Abstract
A male advantage in spatial abilities is predicted to evolve in species where males rely on expansion of home territory to locate dispersed mates during the breeding season. We sought to examine mechanistic underpinnings of this evolved trait by comparing spatial navigational abilities in two species of Peromyscus that employ widely different reproductive strategies. Males and females from outbred stocks of deer mice (P. maniculatus bairdii) in which males engage in territorial expansion and mate search and California mice (P. californicus insignis), in which males do not, were administered tasks that assessed spatial learning and memory, and activity and exploratory behaviours. The maze employed for these studies included four spatial cues that could be used to aid in locating 1 of 12 potential escape holes. As predicted, male deer mice outperformed conspecific females and California mice males in maze performance and memory, and this difference appeared to be due to extent to which animals used spatial cues to guide maze navigation. Consistent with territorial expansion as a component of competition for mates, male deer mice were more active and engaged in more exploratory and less anxiety-related behaviours than conspecific females and California mice males. The results have implications for understanding and studying the cognitive and behavioural mechanisms that have evolved through male-male competition that involves territorial expansion and mate search.
Keywords: California mice, deer mice, spatial learning and memory, search strategy, sexual selection
Sex differences in spatial ability are predicted to evolve in mating systems where males and females differ in home range size during the breeding season, but these sex differences will be minimal in mating systems in which sexually mature males and females share a home range (Gaulin 1992). Comparative studies with various species of Microtus support this hypothesis (Gaulin & FitzGerald 1986, 1989). During the breeding season, male meadow voles (M. pennsylvanicus) expand their home range four- to five-fold and compete by searching for multiple females that are distributed in the habitat (Baird & Birney 1982; Stickel 1968). Home range expansion is considered to be a feature of sexual selection and male-male competition for meadow voles because the sex difference in range size does not appear until puberty and disappears during the non-breeding season (Madison & McShea 1987; Wolff 1989). Additionally, these behaviours may yield a reproductive benefit for males (Spritzer, Meikle & Solomon 2004; Spritzer, Solomon & Meikle 2005). Male prairie (M. ochrogaster) and woodland voles (M. pinetorum, previously referred to as the pine vole), in contrast, do not expand their home range during the breeding season but rather share overlapping home ranges of similar size with their mate. As predicted, field and laboratory studies demonstrate that male meadow voles have enhanced spatial ability relative to conspecific females and male prairie and woodland voles, with no sex differences evident in the two latter species (Gaulin & FitzGerald 1986, 1989).
Studies to date have largely confirmed that sex differences in home range size in natural settings correspond to sex differences for a variety of laboratory measures, including maze tasks designed to measure spatial learning (Galea, Kavaliers & Ossenkopp 1996; Galea et al. 1994; Williams, Barnett & Meck 1990). Maze learning performance is typically assessed by measuring latency to reach the escape hole, path length, errors (i.e., entering a blind hole), and search strategy. The three broad categories of search strategy may be considered as 1) random/mixed that is characterized by unorganized hole searches separated by multiple crossings of the maze center before locating the escape hole; 2) serial/thigmotaxic that is characterized by moving along the periphery of the maze in a clockwise or counter-clockwise direction and visiting at least two blind holes prior to locating the escape hole, and 3) direct that involves moving directly to the quadrant within which the escape hole is located and either immediately entering this hole or moving to an adjacent blind hole prior to entering the correct hole. Predictably, animals employing a direct search strategy demonstrate shorter latencies and path lengths and fewer errors in comparison to animals using random or serial strategies (Harrison et al. 2006; Jasarevic et al. 2011; Mueller & Bale 2007; Rodriguez et al. 2010).
For many of these laboratory maze tasks, males exhibit shorter latencies and path lengths, commit fewer errors, and acquire direct search strategies more rapidly than females (e.g., Galea et al. 1996; Galea et al. 1994; Jasarevic et al. 2011; Rodriguez et al. 2010), although these sex differences are absent in many inbred strains of mice (O'Leary, Savoie & Brown 2011). Direct assessment of the relation between these sex differences and the home range hypothesis (Gaulin, 1992) has been confounded by the use of different tasks across studies and species. In the present study, we used a comparative approach and tested the hypothesis that the male advantage in spatial ability associated with home range expansion is due to their more efficient use of spatial cues to guide navigation. We also tested the hypothesis that home range expansion will be associated with higher activity levels, increased exploratory behaviour, and decreased anxiety-like behaviour.
The genus Peromyscus provides valuable animal models for testing these predictions because it includes closely related species that in response to environmental pressures have evolved the full range of mammalian mating systems (Dewsbury, 1981). Similar to M. pennsylvanicus, polygynous deer mice (P. maniculatus bairdii) males expand their home range during the breeding season to search for mates. In contrast, monogamous California mice males (P. californicus insignis) do not expand their home range but rather exhibit overlapping territories and pair with a single conspecific female (Ribble & Salvioni 1990; Stickel 1968; Wolff 1989). Laboratory studies have confirmed a male advantage in spatial abilities in polygynous Peromyscus species (Galea et al. 1996; Galea et al. 1994; Jasarevic et al. 2011), which only emerges during the breeding season (Pyter, Reader & Nelson 2005; Walton et al. 2011). In particular, male deer mice outperformed conspecific females in navigating to a submerged platform in the Morris water maze (Galea et al. 1996) and in spatial learning as assessed in a Barnes maze (Jasaveric et al., 2011). One possibility is that the males’ advantage was related to use of distant cues located outside the water maze to find the platform (Williams et al. 1990), although this strategy was not directly assessed. On the other hand, Bredy and colleagues (2004) determined that performance of male and female California mice in the Barnes maze did not differ, consistent with the prediction that shared ranges and a lack of male-male competition that involves range expansion will be associated with similar spatial abilities in males and females. Together, the findings suggest that the sex differences observed in deer mice are related to reproductive and not foraging strategies.
These combined results are consistent with the home range hypothesis (Gaulin, 1992), but use of contrasting methods among studies potentially weakens their comparative power. Herein, we report the first direct cross-species comparison of sex differences in spatial learning, activity levels, exploratory and anxiety-like behaviours for deer and California mice. Moreover, the use of the Barnes maze and automated tracking of escape strategy and error patterns, enabled inferences to be drawn about the relative use of spatial cues during spatial learning. If the home range hypothesis is correct, then: 1) Male deer mice will show decreases in latency, reduced number of errors and shorter path lengths to the escape hole, and a more direct search strategy than conspecific females in the Barnes maze. The males will also display a greater frequency of exploratory and overall activity levels and less anxiety-like behaviours in the Elevated Plus Maze (EPM). 2) No comparable sex differences will be detected for these measures in California mice. 3) Male deer mice will demonstrate an advantage on all of these measures relative to male California mice.
METHODS
Animals
Twenty-six (15 males and 11 females) adult deer mice (P. maniculatus bairdii) (60–100 days of age) and 21 (12 males and 9 females) adult California mice (P. californicus) (age 120–150 days) were obtained from the Peromyscus Genetic Stock Center at the University of South Carolina (Columbia, SC). These polygynous and monogamous species differ considerably in the timing of development and reproductive maturation; thus, the age of the animals at the time of testing corresponds to species differences in average age of sexual maturity (King 1968; Layne 1968). At these ages, California mice are substantially larger (44.36 ± 2.10 g, 44.39 ± 1.56 g for males and females, respectively) than deer mice (17.04 ± 0.24 g, 16.76 ± 0.33 g for males and females, respectively). From the time the animals were originally captured from the wild, each species has been carefully bred by the facility to maintain their outbred status, although duration of captivity has varied across these two species with the founder P. maniculatus bairdii and P. californicus captured in 1948 and 1987, respectively. All experiments were approved by University of Missouri Animal Care and Use Committee and performed in accordance with National Institutes of Health Animal Care and Use Guidelines.
Mice were housed in white polypropylene cages (27.8×7.5×13 cm) with a 16:8 h light: dark cycle (lights on at 0600 CST, lights off at 2200 CST) to simulate the breeding season, when sex differences are most apparent in P. maniculatus (Galea et al. 1996). The animals were maintained at a constant temperature and humidity (22 ± 3°C and 50 ± 10%, respectively), and provided ad libitum access to food (AIN-93G rodent diet supplemented with 7 % corn oil; Harlan Teklad, Indianapolis, IN) and filtered water (Jasarevic et al. 2011). Prior to behavioural testing, mice were maintained in same-species and same-sex housing conditions with no more than three siblings, and were singly housed for one week prior to behavioural testing to reduce any social housing effects (Holmes et al. 2000; Palanza, Gioiosa & Parmigiani 2001). All animals were gonadally intact.
Spatial learning and memory
The Barnes maze was used to test spatial learning and memory (Barnes 1979), but modified for Peromyscus, as described previously for deer mice (Jasarevic et al. 2011). This dryland, circular maze measures a rodent's ability to learn intra-maze spatial cues to escape the platform into a home cage (Barnes 1979). The animal is motivated to solve the maze by aversive stimuli, including bright lights and the recording of a natural predator.
The maze consisted of a circular platform (95-cm diameter) constructed of black polypropylene, with 12 escape-holes, one placed every 30° and surrounded by a 50-cm high black curtain barrier to prevent escape and viewing extra-maze cues, e.g., distal objects in the testing room that the animals could possibly employ to locate the target exit hole (Harrison et al. 2006). Although previous reports have shown that mice show a preference for using distal cues, even when proximal cues are present (Harrison et al. 2006), the former are less likely to remain constant during the course of training than the latter. The circular maze was elevated 100 cm above the floor on a polypropylene stand. A small polypropylene ramp was attached to the correct exit hole and led to the home cage of the animal. It was of the same color and texture as the blind exit holes such that it was indistinguishable from the remaining 11 holes from the center of the maze. Four spatial geometric cues (triangle, square, circle, and star) were placed at the same height (~10 cm) every 90° inside the maze wall. Three 100 watt lights (encased in aluminum shells) were suspended ~150cm above the platform to motivate the mice, to escape from the brightly lit open surface after release onto the platform. As pilot data indicated individual differences in habituation to bright lights, after 30 sec, a recording of an owl screech (Tyto alba) was included as an ecologically relevant aversive stimulus (as further detailed below).
Each mouse was assigned an escape-hole number, with hole numbers for consecutively tested mice alternated by 90°, i.e. 3, 6, 9, and 12, to eliminate odor cues. The escape box location remained constant for any individual mouse over all test trials. At the beginning of each training day, the maze was rotated 90° and disinfected with 70% ethanol to eliminate odor cues for consecutively tested mice, but the exit hole number and the positions of the spatial cues relative to the escape hole remained fixed for any individual animal across all acquisition trials and the probe trial. At the beginning of each test day, animals were transferred from the vivarium to the testing room 30 min prior to behavioural testing to minimize any confounding stress. All testing occurred in the light phase (between ~1200 and 1500 h CST), and animals were returned to the vivarium immediately after testing. Animals were provided two shaping trials followed by seven days of two-trial evaluations per day for 5 min (300 sec) each, with a 30-min inter-trial interval. A trial consisted of carefully placing the mouse in the center of the maze, but randomly relative to the location of the spatial cues, in an opaque starting box to allow the tracking system (described below) to detect the center body-point. The starting box was lifted, and a trial initiated once the mouse had begun to move in the maze. If the animal failed to enter the escape box within 5 min, the observer gently guided the animal to the escape-hole. A stimulatory light measuring 1200 lux (versus ~400 lux for vivarium room lighting) was used during all trials. If the animal failed to enter the exit hole within the first 30 sec of a trial (46% of trials), a recording of a barn owl was played to motivate predator avoidance and thus maze escape (Clarke 1983).
On day 10 of testing, 24 h after the last day of acquisition training, a 90 sec probe trial was also run to examine retention of spatial memory. This trial consisted of dividing the Barnes maze into 4 quadrants and blocking all 12 holes, including the target exit. The percentage of time spent in each quadrant was recorded, with the measure of interest being the time spent in the quadrant containing the original escape-hole.
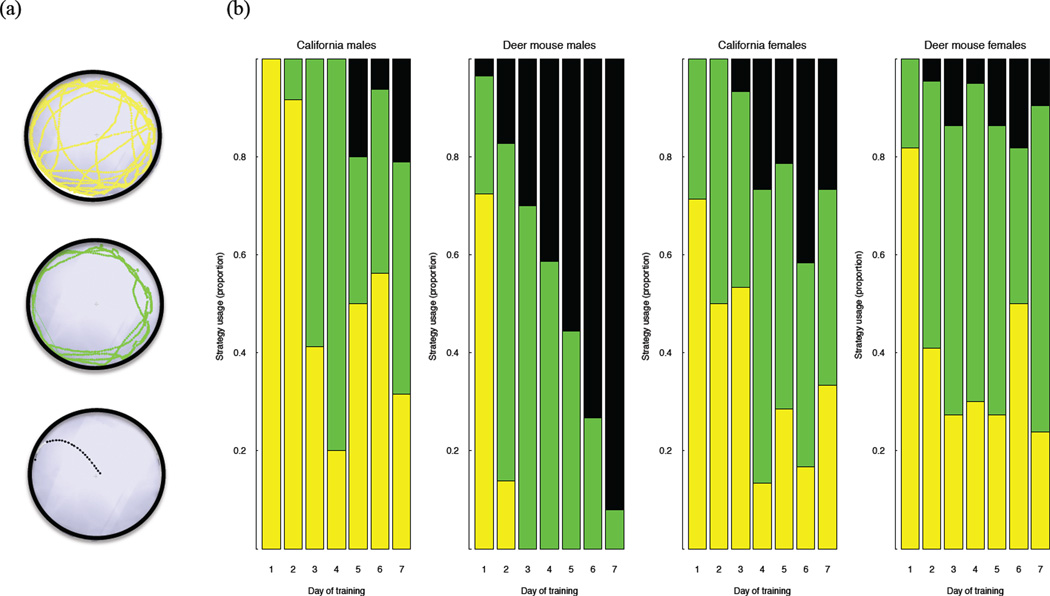
Each trial was recorded with an EthoVision XT video camera (Noldus Technologies, Leesburg, VA), and latency to enter escape-hole and path length was tracked by using accompanying automated tracking EthoVision XT software. Latency performance was averaged across trials on the same day for each individual. However, as noted, California mice are roughly three times the size of deer mice (Layne 1968), thereby, confounding the across-species latency measure. To control for species differences in body size, we focused on differences in path length, escape errors, and search strategy, which were quantified from the video recordings and tracking image composites produced by the EthoVision XT software, respectively. Fig. 1A illustrates the spatial strategies used in these assessments (Harrison et al. 2006; Jasarevic et al. 2011). The random search strategy (coded 1) was operationally defined as localized searches of holes separated by maze center crosses. Serial search strategy (coded 2) was defined as a systematic search of consecutive holes in a clockwise or counterclockwise direction. Finally, direct search strategy (coded 3) was defined as navigating directly to the target quadrant without crossing the center of the maze more than once and with three or fewer errors. Caution must be used, however, for this empirical classification of the direct strategy, because it is possible that animals that are dependent on a serial strategy will have some of their trials coded as direct, as, for example, would occur if the animal started the serial search by chance in the correct quadrant. In this situation, up to 25% of serial strategy trials could be incorrectly scored as a direct strategy. As a result, reliable use of the direct strategy was only attributed to groups (such as deer mice males) that consistently used this strategy on more than 25% of the trials.
Figure 1.
Barnes maze escape strategy of Peromyscus species with one that demonstrates sex differences in spatial navigation (deer mice) and one that does not (California mice). (A) Schematic diagram illustrating different navigational strategies used to locate correct exit hole: random (top), serial (middle) and direct (bottom). (B) Distribution of spatial strategies across sex, species, and day of training. On day 1 of acquisition training, most animals navigated by using a random strategy (black), and on subsequent days, animals increased use of serial search (green). The most efficient direct search strategy (yellow) emerged when the animals began to use spatial intra-maze cues. Across acquisition training, deer mice males quickly adopted the direct search strategy; whereas few animals in the other groups did so.
The combination of trial-by-trial strategy and error information allowed for inferences to be made regarding whether and how the intra-maze spatial cues were used to aide in finding the correct escape-hole. Use of directional cues would entail finding the correct hole based on its location between two of the four intra-maze cues. If the animal never entered an incorrect hole on any given trial more than once, a completely random search strategy would, on average, result in 5.5 errors (11 incorrect holes/2). Therefore, the average number of errors for this strategy would be more than 5.5 if the animal entered the same incorrect hole more than once in the trial. A serial strategy would result in shorter latencies but the same error rate (5.5), presuming the animals were unable to use spatial cues to narrow the range of potential exit holes. If the animal was able to use spatial cues to narrow the search to 9, 6, or 3 escape-holes, the corresponding error rates (assuming incorrect holes are entered only once) would be 4.0, 2.5, and 1.5, respectively. Use of a single cue as a landmark would in effect serve as a positional cue and would result in an average of 0.5 errors for the eight exit holes adjacent to a cue and an average of 1.0 error for the four holes that were not adjacent to the cue. The overall error rate would then be 0.67. The position of the exit hole was necessarily constant for individual animals, as noted. However, these predictions should be useful when within-strategy errors rates are averaged over individuals within species by sex.
Exploratory and anxiety-like behaviour
One week after the animals were tested in the Barnes maze, their exploratory and anxiety-like behaviours were measured by using the elevated plus maze (EPM), as described previously (Fountain et al. 2008; Jasarevic et al. 2011). The EPM was constructed of black polypropylene in a plus configuration with two opposite open arms (30 cm), a middle platform (5 × 5 cm), and two opposing closed arms (30 cm). The maze was supported 100 cm above the floor by a stand constructed of polypropylene. Each animal was placed on the center of the platform and allowed to explore the maze for 300 sec. After each test, the apparatus was cleaned with 70% ethanol. Each trial was recorded with EthoVision XT software (Noldus Technologies, Leesburg, VA), which automatically scores total time spent in open and closed arms and number of closed and open arm entries and center entries. Arm entry was defined as both front paws and shoulders placed into the area. On the occasion an animal jumped off the maze, it was gently placed back in the center, and the trial was continued.
Statistical analysis
Barnes maze path length and errors were analyzed with a 2 (sex) × 2 (species) × 7 (day) repeated-measures ANCOVA, with trial and the proportion of time in closed and open arms and time spent immobile in the EPM as covariates. Latency to reach target exit hole was assessed separately for California and deer mice with a 2 (sex)×7 (day) repeated measures ANCOVA, with trial and the EPM variables as covariates. The discrete Barnes search strategy that was coded 1 (random), 2 (serial), or 3 (direct) was analyzed with a 2 (sex) × 2 (species) × 6 (day) logistic analysis. Day 1 was excluded from these analyses because there was only a single use of strategy 3 on this day, thus preventing accurate contrasts of the probability of using the different strategies. For days 2 to 7, the significant three way sex-by-species-by-day interaction was followed by 2 (sex) × 2 (species) logistic analyses for each day, with trial and the EPM variables as covariates. The outcome of interest was the probability of use of strategy 3 (direct strategy) and thus strategy 1 and strategy 2 (random and serial strategies, respectively) were combined in the final logistic analyses and contrasted with strategy 3 by using a binomial distribution. Mean number of errors associated with each of the three strategies (i.e., random, serial, and direct) was calculated and contrasted with predicted error rates by using one-sample t-tests (see legend to Table 2). Finally, the probe trial was analyzed with a 2 (sex) × 2 (species) ANCOVA, with trial and the EPM variables as covariates.
Table 2.
Error rates across species, sex and search strategy contrasted with predicted values for use of spatial cues by California mice and deer mice to narrow their search for the escape hole*
| Species | Sex | Number of trials |
Mean number of errors (SE) |
CI | Predicted value within CI? |
|||
|---|---|---|---|---|---|---|---|---|
| 5.5 | 4.0 | 2.5 | 1.5 | |||||
| Random strategy | ||||||||
| California | Female | 62 | 7.56 (0.56) | 6.44–8.69 | No | No | No | No |
| Male | 75 | 6.28 (0.49) | 5.30–7.26 | Yes | No | No | No | |
| Deer | Female | 61 | 8.33 (0.67) | 6.98–9.68 | No | No | No | No |
| Male | 27 | 8.85 (0.71) | 7.39–10.32 | No | No | No | No | |
| Serial strategy | ||||||||
| California | Female | 58 | 5.47 (0.51) | 4.44–6.50 | Yes | No | No | No |
| Male | 44 | 4.70 (0.41) | 3.88–5.53 | Yes | Yes | No | No | |
| Deer | Female | 76 | 4.50 (0.33) | 3.84–5.15 | No | Yes | No | No |
| Male | 103 | 3.40 (0.23) | 2.94–3.85 | No | No | No | No | |
| Direct strategy | ||||||||
| California | Female | 17 | 1.82 (0.35) | 1.09–2.56 | No | No | Yes | Yes |
| Male | 9 | 2.78 (0.66) | 1.25–4.30 | No | Yes | Yes | Yes | |
| Deer | Female | 14 | 1.71 (0.32) | 1.02–2.41 | No | No | No | Yes |
| Male | 95 | 1.55 (0.16) | 1.23–1.87 | No | No | No | Yes | |
A random search strategy would, on average, result in 5.5 errors, if the animal never entered the same hole more than once. A serial strategy would result in shorter latencies but the same error rate (5.5), presuming the animals were unable to use spatial cues to narrow the range of potential exit holes. If the animal used spatial cues to narrow the search to nine, six or three escape holes, the corresponding error rates would be 4.0, 2.5 and 1.5. A ‘Yes’ in one of the last four columns indicates that the error rate did not differ significantly from the predicted value (P > 0.05; i.e. the predicted value was within the confidence interval, CI, for the group), and a ‘No’ indicates that the rate was significantly different (P < 0.05).
The EPM variables were included as covariates to ensure that differences in activity level, anxiety-like and exploratory-related behaviours were not influencing performance on the Barnes maze. None of the EPM variables significantly correlated with strategy, path length, or probe variable data (Ps > 0.08) and thus were not included in the final reported analyses. Several effects emerged for latency and error, and thus the EPM variables were retained in the final analyses. Trial effects were not significant for any of the Barnes variables (Ps > 0.1472), except for strategy (P = 0.0003), which correlated with significant trial effects for days 2, 3, and 5 (Ps < 0.0433; trial was not significant for the remaining training days, Ps > 0.2207). The associated means (± standard error of the means, SEM) for sex and species were adjusted for the effects of the EPM variables and trial for analyses in which these variables were included. The proportion of total EPM time spent in open and closed arms and immobile, as well as total number of arm entries were submitted to a 2 sex × 2 (species) ANOVA.
RESULTS
Barnes Maze
Animals in all groups predominantly used a random search strategy on the first day of acquisition training (Fig. 1B). Following the first day, the significant day-by-sex-by-species effect (F5, 479 = 3.14, P = 0.0085) indicated the groups differed in the pattern of strategy use across training. From day 5 forward, deer mice males had a significantly higher probability of employing the direct search strategy (probabilities = 0.56 to 0.83) than deer mice females (probabilities = 0.09 to 0.15) and California mice males (probabilities = 0.06 to 0.21, Ps < 0.018, Table 1). California mice males and females did not differ in strategy use on any day (Ps > 0.1274).
Table 1.
Probability of California mice and deer mice using a direct search strategy to escape the Barnes maze
| Acquisition training |
Mice species |
Sex | Probability of direct search (%) |
t | df | P |
|---|---|---|---|---|---|---|
| Day 4 | California | Male | 0 | 0.04 | 25 | 0.9679 |
| Deer | Female | 5 | 2.42 | 24 | 0.0177 | |
| Deer | Male | 42 | ___ | |||
| Day 5 | California | Male | 19 | 2.41 | 25 | 0.0179 |
| Deer | Female | 12 | 2.97 | 24 | 0.0039 | |
| Deer | Male | 56 | ___ | |||
| Day 6 | California | Male | 6 | 3.16 | 25 | 0.0022 |
| Deer | Female | 15 | 3.39 | 24 | 0.0011 | |
| Deer | Male | 68 | ___ | |||
| Day 7 | California | Male | 21 | 3.88 | 25 | 0.0002 |
| Deer | Female | 9 | 4.30 | 24 | 0.0001 | |
| Deer | Male | 83 | ___ |
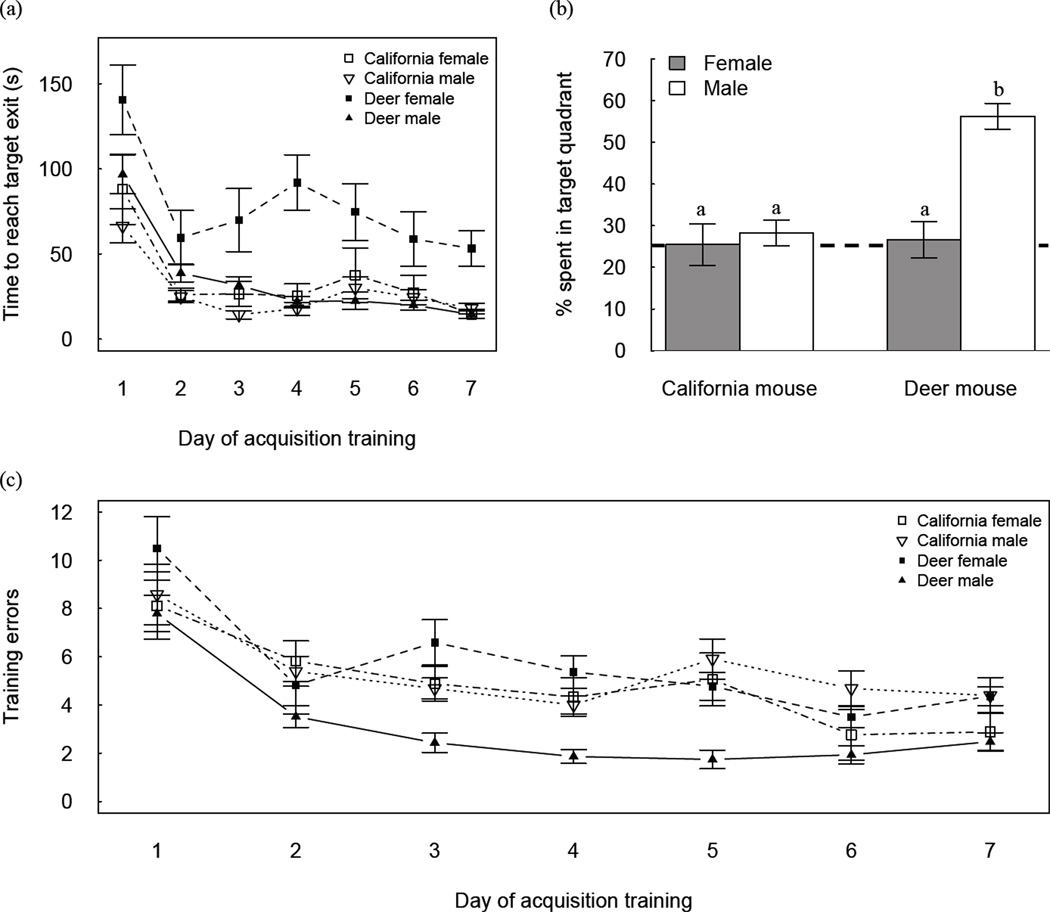
Consistent with the across-day shift in search strategy, latencies to reach the target exit hole decreased across days (Fig. 2A). Within-species comparisons confirmed male deer mice (X̄ = 33 ± 6.8 sec) were quicker than female deer mice (X̄ = 81 ± 8.1 sec) in locating the correct exit hole (F1, 46 = 17.47, P = 0.0001), and no overall sex difference was evident for California mice (F1, 32 = 0.41, P = 0.526; X̄ = 40 ± 6.5, 34 ± 6.1 sec for females and males, respectively).
Figure 2.
Sex-by-species differences in spatial learning and memory in the Barnes maze. (A) Latency to escape maze by day of acquisition training. Deer mice males were quicker to find the correct escape hole than deer mice females. (B) Percent time in the target quadrant during the 90 s probe trial test of spatial memory retention 24 h after the last day of acquisition training. (C) Sex-by-species differences in escape errors by day during the acquisition training in the Barnes Maze.
During the probe test of spatial memory retention (Fig. 2B), deer mice males spent 56% of their time in the quadrant of the correct exit hole (P < 0.0001, compared to 25% chance); whereas animals in the three other groups spent between 26% and 28% of their time in the correct quadrant, which does not differ from chance performance (Ps > 0.50). The corresponding sex-by-species interaction was significant (F1, 83 = 25.99, P = 0.0001), with deer mice males spending statistically significantly more time in the correct quadrant than deer mice females and California mice males (Ps < 0.001).
In terms of error rates, the sex (F1, 78 = 8.37, P = 0.0049) and sex-by-species (F1, 78 = 11.82, P = 0.0009) effects were significant (Fig. 2C). Deer mice males committed fewer overall errors (X̄ = 3.0 ± 0.45) than either deer mice females (X̄ = 5.4 ± 0.61, P = 0.0104) or California mice males (X̄ = 5.8 ± 0.42, P = 0.0001). The error rates of male and female (X̄ = 5.0 ± 0.50) California mice did not differ (P = 0.5945).
Mean number of errors, i.e. incorrect holes, associated with each of the three strategies, i.e., random, serial, and direct, were then contrasted with the predicted error rates for such strategies (Table 2). For example, a purely random search strategy would be expected to result in 5.5 errors, while a narrowing of the search to 9, 6, or 3 escape holes, would provide errors of 4.0, 2.5, and 1.5, respectively. During random searches, the number of errors committed by male California mice did not differ from the predicted 5.5 error rate, but the error rates for all other groups exceeded this value (Ps > 0.05), i.e. they made more errors than expected.
In serial searches, the mean number of errors committed by male and female California mice did not differ from 5.5, again indicating that the majority of these mice had been unable to use spatial cues to narrow the range of potential exit holes. Nevertheless, the confidence interval for male California mice and female deer mice encompassed the 4.0 value, suggesting that some narrowing of the search by some of the mice might have occurred. For the male deer mice, the error rate during serial searches fell between the 2.5 and 4.0, indicating that the animals had narrowed their search to between 6 and 3 escape holes.
Error rates for the direct search have to be interpreted with caution for the male and female California mice and the female deer mice, due to the small number of trials in which they used this strategy (Table 2). Moreover, a serial search could be miscoded as a direct search if, by chance, the animal had started the search in the quadrant of the escape hole. Accordingly, the potential for miscoding could occur in up to 25% of the trials, and only the male deer mice exceeded this threshold (42% of trials were coded as direct searches; P < 0.0001 relative to 25% chance). During direct searches, the error rates of deer mouse males did not differ from the predicted 1.5 value associated with use of spatial cues to narrow the search to one quadrant of the maze.
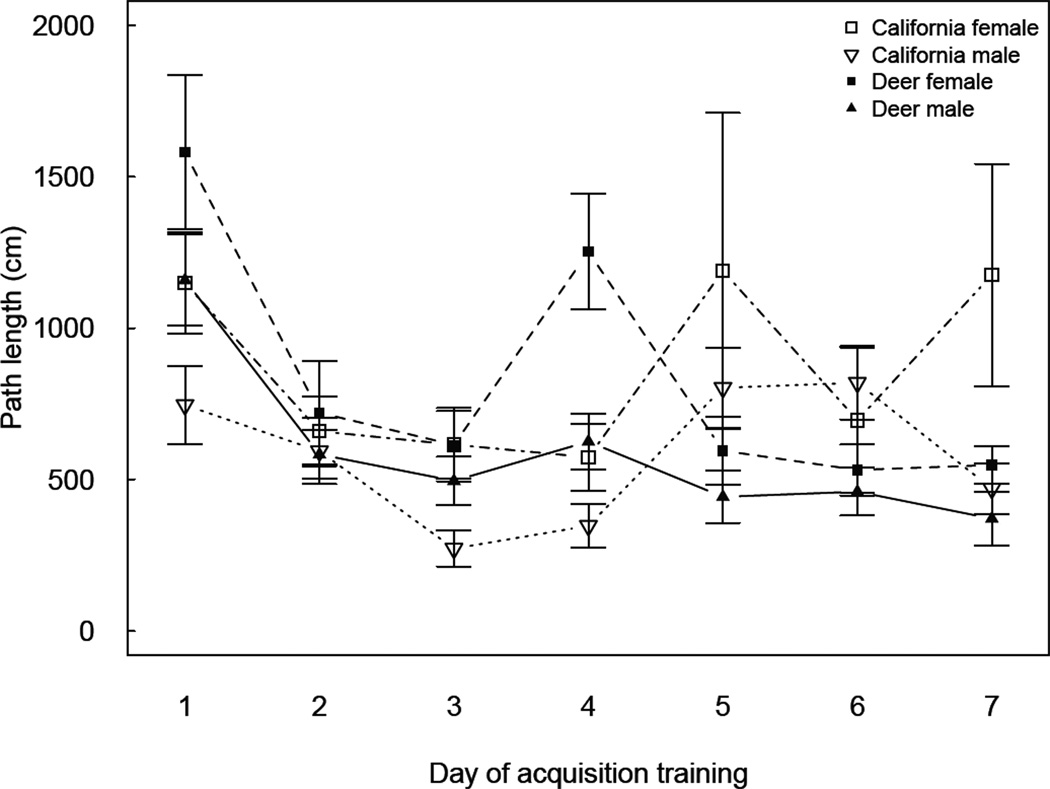
Across days of acquisition training, deer mice males had shorter path lengths than deer mice females (F1,46= 5.82, P = 0.0199, X̄ = 615 ± 75, 844 ± 83 cm for males and females, respectively), and significant shorter path lengths than California mice males on day 5 (X̄ = 454 ± 106, 887 ± 134 cm, t41 = 2.54, P = 0.0150) and 6 (X̄ = 442 ± 98, 815 ± 124 cm, t41 = 2.36, P = 0.0233) (Fig. 3). Across days, California mice males had shorter path lengths than California mice females (F1,26= 8.19, P = 0.0082, X̄ = 607 ± 94, 1123 ± 112 cm for males and females, respectively). The advantage of California mice males was statistically significant, however, on only two of the seven training days; day 1 and day 3 of acquisition training (t41 = 3.29, P = 0.0029; t41 = 2.17, P = 0.0397, respectively).
Figure 3.
Sex-by-species differences in Barnes maze path length. Deer mice males exhibited shorter path lengths than deer mice females across acquisition training (P<0.05), while no sex differences were observed for California mice.
Elevated Plus Maze (EPM)
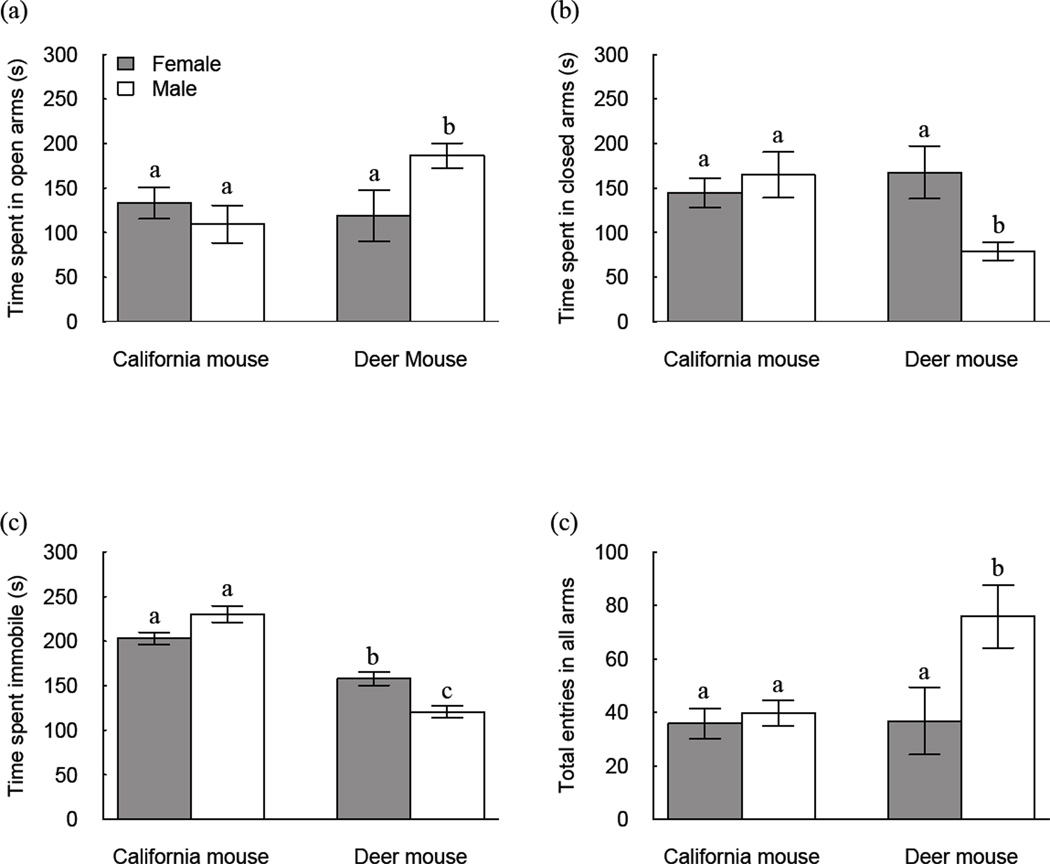
The EPM results revealed sex-by-species effects for proportion of time spent in open (F1,43 = 6.89, P = 0.012) and closed (F1,43 = 5.89, P = 0.0195) arms, as well as time spent immobile (F1,43 = 17.85, P < 0.0001). Deer mice males spent proportionately more of their time in open arms (X̄ = 64 ± 4.6 % of total time) than deer mice females (X̄ = 40 ± 9.5 %, P = 0.0048) and California mice males (X̄ = 37 ± 7.1 %, P = 0.0135) (Fig. 4). Deer mice males spent proportionally less time in closed arms (X̄ = 30 ± 4.4 % of total time) than deer mice females (X̄ = 56 ± 9.9 %, P = 0.0141) and California mice males (X̄ = 55 + 8.6 %, P = 0.013). Deer mice males spent less time immobile (X̄ = 118 ± 6.6 sec) than deer mice females (X̄ = 158 ± 7.5 sec, P = 0.0007) and California mice males (X̄ = 230 ± 9.2 sec, P < 0.0001). Deer mice males committed more total number of arm entries (X̄ = 76 ± 12.5 entries) than deer mice females (X̄ = 37 ± 12.5 entries, P = 0.0089) and California mice males (X̄ = 40 ± 4.8 entries, P = 0.0125). California mice males and females did not differ in terms of proportion of time spent in open and closed arms, or total number of arm entries (Ps < 0.2226), but females (X̄ = 201 ± 10.0 sec) spent less time immobile than males (P = 0.0409).
Figure 4.
Sex-by-species differences in exploratory (A, open arms) and anxiety-like (B, closed arms) behaviour, as well as time spent immobile (C) and total activity (D, total arm entries) in the Elevated Plus Maze (EPM). Means with different letter superscripts differ significantly (P < 0.05).
Elevated Plus Maze covariates for Barnes Maze Performance
The proportion of time spent in open and closed arms and immobile in the EPM was used as a covariate for Barnes maze performance. The only statistically significant correlations that emerged between the EPM and Barnes maze results were for Barnes maze error and latency. The proportion of time in spent in the open arms of the EPM was related to frequency of Barnes maze errors across days of acquisition training (F6, 468 = 2.73, P = 0.0129). Lower proportional time spent in the open arms was associated with fewer errors on day 4 (r93 = −0.37, P = 0.0003) and day 7 (r93 = −0.27, P = 0.0077). Proportion of time spent in closed arms of the EPM was also related to frequency of errors across day of training (F6, 468 = 2.53, P = 0.0205). Higher proportional time in closed arms was associated with increased number of errors on day 4 (r93 = 0.36, P = 0.0003) and day 7 (r93 = 0.28, P = 0.0057).
For California mice, the proportion of time spent immobile in the EPM interacted with latency across day of acquisition training (F6, 192 = 2.83, P = 0.0115). Higher proportional time spent immobile was associated with faster latencies on day 2 (r41 = −0.32, P = 0.0389), day 5, (r41 = −0.39, P = 0.0109), and day 6 (r41 = −0.33, P = 0.034). No other statistically significant Barnes maze latency effects correlated with the open, closed, or time spent immobile variables for either species (Ps > 0.087).
DISCUSSION
Our study provides the first side-by-side and sex-by-species comparisons for P. maniculatus bairdii and P. californicus insignis that enables a systematic testing of the home range hypothesis (Gaulin & FitzGerald 1986). It confirmed the overall predictions that sex differences in spatial ability and learning would be observed in deer mice, no such sex differences would be observed in California mice, and male deer mice would outperform male California mice. Male deer mice performed better on the Barnes maze, as observed by fewer errors, adoption of a direct search strategy during acquisition training, and better spatial memory during probe testing than conspecific females and California mice males. Consistent with our predictions, California mouse males and females did not differ amongst each other on these measures. The only exception was a potential male advantage for path length, but this was statistically significant for only two of the seven acquisition training days. The result may merit a follow up study to determine if a consistent sex difference exists for path length in California mice. The critical point though is that path length is only one component of maze learning and performance, and, for all other components analyzed, search strategy, errors, latencies, and probe-trial memory, California mouse males and females did not differ.
Moreover, our assessment of anxiety-like and exploratory-related behaviours and overall activity level on the EPM extended previous studies on spatial ability in the two species (Bredy et al. 2004; Galea et al. 1996; Galea et al. 1994). These measures were included to test the hypothesis that territorial expansion should, in addition to enhancing spatial ability, be associated with less anxiety-like behaviours and more exploratory-related behaviours in male deer mice as compared to female conspecifics and male California mice. We also predicted no sex differences for these measures in California mice. We confirmed these hypotheses and determined that the spatial learning and spatial memory results were not influenced by anxiety-like or exploratory-related behaviours.
Despite evidence that sex differences in spatial ability have evolved as a result of differences in size of the home range, it is not known whether the differences in spatial ability are performance related (i.e., latency, path length, or error), due to differences in spatial search strategies, or a combination of these parameters (Jonasson 2005; McCarthy & Konkle 2005). Our study indicates that sex differences in spatial learning are related to both performance and search strategy. During acquisition training, female deer mice spent more time and, as a group, demonstrated greater variability than males in locating the target exit hole. These females also committed more errors across training days and spent less time in the correct exit-hole quadrant on the probe trial, suggesting poorer memory retention for solving the maze. California mice males and females performed comparably to each other in terms of errors and time spent in the target quadrant, yet, male California mice were still able to locate the target exit hole about as efficiently as the male deer mice, a finding that we attribute to greater size of the California mice compared to deer mice, which confounded interpretation of latency measures.
Notably, sex-by-species differences in spatial strategy and search error rates emerged across training (Figs. 1 & 2), and these data permit inferences to be drawn about how the animals used intra-maze spatial cues to locate the target exit hole. During random searches, the error rates for animals in all of the groups were either consistent with the predicted random search error rate of 5.5 (California mice males) or exceeded this value (California mice females and deer mice males and females), suggesting these were truly random searches (Harrison et al. 2006). The higher values for random searches were likely due to certain animals entering the same incorrect hole more than once during some trials. Animals engaging in serial searches exhibited shorter latencies than those employing random searches, but the same error rate (5.5) is predicted if the animals were not employing intra-maze cues to narrow the search for the correct exit hole. The associated serial strategy error rates of male and female California mice were consistent with this prediction, although the error rate for California males was also within the range of that expected if intra-maze cues were used to narrow the search to 9 of the 12 exit holes. Future studies will be needed to determine if California mice males utilize intra-maze cues.
The associated serial search error rates of the female deer mice suggest that these animals may have been able to make some use of intra-maze cues to narrow the search to fewer than nine exit holes, but that this group was still not as effective as deer mice males, even when using the same search strategy. The error rates of deer mice males when they used the serial search strategy, in fact, suggested that they were beginning to use intra-maze cues to narrow their search to between nine (expected error rate of 4.0) and six (expected error rate of 2.5) exit holes. This outcome is not surprising if it is assumed that acquisition training leads to steady improvements in use of intra-maze cues rather than a sudden switch in categorical search strategy. The results for the direct search strategy were clear-cut and indicate a more rapid response, across-days, and overall improvement in maze learning for male deer mice compared to female deer mice and male California mice.
Critically, the frequency of use of the direct search strategy was higher than chance (i.e., 25% potential miscoding of serial searches) only for the deer mice males, and the associated error rate (1.55) was strongly consistent with use of intra-maze cues to narrow the search to one quadrant. This conclusion is further supported by their above chance performance on the probe trial and suggests that male deer mice use intra-maze cues in much the same way that male rats (Rattus norvegicus) can use extra-maze cues to guide maze escape or food search strategy (Cheng 2008; Cheng & Newcombe 2005; Rodriguez et al. 2010; Williams et al. 1990). The overall pattern supports the notion that deer mice males use intra-maze cues to guide navigation more effectively than conspecific females and California mice males. The deer mice males’ efficient use of intra-maze cues contributed to their advantages over conspecific females and California mice males in Barnes maze performance (e.g., in use of direct strategy).
The observed sex differences in spatial learning and memory corresponded with sex- and species-differences in activity levels and anxiety-like and exploratory-related behaviours (Fig. 4). Male deer mice exhibited higher overall activity levels, more exploratory behaviour, and less anxiety-like behaviour in the EPM than conspecific females and California mice males, with minimal sex differences for these parameters in California mice. These results are consistent with earlier findings of breeding season increases in open field activity for male meadow voles (M. pennsylvanicus) (Turner et al. 1983). Such increased activity levels and exploratory behaviours are necessary components of male-male competition that requires territorial expansion, and the reduced anxiety-like behaviours may be required to face the increased risk of predation these males would be subjected to while searching for prospective mates (Clarke 1983). Consequently, anxiety-like behaviours, as measured by time spent in the closed arms of the EPM, may be correlated with activities that reduce predation risk in the wild. In essence, given that female deer mice and male California mice do not gain reproductive benefits by territorial expansion, their lower activity levels and higher anxiety-like behaviour may reduce predation risks in natural settings.
Overall, our results demonstrate that male deer mice exhibit enhanced memory retention and greater use of spatial cues to guide search for the escape hole in the Barnes maze than conspecific females. Deer mice males also exhibited increased activity and exploratory behaviours, and less anxiety-like behaviours compared to deer mice females in the EPM. These sex differences likely evolved as components of the polygynous mating system of P. maniculatus bairdii and male-male competition that involves territorial expansion to search for multiple, widely dispersed prospective mates. Support for this hypothesis is strengthened by simultaneous assessment of the related P. californicus, a species in which males mate monogamously and do not expand their territory to search for additional mates (Gubernick & Teferi 2000). As hypothesized, the sex differences observed within deer mice were not evident in California mice, and deer mice males demonstrated the same advantages over California mice males that they had over deer mice females. The striking sex differences in deer mice as well as species differences between the males in spatial learning and anxiety-like behaviours supports the hypothesis that these behaviours have been shaped by sexual selection.
Highlights.
-
➢
We compared navigational abilities of polygynous deer mice and monogamous California mice.
-
➢
Male deer mice outperformed conspecific females and California mice in a Barnes maze.
-
➢
Performance of male deer mice was related to their ability to assimilate spatial clues.
-
➢
Our results provide insights into the evolution of sexually selected cognitive and behavioural traits.
Acknowledgements
We thank Stephen Cobb, Gregory M. Vandas, and Paizlee T. Sieli for assistance with animal husbandry, Mr. Wayne Shoemaker for constructing the behavior testing apparatuses, and three anonymous reviewers for helpful comments that improved the manuscript. This work was supported by a National Institutes of Health Challenge Grant RC1 ES018195 (to C.S.R.), a Mizzou Advantage grant (to C.S.R., D.CG. & R.M.R.), and support from Food for the 21th Century Program to RMR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal AF. Sexual selection and the maintenance of sexual reproduction. Nature. 2001;411:692–695. doi: 10.1038/35079590. [DOI] [PubMed] [Google Scholar]
- Andersson MB. Sexual Selection. Princteon: Princeton University Press; 1994. [Google Scholar]
- Andersson M, Simmons LW. Sexual selection and mate choice. Trends in Ecology & Evolution. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Baird DD, Birney EC. Characteristics of dispersing meadow voles Microtus pennsylvanicus . The American Midland Naturalist Journal. 1982;107:262–283. [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. Journal of Comparative and Physiological Psychology. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Batty ER, Hoban L, Spetch ML, Dickson CT. Rats' use of geometric, featural and orientation cues to locate a hidden goal. Behavioural Processes. 2009;82:327–334. doi: 10.1016/j.beproc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Hormones and Behavior. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Perrigo G. Seasonal regulation of reproduction in muroid rodents. American Zoologist. 1987;27 [Google Scholar]
- Chai XJ, Jacobs LF. Effects of cue types on sex differences in human spatial memory. Behavioural Brain Research. 2010;208:336–342. doi: 10.1016/j.bbr.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Cheng K, Newcombe NS. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychonomic Bulletin & Review. 2005;12:1–23. doi: 10.3758/bf03196346. [DOI] [PubMed] [Google Scholar]
- Cheng K. Whither geometry? Troubles of the geometric module. Trends in Cognitive Sciences. 2008;12:355–361. doi: 10.1016/j.tics.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Clarke J. Moonlight's influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behavioral Ecology and Sociobiology. 1983;13:205–209. [Google Scholar]
- Clutton-Brock T. Sexual selection in males and females. Science. 2007;318:1882–1885. doi: 10.1126/science.1133311. [DOI] [PubMed] [Google Scholar]
- Daly M, Wilson M. Sex, Evolution and Behavior. 2nd edn. Boston: Willard Grant; 1983. [Google Scholar]
- Darwin C. The Descent of Man, and Selection in Relation to Sex. New York: D. Appleton and company; 1871. [Google Scholar]
- Dewsbury DA. An exercise in the prediction of monogamy in the field from laboratory data on 42 species of muroid rodents. The Biologist. 1981;63:138–162. [Google Scholar]
- Fountain ED, Mao J, Whyte JJ, Mueller KE, Ellersieck MR, Will MJ, Roberts RM, Macdonald R, Rosenfeld CS. Effects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in mice. Biology of Reproduction. 2008;78:211–217. doi: 10.1095/biolreprod.107.065003. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Research. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. The Journal of Experimental Biology. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Perrot-Sinal T, Kavaliers M, Ossenkopp K-P. Relations of hippocampal volume and dentate gyrus width to gonadal hormone levels in male and female meadow voles. Brain Research. 1999;821:381–391. doi: 10.1016/s0006-8993(99)01100-2. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. Representations in animal cognition: an introduction. Cognition. 1990;37:1–22. doi: 10.1016/0010-0277(90)90016-d. [DOI] [PubMed] [Google Scholar]
- Gaulin SJ, FitzGerald RW. Sex differences in spatial ability: An evolutionary hypothesis and test. The American Naturalist. 1986;127:74–88. [Google Scholar]
- Gaulin SJ, FitzGerald RW. Sexual selection for spatial learning ability. Animal Behaviour. 1989;37:322–331. [Google Scholar]
- Gaulin SJC. Evolution of sex differences in spatial ability. Yearbook of Physical Anthropology. 1992;35:125–151. [Google Scholar]
- Gubernick DJ, Teferi T. Adaptive significance of male parental care in a monogamous mammal. Proceedings of the Royal Society: Biological sciences. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learning & Memory. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiology & Behavior. 2000;71:509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Gaulin SJ, Sherry DF, Hoffman GE. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Jr, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neuroscience and Biobehavioral Reviews. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- King JA. Psychology. In: King JA, editor. Biology of Peromyscus (Rodentia) Stillwater, OK: American Society for Mammologists; 1968. pp. 496–537. [Google Scholar]
- Layne JN. Ontogeny. In: King JA, editor. Biology of Peromyscus (Rodentia) Stillwater, OK: American Society for Mammologists; 1968. pp. 148–248. [Google Scholar]
- Madison DM, McShea WJ. Seasonal changes in reproductive tolerance, spacing and social organization in meadow voles: a microtine model. American Zoologist. 1987;27:899–908. [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Frontiers in Neuroendocrinology. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiology & Behavior. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- O'Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behavioural Brain Research. 2011;216:531–542. doi: 10.1016/j.bbr.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiology & Behavior. 2001;73:411–420. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- Ribble DO, Salvioni M. Social organization and nest co-occupancy in Peromyscus californicus, a monogamous rodent. Behavioral Ecology and Sociobiology. 1990;26:9–15. [Google Scholar]
- Rodriguez CA, Torres A, Mackintosh NJ, Chamizo VD. Sex differences in the strategies used by rats to solve a navigation task. Journal of Experimental Psychology. 2010;36:395–401. doi: 10.1037/a0017297. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Meikle DB, Solomon NG. The relationship between dominance rank and spatial ability among male meadow voles (Microtus pennsylvanicus) Journal of Comparative Psychology. 2004;118:332–339. doi: 10.1037/0735-7036.118.3.332. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Solomon NG, Meikle DB. Influence of scramble competition for mates upon the spatial ability of male meadow voles. Animal Behaviour. 2005;69:425–436. [Google Scholar]
- Stickel LF. Home range and travels. In: King JA, editor. Biology of Peromyscus. Stillwater: American Society of Mammalogists; 1968. [Google Scholar]
- Symons D. The evolution of human sexuality. New York: Oxford University Press; 1979. [Google Scholar]
- Turner B, Iverson S, Severson K. Seasonal changes in open-field behavior in wild male meadow voles (Microtus pennsylvanicus) Behavioral and Neural Biology. 1983;39:60–77. doi: 10.1016/s0163-1047(83)90637-4. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Sexual selection, social competition, and speciation. Quarterly Review of Biology. 1983;58:155–183. [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behavioral Neuroscience. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Wolff JO. Social Behavior. In: Kirkland GK, Layne JL, editors. Advances in the Study of Peromyscus (Rodentia) Lubbock: Texas Tech University Press; 1989. pp. 271–292. [Google Scholar]