Abstract
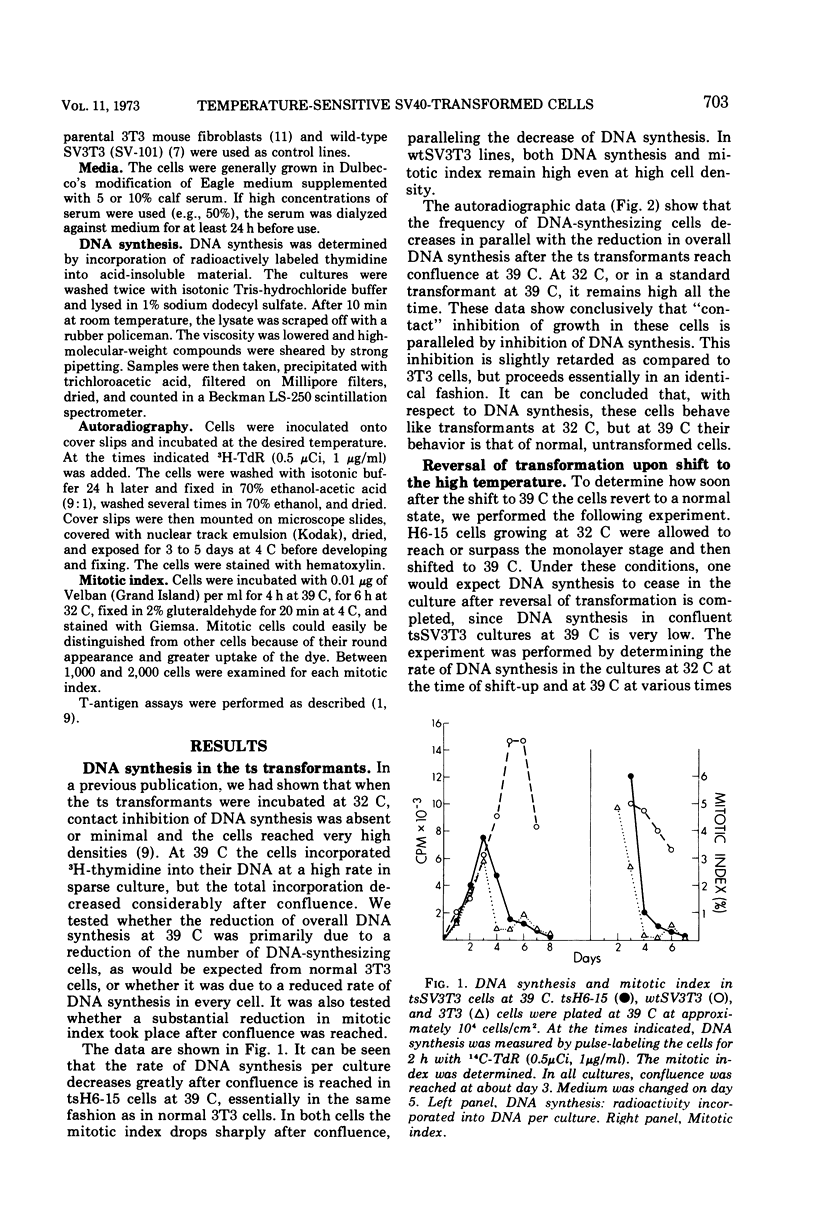
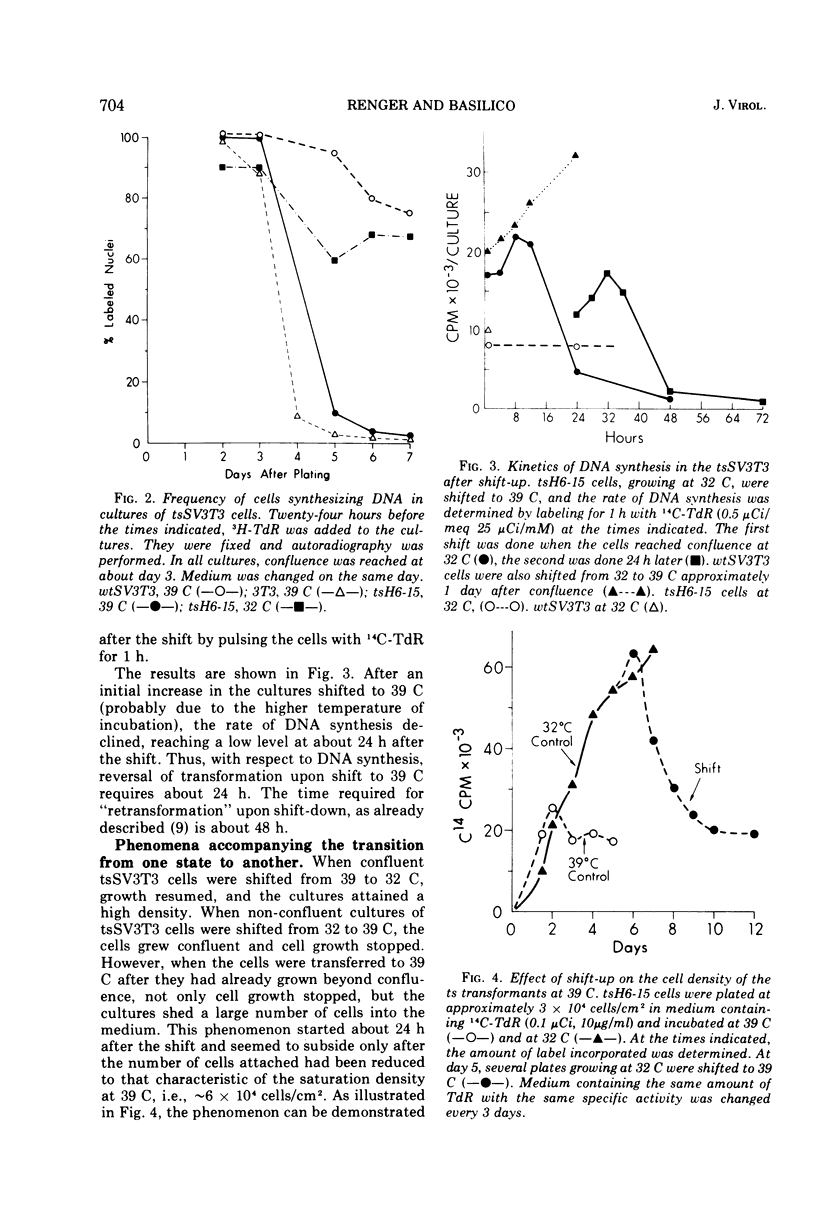
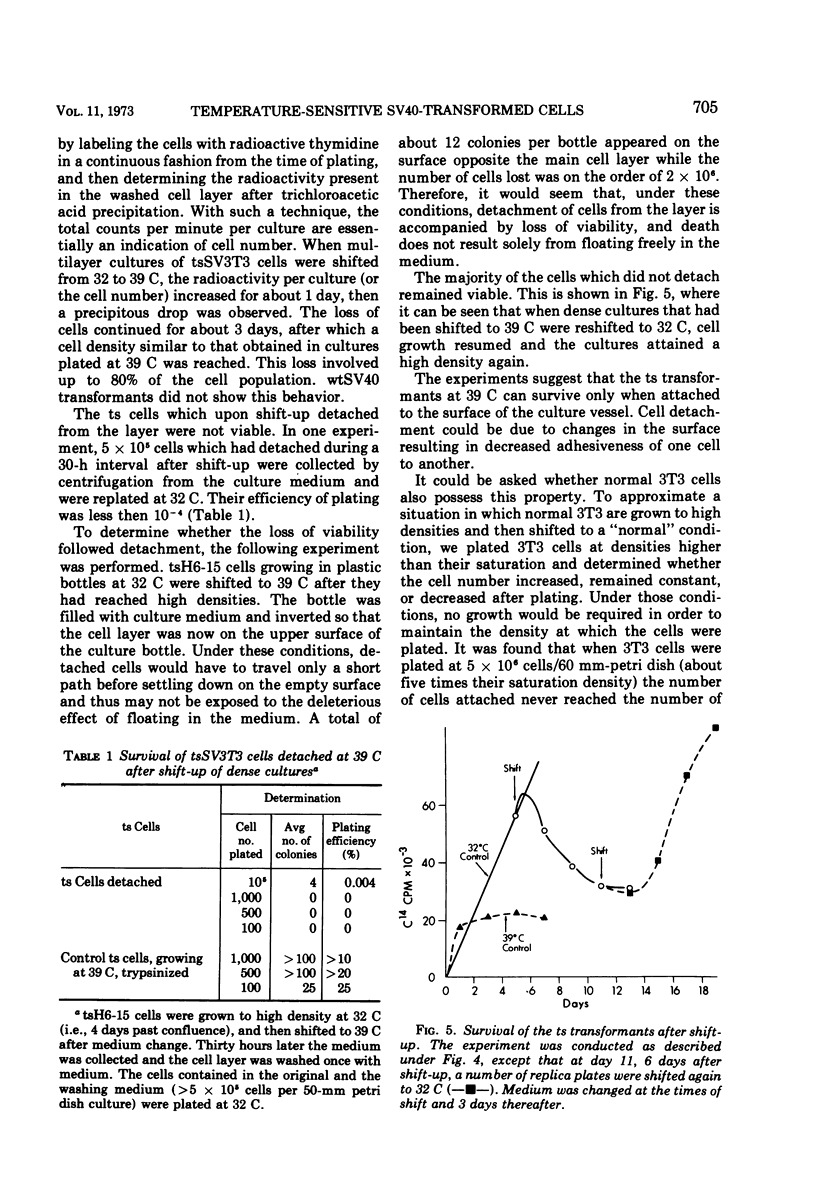
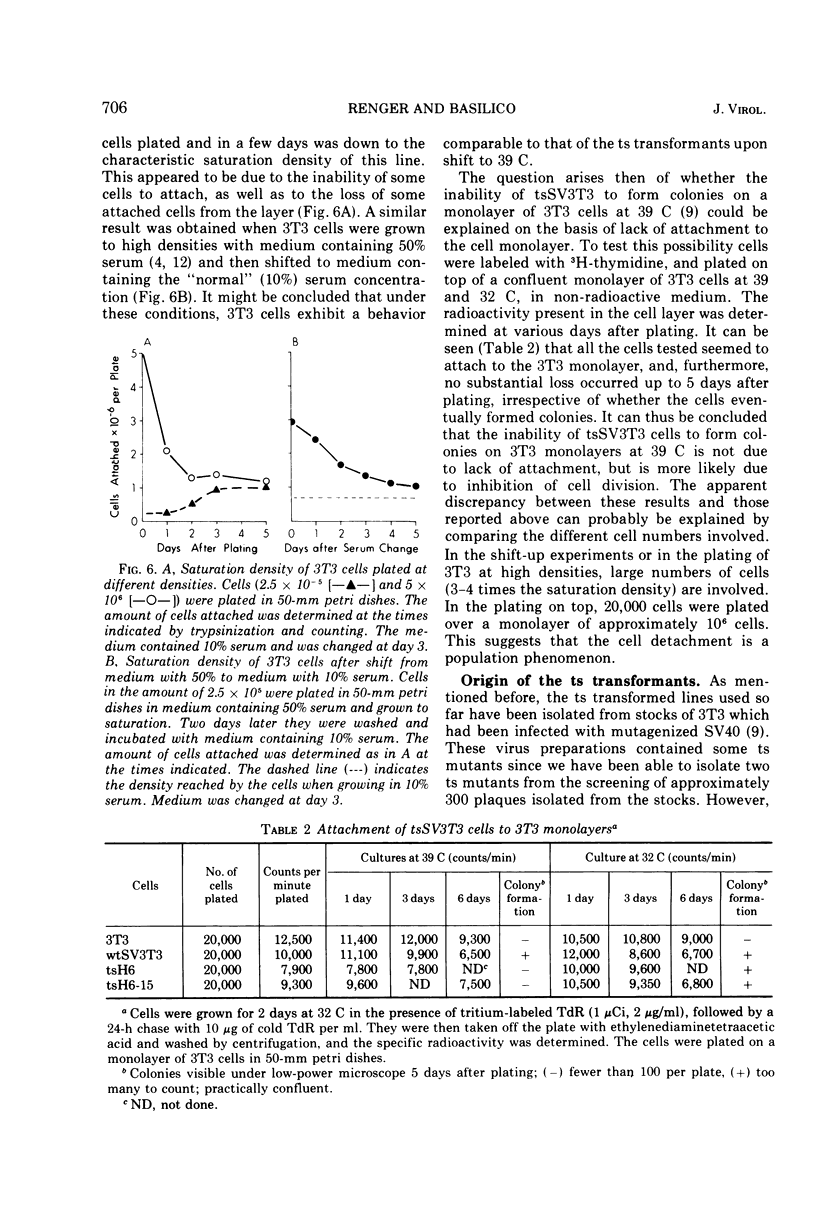
Temperature-sensitive simian virus (SV 40)-transformed 3T3 cells (tsSV3T3), which express the transformed phenotype when growing at 32 C but not at 39 C, were used to study changes in growth behavior during shift-up or shift-down experiments. In cultures of tsSV3T3 cells which had reached or were beyond monolayer density at 32 C, DNA synthesis reached very low levels within 24 to 48 h after shift-up. When cells which had been allowed to grow to high densities at 32 C were shifted to 39 C, not only cell growth stopped, but within two to three days the cultures shed a large number of cells into the medium. These cells were nonviable, and shedding stopped only when the number of cells attached had been reduced to that characteristic of the saturation density at 39 C. The remaining attached cells were viable and after the shift to 32 C were again able to grow from the monolayer to high cell densities. This behavior has been compared with that of normal 3T3 and wild-type SV3T3 cells under different conditions. We have also isolated new tsSV3T3 lines, using cells which had been infected with non-mutagenized wild-type SV40. This further demonstrates that the temperature sensitivity of these lines is due to a cellular rather than a viral mutation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Matsuya Y., Green H. The interaction of polyoma virus with mouse-hamster somatic hybrid cells. Virology. 1970 Jun;41(2):295–305. doi: 10.1016/0042-6822(70)90082-6. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Renger H. C., Basilico C., Burger M. M. Surface changes in temperature-sensitive Simian virus 40-transformed cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):347–349. doi: 10.1073/pnas.70.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C. Retransformation of ts SV40 transformants by murine sarcoma virus at non-permissive temperature. Nat New Biol. 1972 Nov 1;240(96):19–21. doi: 10.1038/newbio240019a0. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Nelson-Rees W. A. Direct isolation and characterization of "flat" SV40-transformed cells. Nat New Biol. 1971 Oct 27;233(43):263–265. doi: 10.1038/newbio233263a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G., Matsuya Y., Bloom S., Robbins A., Green H. Stimulation of RNA synthesis and cell division in resting cells by a factor present in serum. Wistar Inst Symp Monogr. 1967;7:87–101. [PubMed] [Google Scholar]


