with previous reviews of psychological interventions that included parents of children with diabetes, which reported positive effects on child symptoms and family functioning (Armour 2005; Grey 2000; Harris 2010; McBroom 2009). Previous reviews of psychological interventions that included parents of children with asthma or skin diseases were inconclusive due to lack of trials that met inclusion criteria (Ersser 2007; Yorke 2009). Notably, disagreements between the present meta-analysis and previous reviews may be attributed to differences in inclusion criteria, selection of outcome measures and/or selection of comparator group.
Agreements and disagreements with other reviews: individual psychological therapies for combined illness conditions
One prior review indicated that psychological interventions which included coping skills training for adolescents and young adults with chronic illness (cancer, diabetes, juvenile idiopathic arthritis, sickle cell disease and asthma) and their parents/families had mixed effects on child psychosocial functioning and family functioning (Sansom-Daly 2011). We were unable to find any previous reviews that compared results from individual psychological therapies across chronic illness conditions for parent outcomes or child symptoms. Therefore, we cannot draw conclusions regarding consistency of our results by treatment type for parent outcomes or child symptoms with previous reviews.
Authors' conclusions
Implications for practice
More work is needed to develop and provide psychological interventions that directly target parents of children with chronic illness. Few interventions included in this review provided intensive treatment to parents that specifically targeted parent outcomes. We suggest that interventions which target specific strategies aimed at parent mental health and behaviour (e.g. problem solving skills training) are more likely to achieve those effects than interventions which include parents but do not purposefully target strategies in these outcome domains. Targeted relapse prevention strategies have not been attempted, and may be necessary to maintain treatment effects in the long term.
Implications for research
There are relatively few studies of psychological interventions that target parents of children with a chronic illness. For example, there were no studies of children with gynaecological disorders or irritable bowel diseases that met criteria for inclusion in this review. There was also only one study of children with skin diseases that met the inclusion criteria, meaning we were not able to conduct any meta-analyses for this condition. Furthermore, studies in this area need to be conducted to a higher level of quality so that gaps in the evidence base can be filled and the effectiveness of psychological interventions for parents of children with chronic illness can be better understood. The next generation of trials should improve by taking account of the limitations identified in this review, including:
larger sample sizes;
following CONSORT guidelines (Schulz 2010);
the clearer identification of primary outcomes;
designing treatment content to specifically target change in the primary outcomes;
more consistency of measurement and greater consensus within the field around appropriate measure use within and across illness groups;
lodging treatment manuals and data in a shared database to facilitate replication of intervention trials and re-analysis of results.
This review has also highlighted several future directions for research that examines interventions targeting parents of children with chronic illness. Problem solving therapy (PST) looks particularly promising for improving parent mental health and parent behaviour. Research is needed to evaluate this intervention in populations other than cancer and traumatic brain injury (TBI), such as chronic pain. Replication studies are also needed for interventions that have been evaluated by only one research team, such as multisystemic therapy for families of children with diabetes and PST for families of children with TBI. We recognise that this goal may be difficult to achieve given the high degree of competition for funding and lack of interest among funding agencies for replication studies. Research is also needed to evaluate interaction effects such as the impact of changes in parent outcomes on child outcomes, as well as evaluation of specific treatment characteristics such as the intensity of intervention delivered to children versus parents. We also do not know anything about the effects of the interventions included in this review on fathers or siblings, which is a common critique of the field of paediatric psychology.
Our final recommendation for future research in this area is in regards to duplication and piecemeal publication. Editorial policies are needed to inform authors regarding reporting standards for multiple publications from the same trial. Editors play a crucial role in creating and enforcing these policies, and need to take a pro-active approach to identifying such papers during the review process (Committee on Publication Ethics 2011; World Association of Medical Editors 2012).
Acknowledgments
We would like to thank Dr Zeinab Mousavi, Dr Gerrit Hirschfeld, Dr Anna Huguet, Dr Rikard Wicksell, Dr Jordi Miro and Megan Walker for their time in helping to translate papers and extract data.
We also thank all authors who responded to emails requesting further studies or data.
We would also like to thank Jane Hayes for all her help designing and carrying out the search strategies.
Sources of support
Internal sources: • University of Bath, UK
External sources: • National Institutes of Health/National Institutes for Child Health and Human Development, USA Grant number: R21HD065180 (PI: Palermo)
Published notes
Characteristics of studies
Characteristics of included studies
| Allen 1998 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment, 3 months and 1 year. | |
| Participants | End of treatment n = 27, 3-month follow-up = 27, 12-month follow-up = 21 Start of treatment n = 27 Sex of children: 11 M, 16 F Sex of parents: not reported Mean age of children = 12.2 Mean age of parents = not reported Source = referred by paediatricians and neurologists in the community and recruited by newspaper ad Diagnosis of child = migraine headache Mean years of illness = 4.4 years |
|
| Interventions | “Thermal Biofeedback plus Parent Pain Behaviour Management” (CBT) “Thermal Biofeedback” Mode of delivery: individual, face to face Intervention delivered by: authors Training: not reported Duration of intervention (child, hrs) = 6 × 40 minutes = 4 hours Duration of intervention (parent, hours) = not reported |
|
| Outcomes | * Extracted measures Child measures Pain diary* Coping Assistance Questionnaire Child Perception Abbreviated Acceptability Rating Profile Parent measures Parent Perception of Pain Interference Questionnaire* Coping Assistance Questionnaire for Parents* Abbreviated Acceptability Rating Profile |
|
| Yates Quality Scale | Study quality (out of 35): 14 (low quality) Treatment quality (out of 9): 5 (high quality) Design quality (out of 26): 9 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized, controlled group-outcome design, subjects were assigned to either thermal biofeedback intervention.…., or the same biofeedback ntervention plus pain behavior management guidelines”. Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Ambrosino 2008 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 1 month (end of treatment), 3 months, 6 months and 12 months post intervention. | |
| Participants | End of treatment n = 81 children, 3-month follow-up = 79 children, 6-month follow-up = 72, 12-month follow-up = 72 Start of treatment n = 87 parents and children received intervention at start Sex of children: 34 M, 53 F Sex of parents: 5 M, 82 F Mean age of children = 9.91 (+/-1.44) Mean age of parents = 40.01 (+/- 5.40) Source = Yale Pediatric Diabetes Program Diagnosis of child = type 1 diabetes Mean years of illness = 3.71 +/- 2.91 years |
|
| Interventions | “Coping Skills Training (CST)” (CBT) “Group Education (GE)” Mode of delivery: groups, face to face, parents met separately Intervention delivered by: mental health professionals Training: not reported Duration of intervention (child, hours) = 6 × 1.5 = 9 hours Duration of intervention (parent, hours) = 6 × 1.5 = 9 hours |
|
| Outcomes | * Extracted measures Child measures Metabolic control* Child Depression Inventory (CDI)* Disease-related variables Issues in Coping with IDDM - Child scale Self-Efficacy for Diabetes Scale Diabetes Quality of Life Scale for Youth (DQOL) Diabetes Family Behavior Scale (DFBS) Parent measures Center for Epidemiologic Depression Scale (CES-D)* Family Adaptability and Cohesion Scale (FACES II)* Issues in Coping with IDDM - Parent scale Diabetes Responsibility and Conflict Scale |
|
| Yates Quality Scale | Study quality (out of 35): 29 (high quality) Treatment quality (out of 9): 7 (high quality) Design quality (out of 26): 22 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were randomised initially by a sealed envelope technique and later by computer to either the coping skills therapy of group eduction.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Participants were randomised initially by a sealed envelope technique and later by computer to either the coping skills therapy of group eduction.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “All follow-up data were collected by trained research assistants.” Comment: blinding unclear, probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Askins 2009 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and at 3 months. | |
| Participants | End of treatment n = 131 mothers, 3-month follow-up = 123 mothers Start of treatment n = 197 mothers Sex of children: 103 M, 94 F Sex of parents: 0M, 197 F Mean age of child = 8.1 Mean age of parents = 36.3 Source = 4 paediatric cancer centres in USA Diagnosis of child = cancer Mean years of illness = average 6 weeks since diagnosis, range 2 to 16 weeks from diagnosis |
|
| Interventions | “Problem-Solving Skills Training” (PST) “Problem-Solving Skills Training + Personal Digital Assistant” Mode of delivery: individual, face to face Intervention delivered by: therapists with graduate training in Clinical Psychology Training: special training in PSST Duration of intervention (child, hours) = 0 Duration of intervention (parent, hours) = 8 × 1 =8 hours |
|
| Outcomes | * Extracted measures Parent measures Social Problem-Solving Inventory-Revised (SPSI-R)* Beck Depression Inventory-ll (BDI-II)* Profile of Mood States (POMS) Impact of Event Scale-Revised (IES-R) |
|
| Yates Quality Scale | Study quality (out of 35): 27 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 18 (high quality) |
|
| Notes | The comparison looks like a non inferiority trial but it was not designed in this way so we have included it despite the lack of a control group | |
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computerized randomisation to one of the three treatment arms was performed at the data management centre.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Computerized randomisation to one of the three treatment arms was performed at the data management centre.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Barakat 2010 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 12 months. | |
| Participants | End of treatment n = 37, 12-month follow-up = 34 Start of treatment n = 42 received session 1 Sex of children: 15 M, 12 F Sex of parents: not reported Mean age of child = 14.17 (1.75) Mean age of parents = not reported Source =“Comprehensive sickle cell centre” Diagnosis of child = sickle cell disease Mean years of illness = lifetime |
|
| Interventions | “Pain Management Intervention” (CBT) “Disease Education Intervention” Mode of delivery: individual families, face to face Intervention delivered by: Clinical Psychology doctoral students Training: not reported Duration of intervention (child, hours) = 4 × 90 minutes = 6 hours Duration of intervention (parent, hours) = 4 × 90 minutes = 6 hours |
|
| Outcomes | * Extracted measures Child measures Pain diary (% days with pain and % interference with activities)* Coping Strategies Questionnaire Family Cohesion Scale* Health-related Hindrance Inventory Health Service Use per Medical Chart Review School Attendance Records |
|
| Yates Quality Scale | Study quality (out of 35): 27 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 18 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A 2-group, randomised treatment design was used.” Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported. Aims, measures and results only partially concordant. Comment: probably some reporting bias. |
| Barry 1997 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 3 months. | |
| Participants | End of treatment n = 29, 3-month follow-up = 29 Start of treatment n = 36 Sex of children: 10 M, 19 F Sex of parents: not reported Mean age of child = 9.4 Mean age of parents = not reported Source = ads in elementary schools and community health centres, referrals from paediatricians and family physicians Diagnosis of child = headache Mean years of illness = 2 headaches/month |
|
| Interventions | “Cognitive Behavioural Therapy” (CBT) “Wait-list Control” Mode of delivery: group, face to face Intervention delivered by: mental health professionals Training: not reported Duration of intervention (child, hours) = 2 × 90 minutes = 3 hours Duration of intervention (parent, hours) = 2 × 90 minutes = 3 hours |
|
| Outcomes | * Extracted measures Child measures Pain diary* |
|
| Yates Quality Scale | Study quality (out of 35): 16 (low quality) Treatment quality (out of 9): 5 (high quality) Design quality (out of 26): 11 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Each parent-child pair was initially matched with another pair based on the child's age, sex and headache pain as indicated by the parents' ratings of average duration, frequency, and intensity of headaches. Subsequently, one of each of the matched parent-child pairs was randomly assigned to either the treatment condition or the waiting list control condition.” Comment: method not described. |
| Allocation concealment (selection bias) | High risk | “Each parent-child pair was initially matched with another pair based on the child's age, sex and headache pain as indicated by the parents' ratings of average duration, frequency, and intensity of headaches.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | High risk | Data were not fully reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Celano 2012 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 6 months. | |
| Participants | End of treatment n = 40, 6-month follow-up = 37 Start of treatment n = 43 Sex of children: 26 M, 15 F Sex of parents: 85% female Mean age of child = 10.5(1.6) Mean age of parents = not reported Source = urban children's hospital and residential camp for children with asthma Diagnosis of child = asthma Mean years of illness = more than 1 year |
|
| Interventions | “Home based family intervention” “Enhanced treatment as usual” Mode of delivery: individual families, face to face Intervention delivered by: trained asthma counsellors, post-doctoral psychology fellow and respiratory therapist Training: not reported Duration of intervention (child, hours) = 4 to 6 sessions, average 78 minutes per session Duration of intervention (parent, hours) = 4 to 6 sessions, average 78 minutes per session |
|
| Outcomes | * Extracted measures Child measures Family Asthma Management System Scale Metered Dose Inhaler Checklist Cotinine/creatinine ratio Number of school days missed Asthma symptom days* Urgent health care visits Medical records reviewed Parent measures Family Asthma Management System Scale Parenting Stress Index (PSI-SF) Brief Symptoms Inventory (for parent distress)* |
|
| Yates Quality Scale | Study quality (out of 35): 19 (high quality) Treatment quality (out of 9): 6 (high quality) Design quality (out of 26): 13 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation….by blocked randomisation within age group (8 to 10 vs. 11 to 13).” Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Trained assistants blind to group assignment.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Connelly 2006 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 2 months. | |
| Participants | End of treatment n = 31, 2-month follow-up = 31 Start of treatment n = 37 Sex of children: 19 M, 18 F Sex of parents: not reported Mean age of child = 9.2 (1.7) Mean age of parents = not reported Source = outpatient neurology clinic at a large children's hospital in Midwestern USA Diagnosis of child = headache Mean years of illness = 2 years 3 months (2 years 2 months) |
|
| Interventions | “Headstrong CD ROM” (CBT) “Wait-list Control” Mode of delivery: computer and phone calls Intervention delivered by: CD ROM Training: not reported Duration of intervention (child, hours) = 4 × 1 hr = 4 hours Duration of intervention (parent, hours) = 1 × 1 hr = 1 hours |
|
| Outcomes | * Extracted measures Child measures Headache diary* Pediatric Migraine Disability Assessment* Parent measures Headache diary Pediatric Migraine Disability Assessment |
|
| Yates Quality Scale | Study quality (out of 35): 25 (high quality) Treatment quality (out of 9): 7 (high quality) Design quality (out of 26): 18 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomly assigned to one of two groups by a research assistant using a uniform random numbers table.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Randomly assigned to one of two groups by a research assistant using a uniform random numbers table.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Study neurologists remained blind to randomisation condition throughout the study. Chance of unblinding were limited because follow-up appointments with the study neurologist were scheduled for 2 months following the initial assessment.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Duarte 2006 | ||
| Methods | RCT. 2 arms. Assessed at first, second, third and fourth session (sessions were monthly). | |
| Participants | End of treatment n = 32 children Start of treatment n = 32 children Sex of children: 10 M,22 F Sex of parents: not reported Mean age of children = 9.15 (2.1) Mean age of parents = not reported Source = Pediatric Gastroenterology Reference Service Diagnosis of child = recurrent abdominal pain Mean years of illness = 25 +/- 17.5 months |
|
| Interventions | “Cognitive-behavioural family intervention” (CBT) “Control group” Mode of delivery: face to face (group/individual not reported) Intervention delivered by: general health professionals Training: not reported Duration of intervention (child, hours) = 4 × 50 minutes = 3 hours, 20 minutes Duration of intervention (parent, hours) = 4 × 50 minutes = 3 hours, 20 minutes |
|
| Outcomes | * Extracted measures Child measures Pain diary* Visual analogue scale Pressure Pain Threshold |
|
| Yates Quality Scale | Study quality (out of 35): 7 (low quality) Treatment quality (out of 9): 4 (low quality) Design quality (out of 26): 3 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly allocated to 2 groups.” Comment: probably done but unclear method |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | Unclear risk | Data were incompletely reported. Aims, measures and results were fully concordant. Comment: probably some reporting bias. |
| Ellis 2004 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 6 months after study entry (end of treatment). | |
| Participants | End of treatment = 25 Start of treatment n = 31 Sex of children: 14 M, 11 F Sex of parents: all female Mean age of children = 13.6 (1.6) Mean age of parents = 0 M, 31 F Source = endocrinology clinic within a tertiary care children's hospital Diagnosis = type 1 diabetes Mean years of illness = at least 1 year |
|
| Interventions | “Multisystemic Therapy” (MST) “Standard Care Control” Mode of delivery: individual families, face to face and phone contact Intervention delivered by: mental health professionals Training:Completed 1 week MST training Duration of intervention (child) = mean 6.5 months, 46 sessions Duration of intervention (parent) = mean 6.5 months, 46 sessions |
|
| Outcomes | * Extracted measures Child measures Metabolic control* Twenty-Four Hour Recall Interview Frequency of blood glucose testing from blood glucose meter The Diabetes Management Scale (DMS) Health Service Use per Medical Chart Review Parent measures Satisfaction with treatment The Diabetes Management Scale (DMS) |
|
| Yates Quality Scale | Study quality (out of 35): 26 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 17 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomisation to treatment or control group was completed immediately after baseline data collection by the project statistician.” Comment: no description provided. |
| Allocation concealment (selection bias) | Low risk | “Randomisation to treatment or control group was completed immediately after baseline data collection by the project statistician.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “All data was collected by a trained research assistant who was blind to the adolescent's treatment status.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Ellis 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 7 months after study entry (end of treatment), 12 months after study entry (6-month follow-up). | |
| Participants | End of treatment n = 110, 6-month follow-up = 85 Start of treatment n = 127 children and their families Sex of children: 62 M, 65 F Sex of parents: not reported Mean age of children = 13.25 (+/-1.95) Mean age of parents = 38.8 (+/-6.8) Source = endocrinology clinic within a tertiary care children's hospital Diagnosis = type 1 diabetes Mean years of illness = 5.25 (+/- 4.35) years |
|
| Interventions | “Multisystemic Therapy” (MST) “Standard Care Control” Mode of delivery: individual families, face to face and phone contact Intervention delivered by: mental health professional Training: 1-week training in MST and diabetes education Duration of intervention (child) = mean 5.7 months, 48 sessions Duration of intervention (parent) = mean 5.7 months, 48 sessions |
|
| Outcomes | * Extracted measures Child measures HbA1c Levels* Diabetes Stress Questionnaire* Family Relationship Index (FRI) of the Family Environment Scale (FES)* Frequency of Blood Glucose Testing from blood glucose meter Twenty-Four Hour Recall Interview Health Service Use per Medical Chart Review (hospitalisations, emergency department visits) Diabetes Family Behavior Checklist (DFBC) Diabetes Family Responsibility Questionnaire Parental overestimation of adolescent responsibility score Parent measures Family Relationship Index (FRI) of the Family Environment Scale (FES)* Diabetes Family Behavior Checklist (DFBC) Diabetes Family Responsibility Questionnaire |
|
| Yates Quality Scale | Study quality (out of 35): 23 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 15 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Random assignment to treatment group was completed after baseline data collection.” Comment: no method described. |
| Allocation concealment (selection bias) | Unclear risk | “To ensure equivalence across treatment conditions, random assignment was stratified according to HbA1c level at the baseline visit.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were incompletely reported. Aims, measures and results fully concordant. Comment: probably some reporting bias. |
| Grey 2011 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 3 months, 6 months and 12 months post-treatment. Data came from 2 separate randomised clinical trials of coping skills training interventions: one trial included parents and their 8 to 12-year old children, and the other trial included parents of children under 8 years of age. | |
| Participants | End of treatment n = 129, 3 months = 121, 6 months = 120, 12 months = 112 Start of treatment n = 129 Sex of children: 53 M, 74 F Sex of parents: not reported Mean age of children = 8.0 (2.8) Mean age of parents = not reported Source = paediatric diabetes clinic at a university-based medical centre Diagnosis = type 1 diabetes Mean years of illness = at least 6 months |
|
| Interventions | “Coping skills training group” (CBT) “Group diabetes education” Mode of delivery: group, face to face Intervention delivered by: mental health professional Training: not reported Duration of intervention (child) = not reported. Duration of intervention (parent) = 1.5 hours × 6 sessions = 9 hours (treatment), 1.5 hours × 4 sessions = 6 hours (control) |
|
| Outcomes | * Extracted measures Child measures HbA1c Levels Parent measures Issues in Coping with IDDM-Parent Scale The Center for Epidemiologic Studies-Depression Scale The Diabetes Responsibility and Conflict Scale The Parents Diabetes Quality of Life Questionnaire |
|
| Yates Quality Scale | Study quality (out of 35): 24 (high quality) Treatment quality (out of 9): 7 (high quality) Design quality (out of 26): 17 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Data from two separate randomised trials…Participants were randomised using a sealed envelope technique” Comment: combined 2 studies together to produce results. |
| Allocation concealment (selection bias) | Low risk | “Participants were randomised using a sealed envelope technique.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Data were collected….by trained research assistants who were blinded to group assignment.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Aims, measures and results were fully concordant, but data presented were combined. Comment: probably some reporting bias. |
| Hicks 2006 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 1-month follow-up and 3-month follow-up. | |
| Participants | End of treatment n = 37, 1-month follow-up = 37, 3-month follow-up = 32 Start of treatment n = 47 Sex of children: 17 M, 30 F Sex of parents: not reported Mean age of children = 11.7 (2.1) Mean age of parents = not reported Source = media, posters in physicians offices and advertisements in school newsletters Diagnosis = recurrent head or abdominal pain Mean years of illness = 3 years |
|
| Interventions | “Online cognitive-behavioral treatment programme” (CBT) “Wait list Control” Mode of delivery: individual, online web programme, email and phone contact Intervention delivered by: Internet and researcher Training: not reported Duration of intervention (child) = mean 3 hours on the phone, duration to complete online programme not described Duration of intervention (parent) = not described |
|
| Outcomes | * Extracted measures Child measures Pain diary* Pediatric Quality of Life Inventory Treatment expectation Treatment satisfaction Parent measures Pediatric Quality of Life Inventory Treatment expectation Treatment satisfaction |
|
| Yates Quality Scale | Study quality (out of 35): 24 (high quality) Treatment quality (out of 9): 6 (high quality) Design quality (out of 26): 18 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 47 participants were stratified by age and pain severity and randomly assigned by blocks to either the treatment condition or the standard medical care wait-list condition.” Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | “The 47 participants were stratified by age and pain severity and randomly assigned by blocks to either the treatment condition or the standard medical care wait-list condition.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were incompletely reported. Aims, measures and results fully concordant. Comment: probably some reporting bias. |
| Hoekstra-Weebers 1998 | ||
| Methods | RCT. 2 arms. Pre-treatment (at diagnosis), post-treatment, 6-month follow-up | |
| Participants | End of treatment and 6-month follow-up n = 81 parents, 41 children Start of treatment n = 120 parents, 61 children Sex of parents: 40 M, 41 F Sex of children: 23 M, 18 F Mean age of parents = 36.6 (5.4) Mean age of children = 6.4 (4.7) Source = paediatric oncology clinic Diagnosis = cancer Mean years of illness = 2 to 21 days post diagnosis |
|
| Interventions | “Psychoeducational and Cognitive-Behavioral Intervention” (CBT) “Standard Care Control” Mode of delivery: individual, face to face Intervention delivered by: Master's student in Psychology Training: not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 8 × 90 minutes = 12 hours |
|
| Outcomes | * Extracted measures Parent measures Symptom Check List (SCL)* State-Trait Anxiety Inventory-State* Goldberg General Health Questionnaire (GHQ) Social Support List-Discrepancies (SSL-D) Intensity of emotions questionnaire designed by the authors |
|
| Yates Quality Scale | Study quality (out of 35): 20 (high quality) Treatment quality (out of 9): 6 (high quality) Design quality (out of 26): 14 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Parents were randomly assigned…. parents drew one of two envelopes in which a letter indicated in which group they were placed.” Comment: method unclear. |
| Allocation concealment (selection bias) | Unclear risk | “Parents were randomly assigned…. parents drew one of two envelopes in which a letter indicated in which group they were placed.” Comment: probably done but unsure whether envelopes were sealed or numbered. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results fully concordant. Comment: probably no reporting bias. |
| Kashikar-Zuck 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and post-treatment. | |
| Participants | End of treatment n = 27 Start of treatment n = 30 Sex of children: 0 M, 30 F Sex of parents: 3 M, 27 F Mean age of children = 15.83(1.26) Mean age of parents = not reported Source = paediatric rheumatology clinic, Midwestern USA Diagnosis = fibromyalgia syndrome Mean years of illness = over 2 years |
|
| Interventions | “Cognitive Skills Training” (CBT) “Self Monitoring” Mode of delivery: individual, face to face plus phone contact Intervention delivered by: doctoral level paediatric psychology intern or psychology fellow Training: trained by principal investigator Duration of intervention (child) = 6 sessions, hours not reported Duration of intervention (parent) = 3 sessions, hours not reported |
|
| Outcomes | * Extracted measures Child measures Children's Depression Inventory* (CDI) Functional Disability Inventory* (FDI) Visual analogue scale (VAS) Pain Coping Questionnaire (PCQ) Tender point examination |
|
| Yates Quality Scale | Study quality (out of 35): 22 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 14 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer generated pseudo-random number list was used. A simple randomisation technique was used with a 1:1 allocation ratio for 30 subjects as a single block.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “A computer generated pseudo-random number list was used. A simple randomisation technique was used with a 1:1 allocation ratio for 30 subjects as a single block.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “A research assistant who was blind to the study objectives and to the subjects' treatment assignment administered the self-report measures. The rheumatologist or occupational therapist who conducted the tender point assessments was blind to the subjects' treatment assignment.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Kashikar-Zuck 2012 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment, 6-month follow-up | |
| Participants | End of treatment n = 106, follow-up n = 100 Start of treatment n = 114 Sex of children: 9 M, 105 F Sex of parents: not reported Mean age of children = 15.0 (1.8) Mean age of parents = not reported Source = 4 paediatric rheumatology centres, Midwestern USA Diagnosis = fibromyalgia syndrome Mean years of illness = 2 years, 10 months (2 years, 6 months) |
|
| Interventions | “Cognitive behavioural therapy” (CBT) “Fibromyalgia education” Mode of delivery: individual, face to face Intervention delivered by: therapists with postdoctoral training in paediatric psychology Training: 6 to 8-hour training by principal investigator Duration of intervention (child) = 6 hours Duration of intervention (parent) = 2 hours, 15 minutes |
|
| Outcomes | * Extracted measures Child measures Child Depression Inventory* (CDI) Functional Disability Inventory* (FDI) Visual analogue scale* (VAS) Pediatric Quality of Life Inventory (PedsQL) |
|
| Yates Quality Scale | Study quality (out of 35): 33 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 24 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Eligible patients were randomly assigned to 1 of the 2 treatment arms based upon a computer-generated randomisation list. Randomisation was stratified by site.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “When a patient was enrolled, the study therapist contacted the biostatistician to obtain the subject identification number and treatment allocation.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “The principle investigator, study physicians, study coordinator, and assessment staff were all blinded to the patients' treatment condition throughout the trial. Patients were asked not to divulge what treatment they were receiving to the study physician.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Kazak 2004 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and 3 to 5 months post-treatment | |
| Participants | End of treatment n = 116 children Start of treatment n = 150 children Sex of children: 73 M, 77 F Sex of parents: 106 M, 146 F Mean age of children = 14.61 (2.4) Mean age of parents = not reported Source = oncology tumour registry at the Children's Hospital of Philadelphia Diagnosis = childhood cancer survivor Mean years of illness = 5.30 (2.92) years post-treatment |
|
| Interventions | “Surviving Cancer Competently Intervention Program SCCIP” (CBT) “Wait-list Control” Mode of delivery: group, face to face Intervention delivered by: nurses, social workers, psychologists, graduate and postdoctoral psychology trainees Training: 12 hours Duration of intervention (child) = 5 hours direct, 2 hours informal Duration of intervention (parent) = 5 hours direct, 2 hours informal |
|
| Outcomes | * Extracted measures Child measures Post-Traumatic Stress Disorder Reaction Index (PTSD-RI)* Impact of Events Scale-Revised (IES-R) Revised Children's Manifest Anxiety Scale (RCMAS) Parent measures Post-Traumatic Stress Disorder Reaction Index (PTSD-RI)* Impact of Events Scale-Revised (IES-R) State-Trait Anxiety Inventory (STAI) |
|
| Yates Quality Scale | Study quality (out of 35): 24 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 15 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Families were randomised to the treatment or wail-list control condition.” Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were incompletely reported. Aims, measures and results were fully concordant. Comment: probably some reporting bias. |
| Laffel 2003 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment and 1 year. | |
| Participants | End of treatment n = 100 children Start of treatment n = 105 Sex of children: 53 M.47 F Sex of parents: not reported Mean age of children = 12.1 (2.3) Mean age of parents = not reported Source = Joslin Diabetes Center Pediatric and Adolescent Unit Diagnosis = type 1 diabetes Mean years of illness = 2.7 years +/-1.6 years |
|
| Interventions | “Teamwork Intervention” (FT) “Standard Care” Mode of delivery: individual families, face to face Intervention delivered by: research assistant Training: not reported Duration of intervention (child) = 4 sessions over 1 year (hours not reported) Duration of intervention (parent) = 4 sessions over 1 year (hours not reported) |
|
| Outcomes | * Extracted measures Child measures Glycemic Control (A1c)* Diabetes Family Conflict Scale* Clinician Report of Adherence to Diabetes Management Tasks Diabetes Family Responsibility Questionnaire Joint structured interview to assess parental involvement in diabetes management tasks Pediatric Quality of Life Inventory (PedsQL) Parent measures Diabetes Family Conflict Scale* Diabetes Family Responsibility Questionnaire Joint structured interview to assess parental involvement in diabetes management tasks |
|
| Yates Quality Scale | Study quality (out of 35): 12 (low quality) Treatment quality (out of 9): 1 (low quality) Design quality (out of 26): 11 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly assigned according to age and duration.” Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition not adequately described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Lask 1979 | ||
| Methods | RCT. Assessed at pre-treatment, post-treatment and 1 year follow-up. | |
| Participants | End of treatment n = 37 children, 33 families Start of treatment n = 37 children, 33 families Sex of children: not reported Sex of parents: not reported Mean age of children = range 4 to 14 years, mean not reported Mean age of parents = not reported Source = not reported Diagnosis = asthma Mean years of illness = not reported |
|
| Interventions | “Family psychotherapy” (FT) Standard care control group Mode of delivery: individual families, face to face Intervention delivered by: mental health professional Training: not reported Duration of intervention (child) = 6 ×1 hr family psychotherapy = 6 hours Duration of intervention (parent) = 6 × 1 hr family psychotherapy = 6 hours |
|
| Outcomes | * Extracted measures Child measures Diary cards* Peak expiratory flow rate (PEFR) Forced expiratory volume (FEV) Thoracic gas volume (TGV) |
|
| Yates Quality Scale | Study quality (out of 35): 10 (low quality) Treatment quality (out of 9): 2 (low quality) Design quality (out of 26): 8 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Families were then randomly allocated to the experimental (group A) or control group (group B).” Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Lehmkuhl 2010 | ||
| Methods | RCT. 2 arms. Assessed pre and post-treatment. | |
| Participants | End of treatment n = 22 Start of treatment n = 32 Sex of children: 9M,23 F Sex of parents: 2 M, 27 F, 3 unknown Mean age of children = 13.66 (2.43) Mean age of parents = 41.53 (8.14) Source = university-affiliated paediatric endocrinology clinic Diagnosis = type 1 diabetes Mean years of illness = over 6 months |
|
| Interventions | “Telehealth Behavioral Therapy” (CBT) “Wait list control” Mode of delivery: individual, phone calls Intervention delivered by: psychologists and Clinical Psychology interns Training: not reported Duration of intervention (child) = 36 phone calls, 9 to 12 hours Duration of intervention (parent) = 36 phone calls, 9 to 12 hours |
|
| Outcomes | * Extracted measures Child measures A1c Now* Diabetes Family Behavior Scale, Abbreviated (DFBS)* Diabetes Self-Management Profile (DSMP) Diabetes Family Behavior checklist (DFBC) Diabetes Family Responsibility Questionnaire Parent measures Diabetes Self-Management Profile (DSMP) Diabetes Family Behavior checklist (DFBC) Diabetes Family Responsibility Questionnaire Clinician measures Clinical Global Impression Scale (CGIS) Clinical Global Improvement (CGI) |
|
| Yates Quality Scale | Study quality (out of 35): 20 (high quality) Treatment quality (out of 9): 7 (high quality) Design quality (out of 26): 13 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were then randomly assigned to the immediate treatment group or to a 1 month wait-list using a random numbers table.” Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “All assessments were conducted by an independent rater. The rater was a full-time research assistant.” Comment: unclear whether rater was blind. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Levy 2010 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment, 3-month follow-up, 6-month follow-up. | |
| Participants | End of treatment n = 168, 3 months = 143, 6 months = 154 Start of treatment n = 200 Sex of children: 55 M, 145 F Sex of parent: 12M, 188 F Mean age of child = 11.21 (2.55) Mean age of parent = 43.75 (6.35) Source = paediatric Gl Clinics at Seattle Children's Hospital and the Atlantic Health System in Morristown, New Jersey. Seattle area participants were also recruited via local clinics and community-posted flyers. Diagnosis = functional abdominal pain Mean years of illness = 3+ episodes of abdominal pain during a 3-month period |
|
| Interventions | “Cognitive-behavioural treatment” (CBT) “Educational intervention” Mode of delivery: individual families, face to face Intervention delivered by: therapists Training: not reported Duration of intervention (child) = 3 × 75 minutes = 4 hours Duration of intervention (parent) = 3 × 75 minutes = 4 hours |
|
| Outcomes | * Extracted measures Child measures Functional Disability Inventory* (FDI) Faces Pain Scale-Revised* Child Depression Inventory* (CDI) Child Somatization Inventory (CSI) Multidimensional Anxiety Scale for Children (MASC) Parent measures Functional Disability Inventory (FDI) Faces Pain Scale-Revised Child Somatization Inventory (CSI) |
|
| Yates Quality Scale | Study quality (out of 35): 27 (high quality) Treatment quality (out of 9): 7 (high quality) Design quality (out of 26): 20 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was then performed by a different researcher using a computerised random-number generator, stratifying by age.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Randomisation was then performed by a different researcher using a computerised random-number generator, stratifying by age.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Nurse assessors were blind to the treatment assignment of the children.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported after authors responded to data requests. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Ng 2008 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 11 weeks follow-up. | |
| Participants | End of treatment n = 27 Start of treatment n = 46 Sex of children: 25 M, 12 F Sex of parents: not reported Mean age of child = 9.24 (1.48) Mean age of parent = not reported Source = paediatric chest clinic of the Prince of Wales Hospital Hong Kong Diagnosis = asthma Mean years of illness = 5.70 (2.41) |
|
| Interventions | “We Together-We Success Parallel Group for Children with Asthma and their Parents (WTWS)” (FT) “Control Group” (wait list) Mode of delivery: group, face to face Intervention delivered by: not reported Training: not reported Duration of intervention (child) = 11 × 2 hours = 22 hours Duration of intervention (parent) = 11 × 2 hours = 22 hours |
|
| Outcomes | * Extracted measures Child measures Exhaled nitric oxide (eNO)* Spirometry Parent measures Anxiety Subscale of Chinese version Hospital Anxiety and Depression Scale (HADS)* Caretakers' perceived efficacy in the management of child's asthma (self constructed)* The Emotion Scale of Body-Mind-Spirit Well-Being Inventory (BMSWBI) Standard Short Form 12 (SF-12) Chinese (Hong Kong) Version 1 measuring health-related quality of life Patient's adjustment to asthma (self constructed)* |
|
| Yates Quality Scale | Study quality (out of 35): 16 (low quality) Treatment quality (out of 9): 5 (high quality) Design quality (out of 26): 11 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A randomised wait-list-controlled clinical trial design was adopted in this study”. Comment: no method described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were fully reported. No aims or primary outcomes were described in the introduction. Comment: probably some reporting bias. |
| Niebel 2000 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and post-treatment. | |
| Participants | End of treatment n = 47 Start of treatment n = 57 Sex of children: 5 M, 47 F Sex of parents: 0 M, 47 F Mean age of children = 3.9 (2.43) Mean age of parents = 33.9 (1.25) Source = unknown Diagnosis = eczema Mean years of illness = 9.1 years (8.36) |
|
| Interventions | “Direct Behavioural Parental Education” “Standardized Video-based Parental Education” “Dermatologic Standard Treatment” Mode of delivery: group and individual, face to face and video-based Intervention delivered by: mental health professional Training: not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 10 × 2 = 20 hours (direct), 1.67 hours (video-based) |
|
| Outcomes | ||
| Yates Quality Scale | Study quality (out of 35): 11 (low quality) Treatment quality (out of 9): 5 (high quality) Design quality (out of 26): 6 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data not fully reported. Comment: probably no reporting bias. Comment: probably no reporting bias. |
| Olivares 1997 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 9-month follow-up. | |
| Participants | End of treatment n = not reported Start of treatment n = 36 Sex of children: 19M, 17 F Sex of parents: 12M.23 F Mean age of children = not reported Mean age of parents = treatment group = 39.71 (5.47), control group = 40.87 (7.05) Source = not reported Diagnosis = diabetes Mean years of illness = treatment group = 4.76 (3.8) years, control group = 3.72 (2.22) years |
|
| Interventions | “Programme to modify parent behaviour” (CBT) “Wait list control” Mode of delivery: group, face to face Intervention delivered by: not reported Training: not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 8 sessions × 70 min = 9 hours 20 min |
|
| Outcomes | * Extracted measures Knowledge about behaviour modification* Responsibility for diabetes care* Blood glucose level* |
|
| Yates Quality Scale | Study quality (out of 35): 16 (low quality) Treatment quality (out of 9): 4 (low quality) Design quality (out of 26): 12 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data not fully reported. Comment: probably no reporting bias. Comment: probably no reporting bias. |
| Palermo 2009 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 3-month follow-up. | |
| Participants | End of treatment n = 44 Start of treatment n = 48 Sex of children: 13M, 35 F Sex of parents: 7:41 Mean age of children = 14.8 (2.0) Mean age of parents = not reported Source = academic health centre, Pacific Northwest USA Diagnosis = mixed pain conditions Mean years of illness = 30 months |
|
| Interventions | “Internet-delivered family cognitive-behavioral therapy” (CBT) “Wait list control group” Mode of delivery: individual families, Internet Intervention delivered by: Internet and online coach. Online coach was a PhD level postdoctoral psychology fellow. Training: 1 year of experience delivering face-to-face CBT to children with chronic pain. Duration of intervention (child) = 4 hours Duration of intervention (parent) = 4 hours |
|
| Outcomes | * Extracted measures Child measures Pain diary* Child Activity Limitations Interview* (CALI) Revised Child Anxiety and Depression Scale* (RCADS) Treatment Evaluation Inventory - Short Form Parent measures Adult Responses to Children's Symptoms* (ARCS) Treatment Evaluation Inventory - Short Form |
|
| Yates Quality Scale | Study quality (out of 35): 30 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 22 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, we used blocked randomisation with blocks of 10 to assign participants to the two treatment conditions during the course of randomisation. An online random number generator was used to produce the blocked randomisation. Group assignments were identified by ID number in sealed envelopes. Following completion of all pre-treatment assessments, a research coordinator opened the sealed envelope to reveal the group assignment.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, we used blocked randomisation with blocks of 10 to assign participants to the two treatment conditions during the course of randomisation. An online random number generator was used to produce the blocked randomisation. Group assignments were identified by ID number in sealed envelopes. Following completion of all pre-treatment assessments, a research coordinator opened the sealed envelope to reveal the group assignment.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants completed questionnaires online |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Robins 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 6 to 12 months following study entry. | |
| Participants | End of treatment n = 69, follow-up = 69 Start of treatment n = 86 Child sex: 30 M, 39 F Parent sex: not reported Mean age of children = 11.34 (2.4) Mean age of parents = not reported Source = community-based primary care physicians and hospital-based paediatric gastroenterologists Diagnosis = recurrent abdominal pain Mean years of illness = 3+ episodes over 3 months |
|
| Interventions | “Standard Medical Care plus Short-Term Cognitive-Behavioral Family Treatment” (CBT) “Standard Medical Care” Mode of delivery: group, face to face Intervention delivered by: psychology post-doctoral fellow or pre-doctoral intern Training: not reported Duration of intervention (child) = 5 sessions × 40 minutes = 3 hours 20 minutes Duration of intervention (parent) = 3 sessions × 40 minutes = 2 hours |
|
| Outcomes | * Extracted measures Child measures Abdominal Pain Index* (API) Child Somatization Inventory* (CSI) Functional Disability Inventory Child Version* (FDI) School Absences obtained from school attendance records Parent measures Abdominal Pain Index (API) Child Somatization Inventory (CSI) Clinician measures Health service use obtained from physician offices |
|
| Yates Quality Scale | Study quality (out of 35): 22 (high quality) Treatment quality (out of 9): 6 (high quality) Design quality (out of 26): 16 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The remaining sample of 86 were randomly assigned using a coin-flip method.” Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented on significant differences between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported. Aims, measures and results were Dartially concordant. Comment: probably some reporting bias. |
| Sahler 2002 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 3-month follow-up. | |
| Participants | End of treatment n = 81 Start of treatment n = 92 Sex of children: not reported Sex of parents: OM, 92 F Mean age of children = 8.32 (5.5) Mean age of mothers = 35.35 (6.6) Source = 6 children's hospitals in USA Diagnosis = cancer Mean years of illness = 2 to 16 weeks from diagnosis |
|
| Interventions | “Problem solving therapy” (PST) “Standard psychosocial care” Mode of delivery: individual, face to face Intervention delivered by: mental health professional or doctoral candidate in psychology Training: 3-day workshop Duration of intervention (child) = 0 Duration of intervention (parent) = 8 sessions × 1 hr = 8 hours |
|
| Outcomes | * Extracted measures Parent measures Social Problem-Solving Inventory-Cancer* Profile of Mood States* |
|
| Yates Quality Scale | Study quality (out of 35): 23 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 14 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was performed centrally, after stratification by site, using a two-block technique that produced a unique sequence for each site, delivered as a set of consecutively numbered envelopes specifying each subject's assignment”. Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Randomisation was performed centrally, after stratification by site, using a two-block technique that produced a unique sequence for each site, delivered as a set of consecutively numbered envelopes specifying each subject's assignment”. Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported after authors responded to requests. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Sahler 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 6 months after T1. | |
| Participants | End of treatment n = 407 Start of treatment n = 430 Sex of children: 219 M, 210 F Sex of parents: 0M.429 F Mean age of children at diagnosis = 7.6 Mean age of mothers = 35.5 Source = 7 sites is USA + 1 site in Israel Diagnosis = cancer Mean years of illness = 2 to 16 weeks from diagnosis |
|
| Interventions | “Usual psychosocial care plus problem-solving therapy” (PST) “Usual psychosocial care” Mode of delivery: individual, face to face Intervention delivered by: not reported Training: not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 8 × 1 hr = 8 hours |
|
| Outcomes | * Extracted measures Parent measures Profile of Mood States (POMS)* Beck Depression Inventory-ll (BDI-II)* Social Problem-Solving Inventory-Revised (SPSI-R)* NEO-Five Factor Inventory (NEO-FFI) Impact of Event Scale-Revised (IES-R) |
|
| Yates Quality Scale | Study quality (out of 35): 15 (low quality) Treatment quality (out of 9): 5 (high quality) Design quality (out of 26): 10 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (lection bias) | Unclear risk | “Randomisation was performed centrally.” Comment: method not described. |
| Allocation concealment (selection Dias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (etection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| ncomplete outcome data (attrition Dias) | Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Sanders 1994 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment, 6-month follow-up, 12-month follow-up. | |
| Participants | End of treatment n = 44 Start of treatment n = 44 Sex of children: 16 M,28 F Sex of parents: not reported Mean age of children = 9.22 (1.9) Mean age of parents = 39.3 (4.9) Source = not reported Diagnosis = recurrent abdominal pain Mean years of illness = 44 months (37.76) |
|
| Interventions | “Cognitive-behavioral family intervention” (CBT) “Standard paediatric care” Mode of delivery: individual, face to face Intervention delivered by: Clinical Psychologists Training: not reported Duration of intervention (child) = 6 × 50 minutes = 5 hours Duration of intervention (parent) = 6 × 50 minutes = 5 hours |
|
| Outcomes | * Extracted measures Child measures Pain diary* Videotaped vignettes, assessment of children's self coping Parent measures Child Behavior Checklist CBCL* Videotaped vignettes, assessment of maternal care giving* Parent Observation Record (POR) Treatment expectancies Measures of relapse - interview Satisfaction with treatment |
|
| Yates Quality Scale | Study quality (out of 35): 18 (high quality) Treatment quality (out of 9): 5 (high quality) Design quality (out of 26): 13 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The study used a randomised group comparison design with two treatment conditions.” Comment: method not described. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | Unclear risk | Data were incompletely reported. Aims, measures and results were fully concordant. Comment: probably some reporting bias. |
| Seid 2010 | ||
| Methods | RCT. 3 arms. Assessed pre-treatment, post-treatment and 6-month follow-up. | |
| Participants | End of treatment n = 204, 6-month follow-up n = 188 Start of treatment n = 252 Sex of children: 154 M, 98 F Sex of parents: 9M.244F Mean age of children = 7.37 (3.07) Mean age of parents = not reported Source = federally qualified health centres, a commercial HMO, school/daycare, local asthma initiatives and self referrals in San Diego, CA, USA Diagnosis = asthma Mean length of illness = 44 months (37.76) |
|
| Interventions | “Problem-Solving Skills Training + Care Coordination” (PST + Asthma Education) “In Home Asthma Education + Care Coordination” (Asthma Education) “Standard care wait-list control” Mode of delivery: individual families, face to face Intervention delivered by: Master's level health educator (PST), paraprofessional asthma home visitors (care co-ordination) Training: 2-week training Duration of intervention (PST + Asthma Education) = 6 × 45 to 60 minutes Duration of intervention (Asthma Education) = 5 × 45 to 60 minutes |
|
| Outcomes | * Extracted measures Child measures Pediatric Quality of Life Inventory Asthma Module Asthma Symptoms Scale (PedsQL Asthma) Parent measures Pediatric Quality of Life Inventory (PedsQL)* Health Service Use self report |
|
| Yates Quality Scale | Study quality (out of 35): 30 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 21 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Blocked randomisation, stratified by site of care and disease severity was used. Prepared randomisation lists were created by the statistician and concealed until intervention assignment.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Blocked randomisation, stratified by site of care and disease severity was used. Prepared randomisation lists were created by the statistician and concealed until intervention assignment.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Bilingual, bicultural research staff, blinded to the intervention group, administered surveys in English or Spanish in participants' homes.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias. |
| Stehl 2009 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and 1 month post-treatment. | |
| Participants | End of treatment n = 48 families, 92 caregivers Start of treatment n = 76 families, 152 caregivers received intervention Sex of children: 41 M, 35 F Sex of parents = not reported Mean age of children = 6 years Mean age of primary caregiver = 36 years Source = oncology service Diagnosis = cancer Mean years of illness = after diagnosis |
|
| Interventions | #x0201C;Surviving Cancer Competently Intervention Program-Newly Diagnosed” (CBT) “Standard Psychosocial Care” Mode of delivery: group, face to face, CD-ROM based multiple family discussion groups Intervention delivered by: psychology fellows, psychology intern, Master's level psychologist and doctoral-level nurse Training: 18 hours of didactic and experiential training Duration of intervention (children) = 3 × 45 minutes + 3 booster sessions Duration of intervention (parents) = 3 × 45 minutes + 3 booster sessions |
|
| Outcomes | * Extracted measures Parent measures State Trait Anxiety Inventory* (STAI) Impact of Event Scale-Revised (IES-R) Acute Stress Disorder Scale (ASDS) Programme Evaluation Clinicians' measures Social Work Activity Form Child Life Activity Form Intensity of Treatment Rating Scale (ITR-2) |
|
| Yates Quality Scale | Study quality (out of 35): 25 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 17 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was completed by a predetermined concealed random assignment list maintained by a staff member unaware of patient identity.” Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | “Randomization was completed by a predetermined concealed random assignment list maintained by a staff member unaware of patient identity.” Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Add data collection took place at the hospital at a time and location of convenience for the family and was conducted by research assistants.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data fully reported. Aims, measures and results are fully concordant. Comment: probably no reporting bias. |
| Wade 2006 | ||
| Methods | RCT. 2 arms. Assessed pre and post-treatment | |
| Participants | End of treatment n = 32 children and their parents Start of treatment n = 37 children and their parents Sex of children: 21 M, 11 F Sex of parents: not reported Mean age of children = 10.83 (2.94) Mean age of parents = not reported Source = trauma registry at Cincinnati Children's Hospital Medical Center Diagnosis = traumatic brain injury Mean years of illness = 8.78 (4.53) |
|
| Interventions | “Family-centered problem-solving intervention” (PST) “Usual Care” Mode of delivery: individual families, face to face Intervention delivered by: 5th year Clinical Psychology graduate student Training: 2 months Duration of intervention (children) = 7 × 75 minutes = 8 hours 45 minutes to 11 hours 40 minutes + up to 4 individualised sessions Duration of intervention (parents) = 7 × 75 minutes = 8 hours 45 minutes to 11 hours 40 minutes + up to 4 individualised sessions |
|
| Outcomes | * Extracted measures Child measures Conflict Behavior Questionnaire* Treatment satisfaction Parent measures Child Behavior Checklist* (CBCL) Conflict Behavior Questionnaire* (CBQ) Brief Symptom Inventory* (BSI) Treatment satisfaction |
|
| Yates Quality Scale | Study quality (out of 35): 22 (high quality) Treatment quality (out of 9): 9 (high quality) Design quality (out of 26): 13 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Families were randomly assigned to the family-centred problem-solving intervention or usual care group using a random numbers table.” Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | High risk | “Interviewers were also upper-level psychology graduate students who received extensive training.” Comment: no suggestion that they were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Wade 2006b | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and at session 7 of 8. | |
| Participants | End of treatment n = 41 (40 analysed) Start of treatment n = 46 Sex of children: 23 M, 17 F Sex of parents: not reported Mean age of children = 11.00 (3.27) Mean age of parents = not reported Source = trauma registry at Cincinnati Children's Hospital Medical Center Diagnosis = traumatic brain injury Mean years of illness = 13.73 (7.10) months since injury |
|
| Interventions | “Family Problem Solving” (PST) “Internet Resources Control” Mode of delivery: individual, online and video conferencing Intervention delivered by: Clinical Psychology graduate student Training: 2 months Duration of intervention (children) = 8 core modules, 6 supplementary modules, time not reported Duration of intervention (parents) = 8 core modules, 6 supplementary modules, time not reported |
|
| Outcomes | * Extracted measures Parent outcomes Family Assessment Device (FAD) Family Burden of Injury Interview subscales (FBII) Likert scales of global family problem-solving, communication and behaviour management Child Behavior Checklist Internalizing Problems* (CBCL) Home and Community Social Behavior Scale (HCSBS) Social Problem-Solving Index (SPSI-short version) Symptom Checklist-90-Revised (SCL-90-R) Global Severity Index (GSI) Center for Epidemiologic Studies Depression Scale* (CES-D) Anxiety Inventory (Al) Online usage questionnaire Website Evaluation Questionnaire (WEQ) |
|
| Yates Quality Scale | Study quality (out of 35): 25 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 17 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Families were randomly assigned to family problem-solving or internet resources comparison via a computer programme.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Given the nature of the study, neither the participants nor the research assistant was blind to group assignment. The primary outcome measures were based on parent and child report and therefore not dependent on the judgments of the research staff.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was not reported, but no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Wade 2011 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and post-treatment. | |
| Participants | End of treatment n = 35 Start of treatment n = 42 Sex of children: 17M.23 F Sex of parents: not reported Mean age of children = 14.25 (2.29) Mean age of parents = not reported Source = inpatient rehabilitation unit of 2 urban children's hospitals Diagnosis = traumatic brain injury Mean years of illness = 9.54 (4.97) months since injury |
|
| Interventions | “Teen Online Problem Solving” (PST) “Internet Resource Comparison” Mode of delivery: individual, internet and video conferencing Intervention delivered by: staff psychologist + Clinical Psychology graduate students Training: multi-day training Duration of intervention (children) = 10 core modules, 6 supplementary sessions, time not reported Duration of intervention (parents) = 10 core modules, 6 supplementary sessions, time not reported |
|
| Outcomes | * Extracted measures Child measures Youth Self Report* (YSR) Interaction Behaviour Questionnaire* (IBQ) Behavioral Rating Inventory of Executive Functioning Parent measures Child Behaviour Checklist* (CBCL) Interaction Behaviour Questionnaire* (IBQ) Behavioral Rating Inventory of Executive Functioning (BRIEF) |
|
| Yates Quality Scale | Study quality (out of 35): 22 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 14 (low quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Families were randomly assigned to either teen online problem-solving or internet resource group by use of a randomisation scheme that stratified participants on the basis of the adolescent's gender and race/ethnicity to ensure comparable diversity in each group.” Comment: method is not fully described. |
| Allocation concealment (selection bias) | Unclear risk | “Families were randomly assigned to either teen online problem-solving or internet resource group by use of a randomisation scheme that stratified participants on the basis of the adolescent's gender and race/ethnicity to ensure comparable diversity in each group.” |
| Blinding of outcome assessment (detection bias) | Low risk | “Given the nature of the study we were unable to conceal group assignment from the participants and research staff; however, the primary outcome measures were based on parent and teen report and therefore not dependent on judgments of research staff.” Comment: non-blinding of participants and research staff justified. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, no data were presented on equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Wysocki 1999 | ||
| Methods | RCT. 3 arms. Assessed pre-treatment, 3 months (post-treatment), 6-month follow-up and 12-month follow-up. | |
| Participants | End of treatment n = 115 (post-treatment), 113 (6-month follow-up), 108 (12-month follow-up) Start of treatment n = 119 children Sex of children: 50 M, 69 F Sex of parents: 82 M, 117 F Mean age of children = 14.3 (1.4) Mean age of parents = not reported Source = Missouri and Florida Diagnosis = type 1 diabetes Mean years of illness = 5.0 (3.8) |
|
| Interventions | “Behavioral Family Systems Therapy (BFST)” (FT) “Education and Support Group” (ES) “Standard Care” Mode of delivery: individual for BFST, group for ES, face to face Intervention delivered by licensed Clinical Psychologists Training: 150 hours Duration of intervention (children) = 10 sessions, time not reported Duration of intervention (parents) = 10 sessions, time not reported |
|
| Outcomes | * Extracted measures Child measures Parent-Adolescent Relationship Questionnaire (PARQ)* Issues Checklist (IC) 24 Hour Recall Interview of Conflict Situations Teen Adjustment to Diabetes Scale (TADS)* Diabetes Responsibility and Conflict (DRC) 24 Hour Recall Interview of IDDM Self-Care Self-Care Inventory (SCI) Glycated haemoglobin* Parent measures Parent-Adolescent Relationship Questionnaire (PARQ)* Issues Checklist (IC) 24 Hour Recall Interview of Conflict Situations Teen Adjustment to Diabetes Scale (TADS) Diabetes Responsibility and Conflict (DRC) 24 Hour Recall Interview of IDDM Self-Care Self-Care Inventory (SCI) Parent-reported health service use |
|
| Yates Quality Scale | Study quality (out of 35): 23 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 15 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The research scientist at the opposing centre randomly assigned each family, without knowledge of the family's baseline status on any of the outcome measures to one of three conditions.” Comment: method not fully described. |
| Allocation concealment (selection bias) | Unclear risk | “Randomisation was stratified by the adolescent's gender and treatment centre so that each centre enrolled a similar number of boys and girls into the three groups.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | “A research assistant administered questionnaires at evaluation sessions; the research assistant completed telephone interviews during the 2 weeks preceding each of the four evaluations.” Comment: blinding not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented on equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data not fully reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| Wysocki 2006 | ||
| Methods | RCT. 3 arms. Assessed at pre-treatment, 6 months (post-treatment), 12-month follow-up, 18-month follow-up. | |
| Participants | End of treatment n = 92 (post-treatment), 88 (12-month follow-up), 85 (18-month follow-up) Start of treatment n = 104 children (number of caregivers not reported) Sex of children: 57M,47 F Sex of parents: not reported Mean age of children = 14.2 (1.9) Mean age of parents = not reported Source = 2 paediatric centres in the Southeast and Midwest USA Diagnosis = type 1 diabetes or insulin-treated type 2 diabetes Mean years of illness = 5.5 (3.4) |
|
| Interventions | “Behavioral Family Systems Therapy for Diabetes (BFST-D)” (FT) “Educational Support Group” “Standard Care” Mode of delivery: individual families, face to face Intervention delivered by: licensed Clinical Psychologist, Social Worker Training: trained in BFST-D Duration of intervention (BFST-D) = 12 sessions, time not reported Duration of intervention (ES) = 12 × 1.5hr sessions |
|
| Outcomes | * Extracted measures Child measures Parent-Adolescent Relationship Questionnaire (PARQ)* Glycosylated haemoglobin (HbA1c)* Diabetes Responsibility and Conflict (DRC) Diabetes Self-Management Profile (DSMP) Family problem solving discussions coded using Interaction Behavior Code Parent measures Parent-Adolescent Relationship Questionnaire (PARQ)* Diabetes Responsibility and Conflict (DRC) Diabetes Self-Management Profile (DSMP) Family problem solving discussions coded using Interaction Behavior Code |
|
| Yates Quality Scale | Study quality (out of 35): 26 (high quality) Treatment quality (out of 9): 8 (high quality) Design quality (out of 26): 18 (high quality) |
|
| Notes | ||
| Risk of bias table | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A three-group, randomised treatments design was used.” Comment: method not described fully. |
| Allocation concealment (selection bias) | Unclear risk | “Families were stratified by HbA1c” |
| Blinding of outcome assessment (detection bias) | Low risk | “Raters were unaware of the family's identity or group assignment or of when the recording was made.” Comment: probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition was reported, but no data were presented on equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias. |
| footnote | ||
| CBT: cognitive behavioural therapy; Gl: gastrointestinal; HMO: health maintenance organisation; MST: multisystemic therapy; PSST: problem solving skills training; PST: problem solving therapy; RCT: randomised controlled trial | ||
Characteristics of excluded studies
| Aleman 1992 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Anderson 1999 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Betancourt 2004 | |||
| Reason for exclusion | Identified participants prospectively | ||
| Braga 2005 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Bruzzese 2008 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Burke 1997 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Burke 2001 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Cakan 2007 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Canino 2008 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Carey 2008 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Chernoff 2002 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Chiang 2009 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Ellis 2007 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Ellis 2008 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Evans 1999 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Field 1998 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Forsander 1995 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Forsander 2003 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Garbutt 2010 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Gerber 2010 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Giallo 2008 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Glang 2007 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Gustafsson 1986 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Harris 2001 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Haus 1976 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Hernandez 1998 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Hommel 2012 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Hovell 1994 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Humphreys 2000 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Ireys 1996 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Ireys 2001 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Jay 1990 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Johnson 1987 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Kamps 2008 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Kaslow 2000 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Kazak 1996 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Kazak 2005 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Ketchen 2006 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Klinnert 2005 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Klinnert 2007 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Kroner-Herwig 1998 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Kupfer 2010 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Lasecki 2008 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Logan 1997 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Mendez 1997 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Nelson 2011 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Perez 1999 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Rasoli 2008 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Sanders 1989 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Sanders 1996 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Satin 1989 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Scholten 2011 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Sieberg 2011 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Staab 2002 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Sullivan-Bolyai 2010 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Szczepanski 2010 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Wade 2010 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Walders 2006 | |||
| Reason for exclusion | Insufficient psychotherapeutic content | ||
| Walker 1996 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| Warner 2011 | |||
| Reason for exclusion | Inadequate n: the number of patients in any treatment arm was fewer than 10 | ||
| Wysocki 1997 | |||
| Reason for exclusion | Aim of study was irrelevant to this review | ||
| footnotes | |||
| Characteristics of studies awaiting classification | |||
| footnotes | |||
| Characteristics of ongoing studies | |||
| footnotes | |||
Other published versions of this review
Classification pending references
Data and analyses
| 1 Painful Conditions post treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 1.1 Parent Behaviour | 2 | 92 | Std. Mean Difference(IV, Random, 95% CI) | −0.34[−1.18, 0.50] |
| 1.2 Child Behaviour/Disability | 6 | 429 | Std. Mean Difference(IV, Random, 95% CI) | −0.18[−0.43, 0.07] |
| 1.3 Child Mental Health | 4 | 356 | Std. Mean Difference(IV, Random, 95% CI) | −0.02[−0.35, 0.30] |
| 1.4 Child Symptoms | 8 | 512 | Std. Mean Difference(IV, Random, 95% CI) | −0.29[−0.55, −0.03] |
| 2 Painful Conditions Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 2.1 Child Behaviour/Disability | 3 | 289 | Std. Mean Difference(IV, Random, 95% CI) | −0.06[−0.43, 0.32] |
| 2.2 Child Mental Health | 2 | 255 | Std. Mean Difference(IV, Random, 95% CI) | 0.04[−0.21,0.28] |
| 2.3 Child Symptoms | 6 | 391 | Std. Mean Difference(IV, Random, 95% CI) | −0.39[−0.86, 0.08] |
| 3 Cancer Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 3.1 Parent Behaviour | 4 | 629 | Std. Mean Difference(IV, Random, 95% CI) | −0.14[−0.35, 0.07] |
| 3.2 Parent Mental Health | 5 | 706 | Std. Mean Difference(IV, Random, 95% CI) | −0.15[−0.37, 0.07] |
| 4 Cancer Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 4.1 Parent Behaviour | 4 | 597 | Std. Mean Difference(IV, Random, 95% CI) | −0.06[−0.27, 0.15] |
| 4.2 Parent Mental Health | 4 | 598 | Std. Mean Difference(IV, Random, 95% CI) | −0.12[−0.32, 0.08] |
| 5 Diabetes Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 5.1 Child Mental Health | 2 | 198 | Std. Mean Difference(IV, Random, 95% CI) | −0.08[−0.63, 0.47] |
| 5.2 Child Symptoms | 6 | 455 | Std. Mean Difference(IV, Random, 95% CI) | −0.18[−0.39, 0.03] |
| 5.3 Family Functioning | 4 | 306 | Std. Mean Difference(IV, Random, 95% CI) | 0.01 [−0.22, 0.24] |
| 6 Diabetes Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 6.1 Child Symptoms | 3 | 239 | Std. Mean Difference(IV, Random, 95% CI) | −0.25[−0.55, 0.06] |
| 7 Asthma Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 7.1 Parent Mental Health | 2 | 74 | Std. Mean Difference(IV, Random, 95% CI) | −0.20[−0.66, 0.26] |
| 7.2 Child Symptoms | 3 | 170 | Std. Mean Difference(IV, Random, 95% CI) | 0.23[−0.07, 0.54] |
| 8 Asthma Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 8.1 Child Symptoms | 2 | 132 | Std. Mean Difference(IV, Random, 95% CI) | −0.16[−0.72, 0.40] |
| 9 Traumatic Brain Injury Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 9.1 Parent Mental Health | 2 | 72 | Std. Mean Difference(IV, Random, 95% CI) | −0.49[−1.14, 0.16] |
| 9.2 Child Behaviour/Disability | 2 | 72 | Std. Mean Difference(IV, Random, 95% CI) | −0.28[−1.12, 0.56] |
| 9.3 Family Functioning | 2 | 67 | Std. Mean Difference(IV, Random, 95% CI) | −0.14[−0.94, 0.67] |
| 10 Cognitive Behavioural Therapy Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 10.1 Parent Behaviour | 4 | 166 | Std. Mean Difference(IV, Random, 95% CI) | −0.02[−0.41,0.38] |
| 10.2 Parent Mental Health | 4 | 224 | Std. Mean Difference(IV, Random, 95% CI) | 0.14[−0.12, 0.41] |
| 10.3 Child Behaviour/Disability | 7 | 459 | Std. Mean Difference(IV, Random, 95% CI) | −0.11[−0.38, 0.15] |
| 10.4 Child Mental Health | 5 | 439 | Std. Mean Difference(IV, Random, 95% CI) | 0.03[−0.23, 0.29] |
| 10.5 Child Symptoms | 11 | 726 | Std. Mean Difference(IV, Random, 95% CI) | −0.25[−0.44, −0.06] |
| 10.6 Family Functioning | 3 | 211 | Std. Mean Difference(IV, Random, 95% CI) | 0.06[−0.22, 0.33] |
| 11 Cognitive Behavioural Therapy Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 11.1 Parent Behaviour | 2 | 85 | Std. Mean Difference(IV, Random, 95% CI) | −0.28[−1.26, 0.70] |
| 11.2 Parent Mental Health | 2 | 115 | Std. Mean Difference(IV, Random, 95% CI) | 0.32[−0.18, 0.82] |
| 11.3 Child Behaviour/Disability | 3 | 289 | Std. Mean Difference(IV, Random, 95% CI) | −0.06[−0.43, 0.32] |
| 11.4 Child Mental Health | 2 | 257 | Std. Mean Difference(IV, Random, 95% CI) | 0.03[−0.21,0.28] |
| 11.5 Child Symptoms | 7 | 472 | Std. Mean Difference(IV, Random, 95% CI) | −0.35[−0.73, 0.04] |
| 11.6 Family Functioning | 2 | 107 | Std. Mean Difference(IV, Random, 95% CI) | −0.16[−0.66, 0.35] |
| 12 Family Therapy Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 12.1 Parent Mental Health | 2 | 74 | Std. Mean Difference(IV, Random, 95% CI) | −0.20[−0.66, 0.26] |
| 12.2 Child Behaviour/Disability | 2 | 107 | Std. Mean Difference(IV, Random, 95% CI) | −0.87[−2.05, 0.31] |
| 12.3 Child Symptoms | 4 | 202 | Std. Mean Difference(IV, Random, 95% CI) | 0.13[−0.14, 0.41] |
| 12.4 Family Functioning | 2 | 132 | Std. Mean Difference(IV, Random, 95% CI) | −0.08[−0.42, 0.26] |
| 13 Family Therapy Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 13.1 Child Symptoms | 2 | 96 | Std. Mean Difference(IV, Random, 95% CI) | −0.02[−0.43, 0.38] |
| 14 Problem Solving Therapy Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 14.1 Parent Behaviour | 3 | 588 | Std. Mean Difference(IV, Random, 95% CI) | −0.22[−0.38, −0.06] |
| 14.2 Parent Mental Health | 5 | 660 | Std. Mean Difference(IV, Random, 95% CI) | −0.27[−0.53, −0.02] |
| 14.3 Child Behaviour/Disability | 2 | 72 | Std. Mean Difference(IV, Random, 95% CI) | −0.28[−1.12, 0.56] |
| 14.4 Family Functioning | 2 | 67 | Std. Mean Difference(IV, Random, 95% CI) | −0.14[−0.94, 0.67] |
| 15 Problem Solving Therapy Follow-up | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 15.1 Parent Behaviour | 3 | 556 | Std. Mean Difference(IV, Random, 95% CI) | −0.09[−0.31,0.14] |
| 15.2 Parent Mental Health | 3 | 557 | Std. Mean Difference(IV, Random, 95% CI) | −0.13[−0.38, 0.12] |
| 16 Multisystemic Therapy Post-treatment | ||||
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
| 16.1 Child Symptoms | 2 | 142 | Std. Mean Difference(IV, Random, 95% CI) | −0.31 [−0.64, 0.03] |
Summary of findings tables
Additional tables
2. Intervention content and therapy classification of included studies.
| Author | Therapy summary | Therapy type |
|---|---|---|
| Allen 1998 Painful condition (migraine) | Thermal biofeedback plus parent behaviour management. Parents were provided with pain behaviour management guidelines which focused on minimising attention to pain, encouraging the child to participate in daily activities, and praising practice of biofeedback. Children received thermal biofeedback training. | CBT |
| Ambrosino 2008 Diabetes | Coping skills training. Parents and children received training in communication skills, social problem solving, recognising links between thoughts/feelings/behaviours, stress management and conflict resolution. The focus of this intervention was to improve participants' general ability to manage daily problems, and did not directly address diabetes management. | CBT |
| Askins 2009 Cancer | PST + PDA. Mothers received problem solving training using the Bright IDEAS framework: Be optimistic about solving problems, Identify the problem, Determine options, Evaluate options and choose one, Act and See if it worked. Mothers were also provided a personal digital assistant (PDA) device that was designed to review and practise problem solving steps and record problems and solutions encountered between sessions. Children did not receive any intervention. | PST |
| Barakat 2010 Painful condition (SCD) | Pain management intervention. Parents and children received education about sickle cell disease (SCD) as well as training in deep breathing, progressive muscle relaxation, cognitive restructuring and guided imagery. | CBT |
| Barry 1997 Painful condition (Headache) | Cognitive behavioural group treatment. Parents received pain education as well as training in relaxation, imagery and positive parenting strategies. Children received pain education as well as training in relaxation, imagery, distraction and cognitive restructuring. | CBT |
| Celano 2012 Asthma | Home-based family intervention. Families received asthma education regarding trigger control resources and feedback on the child's lung functioning and metered does inhaler (MDI)/spacer technique, as well as psychosocial modules targeting family rules and discipline, family communication and caregiver mental health. | FT |
| Connelly 2006 Painful condition (Headache) | Headstrong programme. Using CD-ROMs, children and parents jointly completed a module on management of pain behaviours and creation of a pain-coping plan. Children received headache education and training in guided imagery, deep breathing, progressive muscle relaxation, cognitive restructuring and problem solving. | CBT |
| Duarte 2006 Painful condition (RAP) | Cognitive-behavioural family intervention. Parents and children received education about abdominal pain as well as training in operant techniques with an emphasis on increasing adaptive behaviours when in pain, deep breathing, physical exercise, progressive muscle relaxation, thought stopping, distraction and imagery. | CBT |
| Ellis 2004 Diabetes | Multisystemic therapy (MST). Families received an intensive, family- and community-based intervention designed to target problems related to adherence to diabetes treatment across the multiple systems within which the child and their family operated. A variety of psychological interventions were employed depending on individual need, including cognitive behavioural therapy, parent training and behavioural family systems therapy. | MST |
| Ellis 2005 Diabetes | Multisystemic therapy (MST). See Ellis 2004 above. | MST |
| Grey 2011 Diabetes | Coping skills intervention and training. Parents received training in communication skills, social problem solving, cognitive restructuring, stress management and conflict resolution and were taught to apply these skills to thoughts, feelings and behaviours related to diabetes management. Children did not receive any intervention. | CBT |
| Hicks 2006 Painful condition (RAP) | Online psychological treatment for paediatric recurrent pain. Using a website, parents received training in ways to promote healthy behaviour. Children received pain education as well as training in deep breathing, relaxation, imagery, cognitive strategies and healthy lifestyle choices. Children also received a tape of personalised relaxation exercises and a thought journal. Each week, families were contacted by a researcher via phone or email to check progress and review materials. | CBT |
| Hoekstra-Weebers 1998 Cancer | Intervention programme for parents of paediatric cancer patients. Parents received education regarding the potential impact of the child's illness on the child and family as well as training in emotional expression, cognitive restructuring, problem-focused coping skills, communication and assertiveness skills. Children did not receive any intervention | CBT |
| Kashikar-Zuck 2005 Painful condition (Fibromyalgia) | Coping skills training. Parents received operant training with a focus on encouraging active coping behaviour and independent pain management. Children received education about behavioural pain management as well as training in progressive muscle relaxation, distraction, activity pacing, cognitive techniques and problem solving. | CBT |
| Kashikar-Zuck 2012 Painful condition (Fibromyalgia) | Cognitive behavioural therapy (CBT) for the treatment of juvenile fibromyalgia. This intervention is a revised version of the Coping Skills Training program evaluated in Kashikar-Zuck (2005) Parents received operant training with a focus on encouraging independent pain management, maintaining a normal routine, avoiding status checks and increasing their child's use of coping skills learned in pe programme. Children received education about behavioural pain management as well as training in progressive muscle relaxation, distraction, activity pacing, using self statements, problem solving and relapse prevention strategies. | CBT |
| Kazak 2004 Cancer | Surviving Cancer Competently Intervention Programme (SCCIP). Families received education about the link between thoughts, feelings and behaviours and training in cognitive restructuring. Families also participated in discussion groups about the ways cancer has affected their family, recognising and responding to distress in other family members, and acknowledging and accepting their cancer experience. | CBT |
| Laffel 2003 Diabetes | Teamwork intervention. Parents and children received training in communicating about diabetes and sharing blood glucose results with family members, the need for teamwork between parents end children in diabetes management during adolescence, managing family members' responses to the child's blood glucose levels, sharing diabetes management with family members, and using a diary to help problem solve high and low blood glucose levels. | FT |
| Lask 1979 Asthma | Family psychotherapy. This intervention aimed to improve the psychological well-being of the family by focusing on attitudes towards asthma and its treatment, fear of death and negative emotions experienced by family members. | FT |
| Lehmkuhl 2010 Diabetes | Telehealth behavioural therapy. Using telephone contact, families received diabetes education in addition to training in specific skills targeting diabetes care and family functioning, including problem solving, behavioural contracting, communication skills, cognitive restructuring and family structuring. | FT |
| Levy 2010 Painful condition (FAP) | Social learning and cognitive behavioural therapy. Children and parents received pain education in addition training in deep breathing, progressive muscle relaxation, imagery, operant strategies, cognitive restructuring and relapse prevention strategies. | CBT |
| Na 2008 Asthma | We Together-We success Parallel Group for Children with Asthma and their Parents (WTWS). Parents and children received asthma education and discuss issues regarding mutual respect between family members, psychosocial factors that may impact asthma symptoms, applying concepts from traditional Chinese medicine to asthma management, and fostering the child's independence. | FT |
| Niebel 2000 Skin Diseases (Eczema) | Direct parental education in groups. Parents received asthma education and training in operant strategies, scratch-control techniques, stress management, progressive muscle relaxation, how to poach their children in using progressive muscle relaxation, how to conduct social skills training L/ith their children and relapse prevention. Children did not participate in the intervention. | CBT |
| Palermo 2009 Painful condition (Mixed pain conditions) | Web-based Management of Adolescent Pain (Web-MAP). Using an internet program, parents received education about chronic pain and training in recognising stress and negative emotions, operant strategies, modelling, sleep hygiene and lifestyle, communication and relapse prevention. Children received education about chronic pain and training in recognising stress and negative emotions, deep breathing and relaxation, distraction, cognitive skills, sleep hygiene and lifestyle, staying active and relapse prevention. | CBT |
| Robins 2005 Painful condition (RAP) | Short-term cognitive behavioural therapy. Children and parents received education about pain and stress as well as training in deep breathing, imagery, relaxation and operant strategies. Children also training in tracking the antecedents and consequences of pain episodes and cognitive restructuring. | CBT |
| Sanders 1994 Painful condition (RAP) | Cognitive-behavioural family intervention. Parents received education about behavioural pain management, operant training and relapse prevention. Children received education about behavioural pain management, muscle relaxation, deep breathing, imagery, cognitive restructuring, distraction and relapse prevention. | CBT |
| Sahler 2002 Cancer | Problem solving skills training. Mothers received problem solving training using the Bright IDEAS framework: Be optimistic about solving problems, Identify the problem, Determine options, Evaluate options and choose one, Act and See if it worked. Children did not receive any intervention. | PST |
| Sahler 2005 Cancer | Problem solving skills training. See Sahler 2002 above. | PST |
| Seid 2010 Asthma | Problem solving skills training + care co-ordination. Parents received in-home asthma education, referrals to community resources, co-ordination with medical providers and problem solving training using the Bright IDEAS framework (see Sahler 2002 above). Children did not receive any intervention. | PST |
| Stehl 2009 Cancer | Surviving Cancer Competently Intervention Programme - Newly diagnosed (SCCIP-ND). Parents received education about the link between thoughts, feelings and behaviours, training in cognitive restructuring, and discussion of beliefs about the role cancer will play in the family's future. Parents also watched a CD-ROM of other parents of children with cancer discussing their experiences and responses to diagnosis. Children did not receive any intervention. | CBT |
| Wade 2006 TBI | Family problem solving intervention. Families received problem solving training using the ABCDE framework (Aim, Brainstorm, Choose, Do It and Evaluate) and were encouraged to have a positive attitude towards problem solving. Families also received education on the effects of TBI on child functioning as well as training in behavioural management, communication skills and handling crises. | PST |
| Wade 2006b TBI | Family problem solving intervention. Using an internet program and videoconferencing, families received training in problem solving, communication, behaviour management skills and relapse prevention. Families could also complete supplemental sessions if needed on stress management, working with the school, sibling concerns, anger management, pain management and marital communication. | PST |
| Wade 2011 TBI | Teen Online Problem Solving (TOPS). Using an internet program and videoconferencing, families received training in stress management, problem solving, planning and organisation, communication and self regulation. Families could also complete supplemental sessions if needed on stress management, self care, marital communication, memory difficulties, planning for after high school graduation, sibling concerns, pain management and communication between teens and parents. | PST |
| Wysocki 1999 Diabetes | Behavioural Family Systems Therapy (BFST). Families received training in problem solving skills, communication skills and cognitive restructuring as well as functional and structural family therapy interventions targeting family systems issues that may have interfered with effective problem solving and communication skills. | FT |
| Wysocki 2006 Diabetes | Behavioural Family Systems Therapy for Diabetes (BFST-D). This intervention is a revised version of the BFST intervention evaluated in Wysocki 1999. Families received training in problem solving, communication skills and cognitive restructuring as well as functional and structural family therapy interventions targeting family systems issues related to effective problem solving and communication. Diabetes-specific adaptations included targeting two or more barriers to diabetes management in treatment, training in behavioural contracting, education in how to improve diabetic control based on data from self monitoring of blood glucose levels, simulation of living with diabetes by parents for 1 week, and involvement of peers/teachers/extended family in treatment as needed. | FT |
BFST-D: Behavioural Family Systems Therapy for Diabetes; CBT: cognitive behavioural therapy; FT: family therapy; MST: multisystemic therapy; PST: problem solving therapy; TBI: traumatic brain injury
3. Scorecard of meta-analytic findings at post-treatment by illness condition and type of therapy.
| Scorecard of findings at post-treatment | ||||||
|---|---|---|---|---|---|---|
| Combined psychological therapies for each illness conidition (post Rx) | ||||||
| Parent | Child | |||||
| Behaviour | Mental health | Behaviour/disability | Mental health | Primary symptom | Family functioning | |
| Pain | No effect* | Unknown | No effect | No effect | Effect found | Unknown |
| Cancer | No effect | No effect | Unknown | Unknown | Unknown | Unknown |
| Diabetes | Unknown | Unknown | Unknown | No effect* | No effect | No effect |
| Asthma | Unknown | No effect* | Unknown | Unknown | No effect | Unknown |
| TBI | Unknown | No effect* | No effect* | Unknown | Unknown | No effect* |
| Skin diseases | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Individual psychological therapies for each illness conidition (post Rx) | ||||||
| Parent | Child | |||||
| Behaviour | Mental Health | Behaviour/disability | Mental health | Primary symptom | Family functioning | |
| CBT | No effect | No effect | No effect | No effect | Effect found | No effect |
| FT | Unknown | No effect* | No effect* | Unknown | No effect | No effect* |
| PST | Effect found | Effect found | No effect* | Unknown | Unknown | No effect* |
| MST | Unknown | Unknown | Unknown | Unknown | No effect* | Unknown |
Denotes which analyses only included two studies and should be interpreted with caution.
CBT: cognitive behavioural therapy; FT: family therapy; MST: multisystemic therapy; PST: problem solving therapy; TBI: traumatic brain injury
Feedback: Appendices
| 1 MEDLINE search strategy |
|
Key: mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier, pt = publication type
2 Other search strategies
| Cochrane Central Register of Controlled Trials (CENTRAL) | |
| 1 | MeSH descriptor Psychotherapy explode all trees |
| 2 | MeSH descriptor Problem Solving, this term only |
| 3 | psychotherap* |
| 4 | ((cogniti* or family or behavior* or behaviour* or psychological*) near/5 (intervention* or treatment* or therap*)) |
| 5 | (problem* near/5 solv*) |
| 6 | CBT |
| 7 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6) |
| 8 | MeSH descriptor Parents explode all trees |
| 9 | MeSH descriptor Family explode all trees |
| 10 | MeSH descriptor Caregivers, this term only |
| 11 | (parent* or mother* or father* or family or families or caregiver* or care-giver*) |
| 12 | (#8OR#9OR#10OR#11) |
| 13 | MeSH descriptor Child explode all trees |
| 14 | MeSH descriptor Infant explode all trees |
| 15 | MeSH descriptor Adolescent, this term only |
| 16 | (child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*) |
| 17 | (#13 0R#14 0R#15 0R#16) |
| 18 | MeSH descriptor Pain explode all trees |
| 19 | MeSH descriptor Complex Regional Pain Syndromes explode all trees |
| 20 | MeSH descriptor Rheumatic Diseases explode all trees |
| 21 | MeSH descriptor Neoplasms explode all trees |
| 22 | MeSH descriptor Diabetes Mellitus explode all trees |
| 23 | MeSH descriptor Asthma explode all trees |
| 24 | MeSH descriptor Brain Injuries explode all trees |
| 25 | MeSH descriptor Inflammatory Bowel Diseases explode all trees |
| 26 | MeSH descriptor Anemia, Sickle Cell explode all trees |
| 27 | MeSH descriptor Skin Diseases explode all trees |
| 28 | MeSH descriptor Genital Diseases, Female explode all trees |
| 29 | MeSH descriptor Menstruation Disturbances explode all trees |
| 30 | (pain* or headache*) |
| 31 | (rheumat* or arthriti* or fibromyalgia) |
| 32 | (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*) |
| 33 | diabet* |
| 34 | asthma* |
| 35 | (brain near/5 (trauma* or injur*)) |
| 36 | (bowel* near/5 inflammatory near/5 (condition* or disease* or illness*)) |
| 37 | (sickle cell near/5 (disease* or disorder* or anemia*)) |
| 38 | ((skin near/5 (disease* or disorder*)) or eczema*) |
| 39 | ((gynecologic* or gynaecologic*) near/5 (disease* or disorder*)) |
| 40 | dysmenorrh* |
| 41 | endometriosis |
| 42 | MeSH descriptor Chronic Disease, this term only |
| 43 | ((chronic* or longterm or long-term) near/5 (condition* or ill* or disease*)) |
| 44 | (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43) |
| 45 | (#7 AND #12 AND #17 AND #44) |
| EMBASE via Ovid | |
| 1 | exp psychotherapy/ |
| 2 | exp problem solving/ |
| 3 | psychotherap*.mp. |
| 4 | ((cogniti* or family or behavior* or behaviour* or psychological*) adj5 (intervention* or treatment* or therap*)).mp. |
| 5 | (problem* adj5 solv*).mp. |
| 6 | CBT.mp. |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 |
| 8 | exp parent/ |
| 9 | exp family/ |
| 10 | exp caregiver/ |
| 11 | (parent* or mother* or father* or family or families or caregiver* or care-giver*).mp. |
| 12 | 8 or 9 or 10 or 11 |
| 13 | exp child/ |
| 14 | exp infant/ |
| 15 | exp adolescent/ |
| 16 | (child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*).mp. |
| 17 | 13or14or15or16 |
| 18 | exp pain/ |
| 19 | exp complex regional pain syndrome/ |
| 20 | exp rheumatic disease/ |
| 21 | exp neoplasm/ |
| 22 | exp diabetes mellitus/ |
| 23 | exp asthma/ |
| 24 | exp brain injury/ |
| 25 | exp enteritis/ |
| 26 | exp sickle cell anemia/ |
| 27 | exp skin disease/ |
| 28 | exp gynecologic disease/ |
| 29 | exp menstruation disorder/ |
| 30 | (pain* or headache*).mp. |
| 31 | (rheumat* or arthriti* or fibromyalgia).mp. |
| 32 | (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*).mp. |
| 33 | diabet*.mp. |
| 34 | asthma*.mp. |
| 35 | (brain adj5 (trauma* or injur*)).mp. |
| 36 | (bowel* adj5 inflammatory adj5 (condition* or disease* or illness*)).mp. |
| 37 | (sickle cell adj5 (disease* or disorder* or anemia*)).mp. |
| 38 | ((skin adj5 (disease* or disorder*)) or eczema*).mp. |
| 39 | ((gynecologic* or gynaecologic*) adj5 (disease* or disorder*)).mp. |
| 40 | dysmenorrh*.mp. |
| 41 | endometriosis.mp. |
| 42 | exp chronic disease/ |
| 43 | ((chronic* or longterm or long-term) adj5 (condition* or ill* or disease*)).mp. |
| 44 | 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 |
| 45 | exp controlled clinical trial/ |
| 46 | random*.mp. |
| 47 | trial*.mp. |
| 48 | placebo*.mp. |
| 49 | exp evaluation/ |
| 50 | exp treatment outcome/ |
| 51 | exp comparative effectiveness/ |
| 52 | (outcome* or assess* or evaluat*).mp. |
| 53 | (quantitative adj5 research).mp. |
| 54 | 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 |
| 55 | 7 and 12 and 17 and 44 and 54 |
| PsyclNFO via Ovid | |
| 1 | exp psychotherapy/ |
| 2 | exp family therapy/ |
| 3 | exp problem solving/ |
| 4 | psychotherap*.mp. |
| 5 | ((cogniti* or family or behavior* or behaviour* or psychological*) adj5 (intervention* or treatment* or therap*)).mp. |
| 6 | (problem* adj5 solv*).mp. |
| 7 | CBT.mp. |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 9 | exp Parents/ |
| 10 | exp Family/ |
| 11 | exp Caregivers/ |
| 12 | (parent* or mother* or father* or family or families or caregiver* or care-giver*).mp. |
| 13 | 9 or 10 or 11 or 12 |
| 14 | (child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*).mp. |
| 15 | exp pain/ |
| 16 | exp Rheumatoid Arthritis/ |
| 17 | exp Neoplasms/ |
| 18 | exp Diabetes Mellitus/ |
| 19 | exp Asthma/ |
| 20 | exp traumatic brain injury/ |
| 21 | exp Sickle Cell Disease/ |
| 22 | exp skin disorders/ |
| 23 | exp gynecological disorders/ |
| 24 | (pain* or headache*).mp. |
| 25 | (rheumat* or arthriti* or fibromyalgia).mp. |
| 26 | (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*).mp. |
| 27 | diabet*.mp. |
| 28 | asthma*.mp. |
| 29 | (brain adj5 (trauma* or injur*)).mp. |
| 30 | (bowel* adj5 inflammatory adj5 (condition* or disease* or illness*)).mp. |
| 31 | (sickle cell adj5 (disease* or disorder* or anemia*)).mp. |
| 32 | ((skin adj5 (disease* or disorder*)) or eczema*).mp. |
| 33 | ((gynecologic* or gynaecologic*) adj5 (disease* or disorder*)).mp. |
| 34 | dysmenorrh*.mp. |
| 35 | endometriosis.mp. |
| 36 | ((chronic* or longterm or long-term) adj5 (condition* or ill* or disease*)).mp. |
| 37 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 |
| 38 | exp Clinical Trials/ |
| 39 | random*.mp. |
| 40 | placebo*.mp. |
| 41 | trial*.mp. |
| 42 | exp treatment outcomes/ |
| 43 | (outcome* or assess* or evaluat*).mp. |
| 44 | (quantitative adj5 research).mp. |
| 45 | 38 or 39 or 40 or 41 or 42 or 43 or 44 |
| 46 | 8 and 13 and 14 and 37 and 45 |
Footnotes
Contact person: Emma Fisher, Research Assistant, Department of Health, University of Bath, Bath, BA2 7AY UK, e.a.fisher@bath.ac.uk
Contributions of authors: CE oversaw authoring of the manuscript, arbitrated the selection of studies, interpreted the analyses, was responsible for the methodology and will be responsible for updating the review in the future.
TP interpreted the analyses, drafted the final manuscript and will update the review in the future.
EF developed the search strategy, searched the electronic databases and reference lists, obtained studies, selected relevant studies, extracted data and entered data into RevMan (RevMan 2011), interpreted the analyses and drafted the review.
EL selected the studies to include and extracted data, interpreted the analyses and drafted the review.
Declarations of interest: None known.
Differences between protocol and review: Language throughout the protocol has been altered to improve the flow and increase the accuracy.
The tense of the language used in the methodology has been changed to past in line with Cochrane guidelines.
Measures of treatment effect: this section has been added to provide a clearer description of intended analyses.
The order of the four main analyses has been re-worded for a clearer understanding of the analysis plan. Parent outcomes have been listed before child outcomes as this is the focus of the review. Appendices were added for other search strategies.
Assessment of risk of bias in included studies: this has been expanded to include a fuller description.
References to studies
Included studies
- Allen KD, Shriver MD. Role of parent-mediated pain behavior management strategies in biofeedback treatment of childhood migraines. Behaviour Therapy. 1998;29:477–90. [Google Scholar]
- *.Ambrosino JM, Fennie K, Whittemore R, Jaser S, Dowd MF, Grey M. Short-term effects of coping skills training in school-age children with type 1 diabetes. Pediatric Diabetes. 2008;9:74–82. doi: 10.1111/j.1399-5448.2007.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey M, Whittermore R, Jaser S, Ambrosino J, Lindemann E, Liberti L, et al. Effects of coping skills training in school-age children with type 1 diabetes. Research in Nursing & Health. 2009;32:405–18. doi: 10.1002/nur.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askins MA, Sahler OJ, Sherman SA, Fairclough DL, Butler RW, Katz ER, et al. Report from a multi-institutional randomized clinical trial examining computer-assisted problem-solving skills training for English- and Spanish-speaking mothers of children with newly diagnosed cancer. Journal of Pediatric Psychology. 2008;34(5):551–63. doi: 10.1093/jpepsy/jsn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat LP, Schwartz LA, Salaom KS, Radcliffe J. A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. Journal of Pediatric Hematology Oncology. 2010;32(7):540–7. doi: 10.1097/MPH.0b013e3181e793f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J, von Baeyer CL. Brief cognitive-behavioral group treatment for child's headache. Clinical Journal of Pain. 1997;13(3):215–20. doi: 10.1097/00002508-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Celano MP, Holsey CN, Kobrynski LJ. Home-based family intervention for low-income children with asthma: a randomized controlled pilot study. Journal of Family Psychology. 2012:1–8. doi: 10.1037/a0027218. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Connelly M, Rapoff MA, Thompson N, Connelly W. Headstrong: a pilot study of a CD-ROM intervention for recurrent pediatric headache. Journal of Pediatric Psychology. 2006;31(7):737–47. doi: 10.1093/jpepsy/jsj003. [DOI] [PubMed] [Google Scholar]
- Duarte MA, Penna FJ, Andrade EMG, Cancela CSP, Neto JCA, Barbosa TF. Treatment of nonorganic recurrent abdominal pain: cognitive-behavioral family intervention. Journal of Pediatric Gastroenterology and Nutrition. 2006;43(1):59–64. doi: 10.1097/01.mpg.0000226373.10871.76. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Frey M, Templin T, Rowland M, Greger N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in poor metabolic control: a pilot investigation. Journal of Clinical Psychology in Medical Settings. 2004;11(4):315–24. [Google Scholar]
- *.Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. The effects of multisystemic therapy on diabetes stress among adolescents with chronically poorly controlled type 1 diabetes: findings from a randomized, controlled trial. Pediatrics. 2005;116(6):e826–32. doi: 10.1542/peds.2005-0638. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control. Diabetes Care. 2005;28(7):1604–10. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Templin T, Naar-King S, Frey MA, Cunningham PB, Podolski CL, et al. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: stability of treatment effects in a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2007;75(1):168–74. doi: 10.1037/0022-006X.75.1.168. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Yopp J, Templin T, Naar-King S, Frey MA, Cunningham PB, et al. Family mediators and moderators of treatment outcomes among youths with poorly controlled type 1 diabetes: results from a randomized controlled trial. Journal of Pediatric Psychology. 2007;32(2):194–205. doi: 10.1093/jpepsy/jsj116. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Ellis DA, Idalski A, Frey MA, Cunningham P. Multisystemic therapy decreases parental overestimation of adolescent responsibility for type 1 diabetes management in urban youth. Families, Systems & Health. 2007;25(2):178–89. [Google Scholar]
- Grey M, Jaser SS, Whittemore R, Jeon S, Lindemann E. Coping skills training for parents of children with type 1 diabetes. Nursing Research. 2011;60(3):173–81. doi: 10.1097/NNR.0b013e3182159c8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks CL, von Baeyer CL, McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. Journal of Pediatric Psychology. 2006;31(7):724–36. doi: 10.1093/jpepsy/jsj065. [DOI] [PubMed] [Google Scholar]
- Hoekstra-Weebers JEHM, Heuvel F, Jaspers JPC, Kamps WA, Klip EC. Brief report: an intervention program for parents of pediatric cancer patients: a randomized controlled trial. Journal of Pediatric Psychology. 1998;23(3):207–14. doi: 10.1093/jpepsy/23.3.207. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Swain N, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology. 2005;32(8):1594–602. [PubMed] [Google Scholar]
- Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia. Arthritis and Rheumatism. 2012;64(1):297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak AE, Alderfer MA, Streisand R, Simms S, Rourke MT, Barakat LP, et al. Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: a randomized clinical trial. Journal of Family Psychology. 2004;18(3):493–504. doi: 10.1037/0893-3200.18.3.493. [DOI] [PubMed] [Google Scholar]
- Laffel LMB, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. Journal of Pediatrics. 2003;142:409–16. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- Lask B, Matthew D. Childhood asthma. A controlled trial of family psychotherapy. Archives of Disease in Childhood. 1979;54(2):116–9. doi: 10.1136/adc.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl HD, Storch EA, Cammarata C, Meyer K, Rahman O, Silverstein J, et al. Telehealth behavior therapy for the management of type 1 diabetes in adolescents. Journal of Diabetes Science and Technology. 2010;4(1):199–208. doi: 10.1177/193229681000400125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, et al. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. American Journal of Gastroenterology. 2010;105(4):946–56. doi: 10.1038/ajg.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SM, Li AM, Lou WVQ, Tso IF, Wan PYP, Chan DFY. Incorporating family therapy into asthma group intervention: a randomized waitlist-controlled trial. Family Process. 2008;47(1):115–30. doi: 10.1111/j.1545-5300.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- Niebel G, Kallweit C, Lange I, Folster-Holst R. Direct versus video-aided parent education in atopic eczema in childhood as a supplement to specialty physician treatment. A controlled pilot study [Direkte versus videover-mittelte elternschulung bei atopishchem ekzem in kinderslater als erganzung facharztlicher behandlung: eine kontrollierte pilotstudie] Hautarzt. 2000;51:401–11. doi: 10.1007/s001050051141. [DOI] [PubMed] [Google Scholar]
- Olivares J, Mendez FX, Ros M, Bermejo RM. Effects of a training program for parents with diabetic children with obstacles to comply with therapy [El cuidado de la diabetes mellitus insulino-dependiente: Efectos de un programa de modificacion de conducta en padres] Psicologia Conductual. 1997;5(2):219–35. [Google Scholar]
- Palermo TM, Wilson AC, Peters M, Lweandowski A, Somhegyi H. Randomized controlled trial of an Internet delivered family cognitive behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1-2):205–13. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins PM, Smith SM, Glutting JJ, Bishop CT. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. Journal of Pediatric Psychology. 2005;30(5):397–408. doi: 10.1093/jpepsy/jsi063. [DOI] [PubMed] [Google Scholar]
- Sahler OJZ, Varni JW, Fairclough DL, Butler RW, Noll RB, Dolgin MJ, et al. Problem-solving skills training for mothers of children with newly diagnosed cancer: a randomized trial. Developmental and Behavioural Pediatrics. 2002;23(2):77–86. doi: 10.1097/00004703-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Sahler OJZ, Fairclough DL, Phipps S, Mulhern RK, Dolgin MJ, Noll RB, et al. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: Report of a multisite randomized trial. Journal of Consulting and Clinical Psychology. 2005;73(2):272–83. doi: 10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- Sandes MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. Journal of Consulting and Clinical Psychology. 1994;62(2):306–14. doi: 10.1037//0022-006x.62.2.306. [DOI] [PubMed] [Google Scholar]
- Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ. Problem-solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health-related quality of life outcomes. Journal of Pediatric Psychology. 2010;35(10):1133–43. doi: 10.1093/jpepsy/jsp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehl ML, Kazak AE, Alderfer MA, Rodriguez A, Hwang WT, Pai ALH, et al. Conducting a randomized clinical trial of an psychological intervention for parents/caregivers of children with cancer shortly after diagnosis. Journal of Pediatric Psychology. 2009;34(8):803–16. doi: 10.1093/jpepsy/jsn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Michaud L, Brown TM. Putting the pieces together: preliminary efficacy of a family problem-solving intervention for children with traumatic brain injury. Journal of Head Trauma Rehabilitation. 2006;21(1):59–67. doi: 10.1097/00001199-200601000-00006. [DOI] [PubMed] [Google Scholar]
- Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. Journal of Consulting and Clinical Psychology. 2006;74(3):445–54. doi: 10.1037/0022-006X.74.3.445. [DOI] [PubMed] [Google Scholar]
- Wade SL, Carey J, Wolfe CR. The efficacy of an online cognitive-behavioral family intervention in improving child behavior and social competence following pediatric brain injury. Rehabilitation Psychology. 2006;51(3):179–89. [Google Scholar]
- Wade SL, Walz NC, Carey J, McMullen KM, Cass J, Mark E, et al. Effect on behavior problems of teen online problem-solving for adolescent traumatic brain injury. Pediatrics. 2011;128(4):e947–53. doi: 10.1542/peds.2010-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T, Greco P, Harris MA, Bubb J, White NH. Behavior therapy for families of adolescents with diabetes: maintenance of treatment effects. Diabetes Care. 2001;24:441–6. doi: 10.2337/diacare.24.3.441. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Miller KM, Greco P, Harris MA, Harvey LM, Taylor A, et al. Behavior therapy for families of adolescents with diabetes: effects on directly observed family interactions. Behavior Therapy. 1999;30:507–25. [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N, et al. Randomized trial of behavioral family systems therapy for diabetes: maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30:555–60. doi: 10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents' family relationships, treatment adherence, and metabolic control. Journal of Pediatric Psychology. 2006;31(9):928–38. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Randomized, controlled trial of behavioral family systems therapy for diabetes: maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy. 2008;39:33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]
Excluded studies
- Aleman Mendez S, Palacios AS. An integrated approach to the psychological features of the asthmatic child [Un adordaje integral de los aspectos psicologicos del nino asmatico] Allergologia et Immunopathologia. 1992;20:240–5. [PubMed] [Google Scholar]
- Anderson BJ, Ho J, Brackett J, Laffel LMB. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22(7):713–21. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Betancourt GP, Gutierrez de Pineres Scarpetta C. Psychological intervention pre-postsurgical program for cardiovascular pediatric patients. Saludarte. 2004;3(11):19–34. [Google Scholar]
- Braga L, Da Paz A, Junior, Ylvisaker M. Direct clinician-delivered versus indirect family-supported rehabilitation of children with traumatic brain injury: a randomized controlled trial. Brain Injury. 2005;19(10):819–31. doi: 10.1080/02699050500110165. [DOI] [PubMed] [Google Scholar]
- Bruzzese J, Unikel L, Gallagher R, Evans D, Colland V. Feasibility and impact of a school-based intervention for families of urban adolescents with asthma: results from a randomized pilot trial. Family Process. 2008;47(1):95–113. doi: 10.1111/j.1545-5300.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- Burke SO, Handley-Derry MH, Costello EA, Kauffmann E, Dillon MC. Stress-point intervention for parents of repeatedly hospitalized children with chronic conditions. Research in Nursing & Health. 1997;20(6):475–85. doi: 10.1002/(sici)1098-240x(199712)20:6<475::aid-nur2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Burke SO, Harrison MB, Kauffmann E, Wong C. Effects of stress-point intervention with families of repeatedly hospitalized children. Journal of Family Nursing. 2001;7(2):128–58. [Google Scholar]
- Cakan N, Ellis DA, Templin T, Frey M, Naar-King S. The effects of weight status on treatment outcomes in a randomized clinical trial of multisystemic therapy for adolescents with type 1 diabetes and chronically poor metabolic control. Pediatric Diabetes. 2007;8:206–13. doi: 10.1111/j.1399-5448.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- Canino G, Vila D, Normand ST, Acosta-Perez E, Ramirez R, Garcia P, et al. Reducing asthma health disparities in poor Puerto Rican children: the effectiveness of a culturally tailored family intervention. Journal of Allergy & Clinical Immunology. 2008;121(3):665–70. doi: 10.1016/j.jaci.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JC, Wade SL, Wolfe CR. Lessons learned: the effect of prior technology use on web-based interventions. Cyber Psychology & Behavior. 2008;11(2):188–95. doi: 10.1089/cpb.2007.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff RG, Ireys HT, DeVet KA, Kim YJ. A randomized, controlled trial of a community-based support program for families of children with chronic illness: pediatric outcomes. Archives of Pediatrics and Adolescent Medicine. 2002;156(6):533–9. doi: 10.1001/archpedi.156.6.533. [DOI] [PubMed] [Google Scholar]
- Chiang L, Ma W, Huang J, Tseng L, Hsueh K. Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial. International Journal of Nursing Studies. 2009;46(8):1061–70. doi: 10.1016/j.ijnurstu.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Templin T, Frey MA, Cunningham PB. Improving health outcomes among youth with poorly controlled type 1 diabetes: the role of treatment fidelity in a randomized clinical trial of multisystemic therapy. Journal of Family Psychology. 2007;21(3):363–71. doi: 10.1037/0893-3200.21.3.363. [DOI] [PubMed] [Google Scholar]
- Ellis D, Naar-King S, Templin T, Frey M, Cunningham P, Sheidow A, et al. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: reduced diabetic ketoacidosis admissions and related costs over 24 months. Diabetes Care. 2008;31(9):1746–7. doi: 10.2337/dc07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the national cooperative inner-city asthma study. Journal of Pediatrics. 1999;135(3):332–8. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- Field T, Henteleff T, Hernandez-Reif M, Martinez E, Mavunda K, Kuhn C, et al. Children with asthma have improved pulmonary functions after massage therapy. Journal of Pediatrics. 1998;132(5):854–8. doi: 10.1016/s0022-3476(98)70317-8. [DOI] [PubMed] [Google Scholar]
- Forsander G, Persson B, Sundelin J, Berglund E, Snellman K, Hellstom R. Metabolic control in children with insulin-dependent diabetes mellitus 5y after diagnosis. Early detection of patients at risk for poor metabolic control. Acta Paediatrica. 1998;87:857–64. doi: 10.1080/080352598750013635. [DOI] [PubMed] [Google Scholar]
- *.Forsander G. Family attitudes to different management regimens in diabetes mellitus. Practical Diabetes International. 1995;12(2):80–5. [Google Scholar]
- Forsander GA, Sundelin J, Persson B. Influence of the initial management regimen and family social situation on glycemic control and medical care in children with type I diabetes mellitus. Acta Paediatrica International Journal of Paediatrics. 2000;89(12):1462–8. doi: 10.1080/080352500456651. [DOI] [PubMed] [Google Scholar]
- Forsander GA, Sundelin J. Comparison of two therapeutic regimes for diabetes-stricken children Social and mental resources of the family are often crucial for the prognosis [Två behandlingsregimer vid diabetesdebut hos barn jämförda: Familjens sociala och mentalaresurser avöbr ofta prognosen] Lakartidningen. 2001;89:5484–9. [PubMed] [Google Scholar]
- Sundelin JG, Forsander G, Mattson SE. Family-oriented support at the onset of diabetes mellitus: a comparison of two group conditions during 2 years following diagnosis. Acta Paediatrica International Journal of Paediatrics. 1996;85(1):49–55. doi: 10.1111/j.1651-2227.1996.tb13889.x. [DOI] [PubMed] [Google Scholar]
- Forsander G, Malmodin B, Eklund C, Persson B. Relationship between dietary intake in children with diabetes mellitus type I, their management at diagnosis, social factors, anthropometry and glycaemic control. Scandinavian Journal of Nutrition/Naringsforskning. 2003;47(2):75–84. [Google Scholar]
- Garbutt JM, Banister C, Highstein G, Sterkel R, Epstein J, Bruns J, et al. Telephone coaching for parents of children with asthma: impact and lessons learned. Archives of Pediatrics and Adolescent Medicine. 2010;164(7):625–30. doi: 10.1001/archpediatrics.2010.91. [DOI] [PubMed] [Google Scholar]
- Gerber W, Petermann F, Gerber-von Muller G, Dollwet M, Darabaneanu S, Niederberger U, et al. MIPAS-Family-evaluation of a new multi-modal behavioral training program for pediatric headaches: clinical effects and the impact on quality of life. Journal of Headache Pain. 2010;11:215–25. doi: 10.1007/s10194-010-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber WD, Muller GG, Petermann U, Niederberger U, Petermann F. Do behavioral medicine approaches have an effect on the quality of life and everyday competence of children suffering from chronic headaches? [Verbessern verhaltensmedizinische behandlungsstrategien die lebens-qualitat bei kindern mit chronischen kopfschmerzen?] Zeitschrift fur Klinische Psychologie und Psychotherapie. 2009;38(4):231–9. [Google Scholar]
- Gerber WD, Petermann F, Muller G, Niederberger U, Rentmeister B, Siniatchkin M, et al. MIPAS-family: development and evaluation of a behavioural medicine programme for the treatment of chronic paediatric headaches. Verhaltenstherapie. 2008;18(4):247–55. [Google Scholar]
- Giallo R, Gavidia-Payne S. Evaluation of a family-based intervention for siblings of children with a disability or chronic illness. AeJAMH (Australian e-Journal for the Advancement of Mental Health) 2008;7(2):1–13. [Google Scholar]
- Glang A, McLaughlin K, Schroeder S. Using interactive multimedia to teach parent advocacy skills: an exploratory study. Journal of Head Trauma Rehabilitation. 2007;22(3):196–203. doi: 10.1097/01.HTR.0000271121.42523.3a. [DOI] [PubMed] [Google Scholar]
- Gustafsson PA, Kjellman M, Cederblad M. Family therapy in the treatment of severe childhood asthma. Journal of Psychosomatic Research. 1986;30(3):369–74. doi: 10.1016/0022-3999(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Harris MA, Greco P, Wysocki T, White TH. Family therapy with adolescents with diabetes: a litmus test for clinically meaningful change. Families, Systems & Health. 2001;19:159–68. [Google Scholar]
- Haus BF, Thompson S. The effect of nursing intervention on a program of behavior modification by parents in the home. Journal of Psychiatric Nursing and Mental Health Services. 1976;14(8):9–16. [PubMed] [Google Scholar]
- Hernandez NE, Kolb S. Effects of relaxation on anxiety in primary caregivers of chronically ill children. Pediatric Nursing. 1998;24(1):51–6. [PubMed] [Google Scholar]
- Hommel KA, Hente EA, Odell S, Herzer M, Ingerski LM, Guilfoyle SM, et al. Evaluation of a group-based behavioral intervention to promote adherence in adolescents with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology. 2012;24(1):64–9. doi: 10.1097/MEG.0b013e32834d09f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–6. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. Journal of Pediatric Gastroenterology and Nutrition. 2000;31(1):47–51. doi: 10.1097/00005176-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Ireys HT, Sills EM, Kolodner KB, Walsh BB. A social support intervention for parents of children with juvenile rheumatoid arthritis: results of a randomized trial. Journal of Pediatric Psychology. 1996;21(5):633–41. doi: 10.1093/jpepsy/21.5.633. [DOI] [PubMed] [Google Scholar]
- Ireys HT, Chernoff R, DeVet KA, Kim Y. Maternal outcomes of a randomized controlled trial of a community-based support program for families of children with chronic illnesses. Archives of Pediatrics and Adolescent Medicine. 2001;155(7):771–7. doi: 10.1001/archpedi.155.7.771. [DOI] [PubMed] [Google Scholar]
- Jay SM, Elliott CH. A stress inoculation program for parents whose children are undergoing painful medical procedures. Journal of Consulting and Clinical Psychology. 1990;58(6):799–804. doi: 10.1037//0022-006x.58.6.799. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Whitt JK, Martin B. The effect of fantasy facilitation of anxiety in chronically ill and healthy children. Journal of Pediatric Psychology. 1987;12(2):273–84. doi: 10.1093/jpepsy/12.2.273. [DOI] [PubMed] [Google Scholar]
- Kamps JL, Rapoff MA, Roberts MC, Varela RE, Barnard M, Olson N. Improving adherence to inhaled corticosteroids in children with asthma: a pilot of a randomized clinical trial. Children's Health Care. 2008;37(4):261–77. [Google Scholar]
- Kaslow NJ, Collins MH, Rashid FL, Baskin ML, Griffith JR, Hollins L, et al. The efficacy of a pilot family psychoeducational intervention for pediatric sickle cell disease. Families, Systems, & Health. 2000;18(4):381–404. [Google Scholar]
- Kazak AE, Penati B, Boyer BA, Himelstein B, Brophy P, Waibel K, et al. A randomized controlled prospective outcome study of a psychological and pharmacological intervention protocol for procedural distress in pediatric leukaemia. Journal of Pediatric Psychology. 1996;21:615–31. doi: 10.1093/jpepsy/21.5.615. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Simms S, Alderfer MA, Rourke MT, Crump T, McClure K, et al. Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnose with cancer. Journal of Pediatric Psychology. 2005;30(8):644–55. doi: 10.1093/jpepsy/jsi051. [DOI] [PubMed] [Google Scholar]
- Ketchen B, Hazzard A, Lassiter S, Barber N, Armistead L, Mentz R, et al. STARBRIGHT world: a pilot study of a home-based sickle cell psychoeducational intervention. Children's Health Care. 2006;35(4):321–38. [Google Scholar]
- Klinnert MD, Liu AH, Pearson MR, Ellison M, Budhiraja N, Robinson JL. Short-term impact of a randomized multifaceted intervention for wheezing infants in low-income families. Archives of Pediatric Adolescent Medicine. 2005;159:75–82. doi: 10.1001/archpedi.159.1.75. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Liu AH, Pearson MR, Tong S, Strand M, Luckow A, et al. Outcome of a randomized multifaceted intervention with low-income families of wheezing infants. Archives of Pediatrics and Adolescent Medicine. 2007;161(8):783–90. doi: 10.1001/archpedi.161.8.783. [DOI] [PubMed] [Google Scholar]
- Kroner-Herwig B, Mohn U, Pothmann R. Comparison of biofeedback and relaxation in the treatment of pediatric headache and the influence of parent involvement on outcome. Applied Psychophysiology and Biofeedback. 1998;23(3):143–57. doi: 10.1023/a:1022267104369. [DOI] [PubMed] [Google Scholar]
- Kupfer J, Gieler U, Diepgen TL, Fartasch M, Lob-Corzilius T, Ring J, et al. Structured education program improves the coping with atopic dermatitis in children and their parents - a multicenter, randomized controlled trial. Journal of Psychosomatic Research. 2010;68(4):353–8. doi: 10.1016/j.jpsychores.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Lasecki K, Olympia D, Clark E, Jenson W, Heathfield LT. Using behavioral interventions to assist children with type 1 diabetes manage blood glucose levels. School Psychology Quarterly. 2008;23(3):389–406. [Google Scholar]
- Logan S. Emotionally focused therapy improves marital adjustment in parents of children with chronically ill children. Child: Care, Health and Development. 1997;23(6):479–80. doi: 10.1111/j.1365-2214.1997.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Mendez FJ, Belendez M. Effects of a behavioral intervention on treatment adherence and stress management in adolescent with IDDM. Diabetes Care. 1997;20(9):1370–5. doi: 10.2337/diacare.20.9.1370. [DOI] [PubMed] [Google Scholar]
- Nelson KA, Highstein GR, Garbutt J, Trinkaus K, Fisher E, Smith SR, et al. A randomized controlled trial of parental asthma coaching to improve outcomes among urban minority children. Archives of Pediatric Adolescent Medicine. 2011;165(6):520–6. doi: 10.1001/archpediatrics.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MG, Feldman L, Caballero F. Effects of a self-management educational program for the control of childhood asthma. Patient Education and Counseling. 1999;36:47–55. doi: 10.1016/s0738-3991(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Rasoli R, Etemadi A, Shafidabadi A, Delavar A. Comparing effectiveness of individual and marital emotionally focused intervention based on decreasing relationship distress of couples with chronically ill children. Journal of Family Research. 2008;3(3):683–96. [Google Scholar]
- Sanders MR, Rebgetz M, Morrison M, Bor W, Gordon A, Dadds M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. Journal of Consulting and Clinical Psychology. 1989;57(2):294–300. doi: 10.1037//0022-006x.57.2.294. [DOI] [PubMed] [Google Scholar]
- Sanders MR, Cleghorn G, Shepherd RW, Patrick M. Predictors of clinical improvement in children with recurrent abdominal pain. Behavioural and Cognitive Psychotherapy. 1996;24:27–38. [Google Scholar]
- Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of multifamily group intervention and parent simulation of diabetes. Journal of Pediatric Psychology. 1989;14(2):259–75. doi: 10.1093/jpepsy/14.2.259. [DOI] [PubMed] [Google Scholar]
- Scholten L, Willemen AM, Grootenhuis MA, Maurice-Stam H, Schuengel C, Last BF. A cognitive behavioral based group intervention for children with a chronic illness and their parents: a multicentre randomized controlled trial. BMC Pediatrics. 2011;11(65):1–8. doi: 10.1186/1471-2431-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberg CB, Flannery-Schroeder E, Plante W. Children with co-morbid recurrent abdominal pain and anxiety disorders: results from a multiple-baseline intervention study. Journal of Child Health Care. 2011;15(2):126–39. doi: 10.1177/1367493511401640. [DOI] [PubMed] [Google Scholar]
- Staab D, van Rueden U, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatric Allergy and Immunology. 2002;13(2):84–90. doi: 10.1034/j.1399-3038.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Bova C, Leung K, Trudeau A, Lee M, Gruppuso P. Social support to empower parents (STEP): an intervention for parents of young children newly diagnosed with type 1 diabetes. The Diabetes Educator. 2010;36(1):88–97. doi: 10.1177/0145721709352384. [DOI] [PubMed] [Google Scholar]
- Szczepanski R, Jaeschke R, Spindler T, Ihorst G, Forster J, ASEV Study Group Preschoolers' and parents' asthma education trial (P2AET) - a randomized controlled study. European Journal of Pediatrics. 2010;169(9):1051–60. doi: 10.1007/s00431-010-1173-z. [DOI] [PubMed] [Google Scholar]
- Wade SL, Walz NC, Carey J, Williams KM, Cass J, Herren L, et al. A randomized trial of teen online problem solving for improving executive function deficits following pediatric traumatic brain injury. Journal of Head Trauma Rehabilitation. 2010;25(6):409–15. doi: 10.1097/HTR.0b013e3181fb900d. [DOI] [PubMed] [Google Scholar]
- Walders N, Kercsmar C, Schluchter M, Redline S, Lester Kirchner H, Drotar D. An interdisciplinary intervention for undertreated pediatric asthma. Chest. 2006;129(2):292–9. doi: 10.1378/chest.129.2.292. [DOI] [PubMed] [Google Scholar]
- Walker JG, Johnson S, Manion I, Cloutier P. Emotionally focused marital intervention for couples with chronically ill children. Journal of Consulting and Clinical Psychology. 1996;64(5):1029–36. doi: 10.1037//0022-006x.64.5.1029. [DOI] [PubMed] [Google Scholar]
- Warner CM, Ludwig K, Sweeney C, Spillane C, Hogan L, Ryan J, et al. Treating persistent distress and anxiety in parents of children with cancer: an initial feasibility trial. Journal of Pediatric Oncology Nursing. 2011;28(4):224–30. doi: 10.1177/1043454211408105. [DOI] [PubMed] [Google Scholar]
- Wysocki T, McDonell K, Harris MA, Elder Danda CL, Greco P, Bubb J, et al. Social validity of support group and behavior therapy interventions for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 1997;22(5):635–49. doi: 10.1093/jpepsy/22.5.635. [DOI] [PubMed] [Google Scholar]
Studies awaiting classification
Ongoing studies
Other references
Additional references
- American Psychological Association. Publication Manual of the American Psychological Association. Washington: American Psychological Association; 2011. [Google Scholar]
- Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database of Systematic Reviews. 2012;(2) doi: 10.1002/14651858.CD001916.pub2. Art. No.: CD001916. [DOI] [PubMed] [Google Scholar]
- Armour TA, Norris SL, Jack L, Jr, Zhang X, Fisher L. The effectiveness of family interventions in people with diabetes mellitus: a systematic review. Diabetic Medicine. 2005;22:1295–305. doi: 10.1111/j.1464-5491.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106(3):529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Learning Theory. New Jersey: Prentice-Hall; 1977. [Google Scholar]
- Bandura A. Human agency in social cognitive theory. American Psychologist. 1989;44(9):1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Morsella E. The unconscious mind. Perspectives on Psychological Science. 2008;3(1):73–9. doi: 10.1111/j.1745-6916.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AR, Rush AJ, Shaw B, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- Bergin AE, Suinn RM. Individual psychotherapy and behavior therapy. Annual Review of Psychology. 1975;26:509–56. doi: 10.1146/annurev.ps.26.020175.002453. [DOI] [PubMed] [Google Scholar]
- Bronfenbreener U. The Ecology of Human Development: Experiments and Design and Nature. Massachusetts: Harvard University; 1979. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Committee on Publication Ethics. [accessed 22 march 2012];Code of conduct and best practice guidelines for journal editors. http://publicationethics.org/
- D'Zurilla TJ, Nezu . Social problem solving in adults. In: Kendall PC, editor. Advances in Cognitive-Behavioral Research and Therapy. New York: Academic Press; 1982. pp. 202–74. [Google Scholar]
- D'Zurilla TJ, Goldfried MR. Problem solving and behavior modification. Journal of Abnormal Psychology. 1995;78:107–26. doi: 10.1037/h0031360. [DOI] [PubMed] [Google Scholar]
- D'Zurilla TJ, Nezu AM. Problem-Solving Therapy: A Social Competence Approach to Clinical Intervention. New York: Springer-Verlag; 1999. [Google Scholar]
- D'Zurilla TJ, Nezu AM. A positive approach to clinical intervention. New York: Springer; 2007. Problem-solving therapy. [Google Scholar]
- National survey of children with special health care needs: current health conditions and functional difficulties. Data Resource Center for Child & Adolescent Health. 2010 [Google Scholar]
- Data's shameful neglect. 7261. Vol. 461. Nature; 2009. Nature; p. 145. [DOI] [PubMed] [Google Scholar]
- Drotar D. Editorial: Guidance for submission and review of multiple publications derived from the same study. Journal of Pediatric Psychology. 2010;35(3):225–30. [Google Scholar]
- Eccleston C, Palermo TM, Williams ACDC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD003968. Art. No.: CD003968. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Williams ACDC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD007407. Art. No.: CD007407. [DOI] [PubMed] [Google Scholar]
- Ersser SJ, Latter S, Sibley A, Satherley PA, Welbourne S. Psychological and educational interventions for atopic eczema in children. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD004054. Art. No.: CD000054. [DOI] [PubMed] [Google Scholar]
- Gilliom M, Shaw DS, Beck JE, Schonberg MA, Lukon JL. Anger regulation in disadvantaged preschool boys: strategies, antecedents, and the development of self-control. Developmental Psychology. 2002;38:222–35. doi: 10.1037//0012-1649.38.2.222. [DOI] [PubMed] [Google Scholar]
- Grey M. Interventions for children with diabetes and their families. Annual Review of Nursing Research. 2000;18(1):149–70. [PubMed] [Google Scholar]
- Haley J. Problem Solving Therapy. San Francisco: Jossey-Bass; 1976. [Google Scholar]
- Halfon N, Newackeck PW. Evolving notions of childhood chronic illness. JAMA. 2010;303(7):665–6. doi: 10.1001/jama.2010.130. [DOI] [PubMed] [Google Scholar]
- Harris MA, Freeman KA, Duke DC. Getting (the most) out of the research business: interventions for youth with T1 DM. Current Diabetes Report. 2010;10:406–14. doi: 10.1007/s11892-010-0142-2. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Lee T. Multisystemic treatment of serious clinical problems. In: Kazdin E, Weisz JR, editors. Evidence-based Psychotherapies for Children and Adolescents. Vol. 2003. New York: Guilford Press; pp. 301–24. [Google Scholar]
- Higgins JPT, Green S, editors. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011 Available from www.cochrane-handbook.org.
- Huertas-Ceballos AA, Logan S, Bennett C, Macarthur C. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD003014.pub2. Art. No.: CD03014. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Henry Holt and Company; 1980. [Google Scholar]
- Jordan AL, Eccleston C, Osborn M. Being a parent of the adolescent with complex chronic pain: an interpretative phenomenological analysis. European Journal of Pain. 2007;11:49–56. doi: 10.1016/j.ejpain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, van Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews. 2010;(3) doi: 10.1002/14651858.CD004015. Art. No.: CD004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S (1992-2004) Cancer. 2007;112(2):416–32. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: an investigation of moderating effects on the pathway from pain to disability. Journal of Pediatric Psychology. 2005;30(8):698–707. doi: 10.1093/jpepsy/jsj060. [DOI] [PubMed] [Google Scholar]
- McBroom LA, Enriquez M. Review of family-centered interventions to enhance the health outcomes of children with type 1 diabetes. The Diabetes Educator. 2009;35(3):428–38. doi: 10.1177/0145721709332814. [DOI] [PubMed] [Google Scholar]
- McDougall J, King G, de Wit D, Miller LT, Honh S, Offord DR, et al. Chronic physical health conditions and disability among Canadian school-aged children: a national profile. Disability and Rehabilitation. 2004;26(1):35–45. doi: 10.1080/09638280410001645076. [DOI] [PubMed] [Google Scholar]
- Minuchin S. Families & Family Therapy. Massachusetts: Harvard University; 1974. [Google Scholar]
- Nock MK, Kazdin AE. Parent expectancies for child therapy: assessment and relation to participation in treatment. Journal of Child and Family Studies. 2001;10(2):155–80. [Google Scholar]
- Pai ALH, Drotar D, Zebracki K, Moore M, Youngstorm E. A meta-analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. Journal of Pediatric Psychology. 2006;31(9):978–88. doi: 10.1093/jpepsy/jsj109. [DOI] [PubMed] [Google Scholar]
- Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. Developmental and Behavioral Pediatrics. 2000;21(1):58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119:1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an internet delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146:205–13. doi: 10.1016/j.pain.2009.07.034. 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Eccleston C. Parents of children and adolescents with chronic pain. Pain. 2009;146(1-2):15–7. doi: 10.1016/j.pain.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JM, Bloom SR, Gortmaker SL. The increase of childhood chronic conditions in the United States. JAMA. 2007;297(24):2755–9. doi: 10.1001/jama.297.24.2755. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- Robin AL, Foster SL. Negotiating Parent Adolescent Conflict: A Behavioral-family Systems Approach. New York: Guilford; 1989. [Google Scholar]
- Sansom-Daly UM, Peate M, Wakefield CE, Bryant RA, Cohn RJ. A systematic review of psychological interventions for adolescents and young adults living with chronic illness. Health Psychology. 2011 doi: 10.1037/a0025977. Advance online publication:1-15. [10.1037/a0025977] [DOI] [PubMed] [Google Scholar]
- Savage E, Beirne PV, Ni Chroinin M, Duff A, Fitzgerald T, Farrell D. Self-management education for cystic fibrosis. Cochrane Database of Systematic Reviews. 2011;(7) doi: 10.1002/14651858.CD007641. Art. No.: CD007641. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, for the CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:698–702. [Google Scholar]
- Shapiro B. Building bridges between body and mind: the analysis of an adolescent with paralyzing chronic pain. International Journal of Psychoanalysis. 2003;84:547–61. doi: 10.1516/002075703766644607. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Science and Human Behaviour. Toronto: The Macmillan Company; 1953. Chapter 5: Operant behavior. [Google Scholar]
- Soo C, Tate R. Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD005239.pub2. Art. No.: CD005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623–30. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, Mokkink LB, Grootenhuis MA, Heymans HS, Offringa M. Definitions and measurement of chronic health conditions in childhood. JAMA. 2007;297(24):2741–51. doi: 10.1001/jama.297.24.2741. [DOI] [PubMed] [Google Scholar]
- Wegner DM. Ironic processes of mental control. Psychological Review. 1994;101(1):34–52. doi: 10.1037/0033-295x.101.1.34. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Library Cataloguing-in-Publication Data. 2011. World Health Statistics. [Google Scholar]
- Wicherts JM, Borsboom D, Kats J, Molenaar D. The poor availability of psychological research data for reanalysis. American Psychologist. 2006;61(7):726–8. doi: 10.1037/0003-066X.61.7.726. [DOI] [PubMed] [Google Scholar]
- Wicherts JM, Bakker M, Molenaar D. Willingness to share research data is related to the strength of the evidence and quality of reporting of statistical results. PLoS ONE. 2011;6(11):e26828. doi: 10.1371/journal.pone.0026828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Association of Medical Editors. [accessed 22 March 2012];Publication ethics policies for medical journals. http://www.wame.org.
- Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- Yates SL, Morley S, Eccleston C, Williams A. A scale for rating the quality of psychological trials for pain. Pain. 2005;117:314–25. doi: 10.1016/j.pain.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Yorke J, Shuldham C. Family therapy for asthma in children. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD003272.pub2. Art. No.: CD003272. [DOI] [PMC free article] [PubMed] [Google Scholar]