Abstract
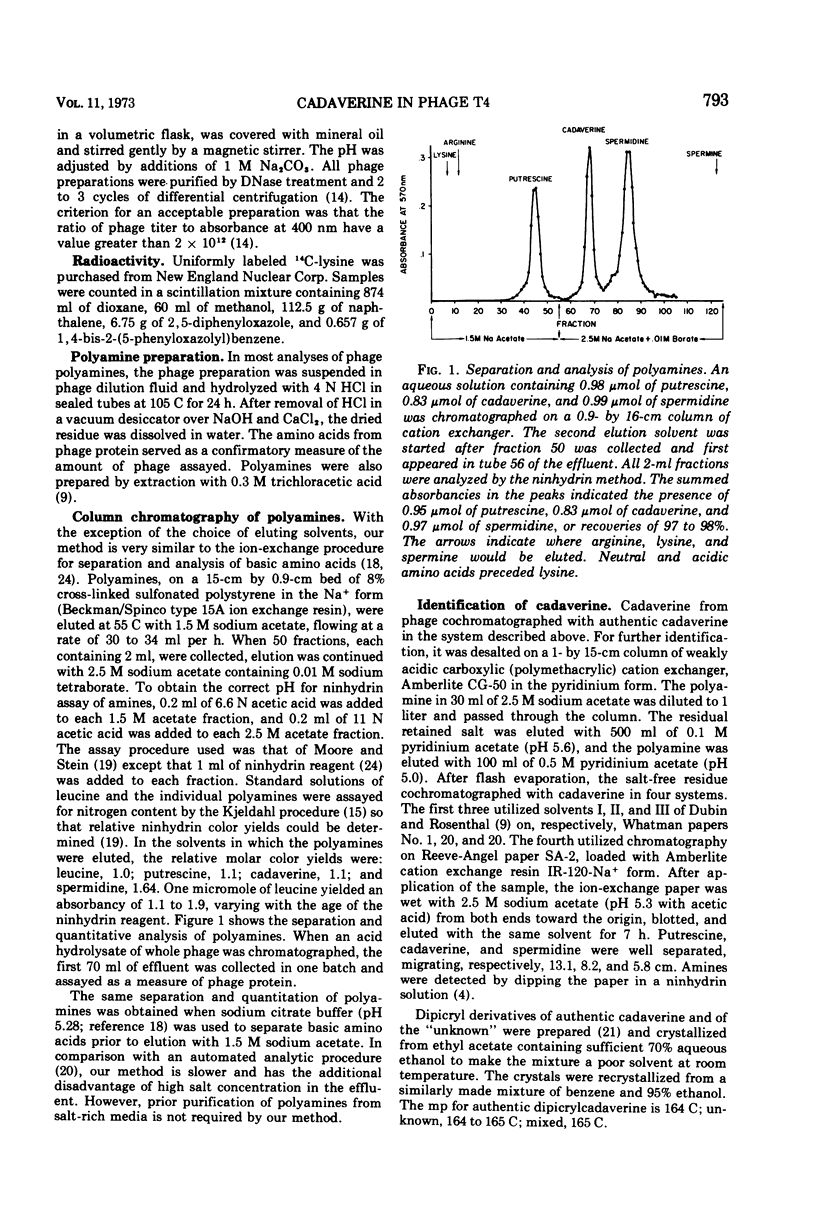
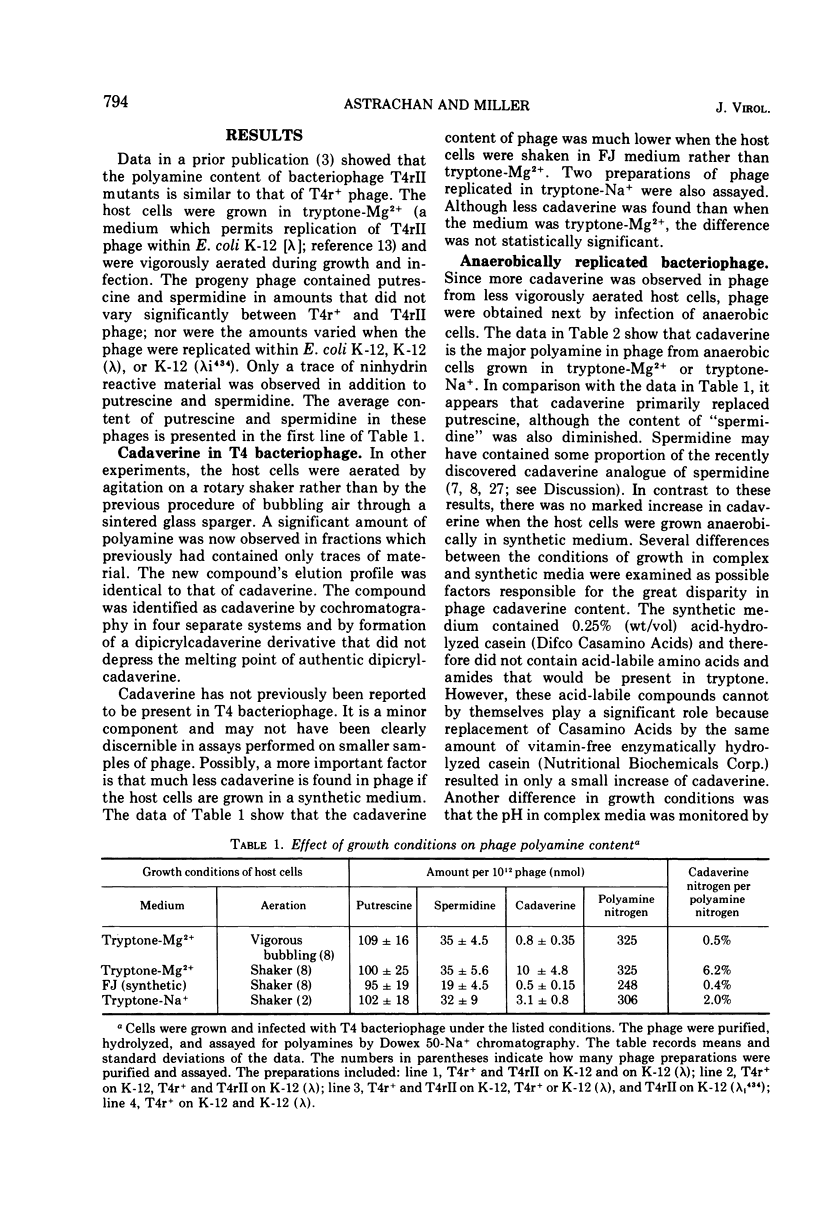
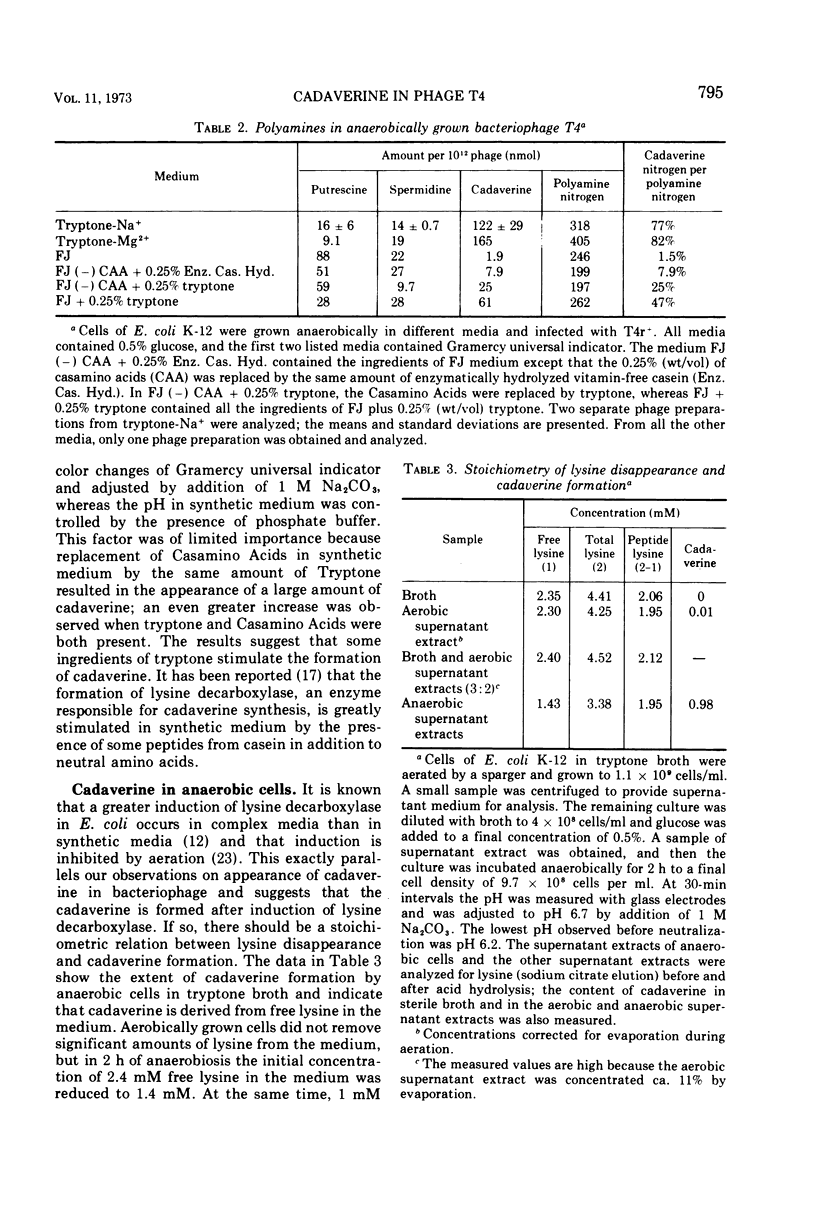
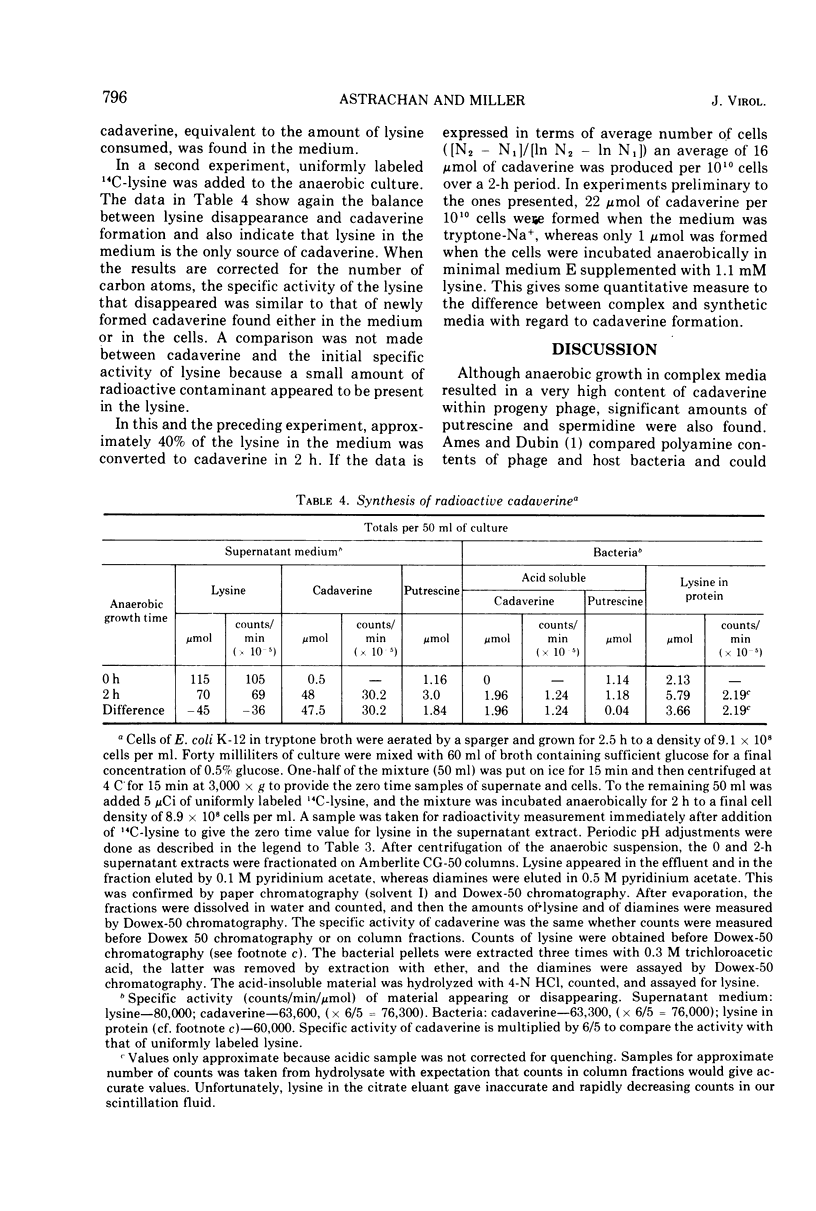
Cadaverine was found in bacteriophage T4 when the host cells of Escherichia coli K-12 were grown in complex media and aerated by agitation. Only traces of cadaverine were found if the host was grown and agitated in synthetic medium or was aerated by vigorous bubbling in a complex medium. When the host cells were grown anaerobically in a complex medium, cadaverine became the major polyamine in the progeny phage. The polyamine content comprised 80% cadaverine, 14% spermidine (or its recently discovered homologue, N-3-aminopropyl-1, 5-diaminopentane), and the remainder putrescine. The conditions that favored appearance of cadaverine are known to be required for induction of lysine decarboxylase. It was shown that lysine was the sole source of bacterial cadaverine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T., ROSENTHAL S. M. Presence of polyamines in certain bacterial viruses. Science. 1958 Apr 11;127(3302):814–815. doi: 10.1126/science.127.3302.814-a. [DOI] [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. II. Requirement for polyamines in T4 infection of a polyamine auxotroph. J Virol. 1972 Mar;9(3):423–430. doi: 10.1128/jvi.9.3.423-430.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940 Mar;34(3):392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coliphage T2. I. Preparation, assay, and some chemical properties. J Gen Physiol. 1957 May 20;40(5):809–825. doi: 10.1085/jgp.40.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R., MEHROTRA B. D., SHARP C. W. Effects of diamines on the thermal transition of DNA. Biochem Biophys Res Commun. 1961 Jan 25;4:79–82. doi: 10.1016/0006-291x(61)90260-1. [DOI] [PubMed] [Google Scholar]
- MARETZKI A., MALLETTE M. F. Nutritional factors stimulating the formation of lysine decarboxylase in Escherichia coli. J Bacteriol. 1962 Apr;83:720–726. doi: 10.1128/jb.83.4.720-726.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L., Okstein C. J. An automated method for polyamine analysis. Anal Biochem. 1969 Sep;30(3):449–453. doi: 10.1016/0003-2697(69)90140-7. [DOI] [PubMed] [Google Scholar]
- Raina A., Jansen M., Cohen S. S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1684–1696. doi: 10.1128/jb.94.5.1684-1696.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHER I. H., MALLETTE M. F. Purification and study of L-lysine decarboxylase from Escherichia coli B. Arch Biochem Biophys. 1954 Dec;53(2):354–369. doi: 10.1016/0003-9861(54)90417-8. [DOI] [PubMed] [Google Scholar]
- TABOR H. The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry. 1962 May 25;1:496–501. doi: 10.1021/bi00909a021. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Dobbs L. G. Metabolism of 1,4-diaminobutane and spermidine in Escherichia coli: the effects of low temperature during storage and harvesting of cultures. J Biol Chem. 1970 Apr 25;245(8):2086–2091. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- VOLKIN E., ASTRACHAN L. Phosphorus incorporation in Escherichia coli ribo-nucleic acid after infection with bacteriophage T2. Virology. 1956 Apr;2(2):149–161. doi: 10.1016/0042-6822(56)90016-2. [DOI] [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


