Abstract
CD27 interactions with its ligand, CD70, are thought to be necessary for optimal primary and memory adaptive immune responses to a variety of pathogens. Thus far all studies addressing the function of the CD27-CD70 axis have been performed either in mice lacking CD27, overexpressing CD70, or in which these receptors were blocked or mimicked by antibodies or recombinant soluble CD70. Because these methods have in some cases led to divergent results, we generated CD70-deficient mice to directly assess its role in vivo. We find that lack of CD70-mediated stimulation during primary responses to LCMV lowered the magnitude of CD8 antigen-specific T cell response, resulting in impaired viral clearance, without affecting CD4 T cell responses. Unexpectedly, CD70-CD27 costimulation was not needed for memory CD8 T cell generation or the ability to mount a recall response to LCMV. Adoptive transfers of wild type (WT) memory T cells into CD70−/− or WT hosts also showed no need for CD70-mediated stimulation during the course of the recall response. Moreover, CD70-expression by CD8 T cells could not rescue endogenous CD70−/− cells from defective expansion, arguing against a role for CD70-mediated T:T help in this model. Therefore, CD70 appears to be an important factor in the initiation of a robust and effective primary response but dispensable for CD8 T cell memory responses.
Introduction
Whereas a T cell's eponymous receptor (TCR) provides antigen-specificity for adaptive immune responses, full T cell activation requires complementary signals from costimulatory receptors and inflammatory cytokines. Costimulation lowers the threshold for T cell activation and supports proliferation and survival by promoting cell cycle progression, upregulating anti-apoptotic molecules, and inducing IL-2 secretion (1–3). These functions were initially attributed to CD28, the first widely appreciated T-cell costimulatory receptor. However, CD28−/− mice provided evidence that, although sufficient for costimulation in vitro, CD28 was not necessary for all aspects of the immune response (4, 5). In particular, CD28 is now not thought to be a major contributor to viral clearance and CD8 T cell memory generation in response to strongly replicating and/or virulent pathogens such as Lymphocytic Choriomeningitis Virus (LCMV) and virulent Vaccinia strains (4, 6). Other costimulatory molecules have been identified, the most prominent of which are members of the tumor necrosis factor receptor (TNFR) superfamily such as CD27, 4-1BB, OX40, HVEM, CD30, and GITR, which have both redundant and unique contributions to the T cell activation process (7). The timing and location of different costimulatory interactions vary substantially (7, 8). CD28 acts during early T cell priming and is quickly outcompeted by the coinhibitory receptor CTLA-4 (9, 10), whereas TNFR superfamily members and their ligands have much more complex regulation of expression during an immune response, seemingly regulating its outcome at defined phases (11, 12). In addition, CD4 and CD8 T cells seem to depend primarily on different TNF/TNFR superfamily members, with CD30/CD30L and OX40/OX40L interactions being more important for CD4 cells and 4-1BB/4-1BBL for CD8 T cells (13–15). CD27-CD70 interactions have been implicated in both CD4 and CD8 T cell responses (16, 17).
CD27 exerts its costimulatory activity after engagement by its only known ligand, membrane-bound CD70. CD27 is highly expressed on naïve T cells and is further upregulated during early T cell activation. It is downregulated in late-phase effectors and/or terminally differentiated effector memory cells (TEM), but it is expressed at high levels in central memory T cells (TCM), a subset endowed with a high proliferative capacity (18–20). CD27 signaling is controlled by the limited expression of CD70, which is constitutively expressed on only a small fraction of thymic medullary cells and gut lamina propria dendritic cells (DC). CD70 is transiently expressed on DC after stimulation via Toll-like receptors (TLRs) and/or engagement of CD40 with CD40L, and on activated T and B cells (21, 22). CD27-CD70 interactions were recognized as a bona fide costimulatory pathway in in vitro studies where anti-CD27 crosslinking or co-culture with CD70-transfected cells supported T cell proliferation in response to otherwise suboptimal stimuli (23–26). More importantly, CD27−/− mice manifested diminished antiviral T responses (16). Notably, in CD27-deficient mice the initial commitment to T cell division was largely unperturbed (likely ascribed to redundancy with CD28), but the effector T cells were more susceptible to apoptosis and consequently accumulated poorly (12, 27). In addition, whereas single knockouts for CD27 or CD28 had a similarly reduced CD8 T cell immune response to influenza, double-deficient mice mounted almost no response, demonstrating the complementary effects of those receptors (27). Importantly, the most prominent defect in CD27−/− mice was a failure to mount productive CD8 T cell recall responses (28, 29), which could at least partially be attributed to the need for CD27-CD70 engagement in CD4 T cell-mediated licensing of DC (17, 30, 31). DC-licensing is one of the proposed mechanisms that allow CD8 T cell differentiation into protective memory cells, i.e. equipped with a full arsenal of anti-apoptotic molecules necessary to survive secondary expansion (17). However, such studies were done in CD27−/− mice or with adoptively transferred CD27−/− cells, so CD70-mediated stimuli were absent during both the primary and secondary response. Thus, it was never formally tested if memory CD8 T cells need CD70 during the course of the recall response itself.
CD70 expression must be tightly controlled, as evidenced by pathological T cell activation in three strains of CD70 transgenic (Tg) mice overexpressing CD70 on B cells, DC, and T cells (32–34). The most severe phenotype was observed in B cell-CD70 transgenic mice where constant T cell hyperstimulation led to progressive depletion of naïve T cells and IFN-γ–mediated B cell ablation, ultimately resulting in a lethal immunodeficiency (35). Although unmanipulated T cell-CD70 transgenic mice had a much milder phenotype, when challenged with influenza they exhibited enhanced primary CD8 T cell responses and T cell exhaustion, providing formal proof that T cells can provide costimulation to other T cells by overexpressing CD70 (34). Constitutive CD70 expression is also found on T cells during chronic infections such as HIV, supporting the hypothesis that HIV-associated T cell exhaustion is an aftermath of exaggerated T cell costimulation via CD70-expressing T cells (36, 37). A similar role in T-cell exhaustion via continued CD27-CD70 interaction was found in a mouse model of chronic LCMV infection, although the cells expressing CD70 were not identified (38). Given the fact that, when activated, several immune subsets (DC, T and B cells) can express CD70, it is difficult to discern the exact role that each of them has in promoting T cell responses or, adversely, causing overactivation or exhaustion. Specifically, the repercussions of the putative CD27-CD70 engagement in T-T costimulation are underexplored.
Although the role of CD27-CD70 engagement is considered important in many infectious and immunization models, a consensus on its importance in the primary immune response to an acute LCMV infection and pathogen clearance has not been reached. Whereas initial studies in both CD27−/− mice and mice injected with anti-CD70 reported that the CD8 T cell response to LCMV was normal (29, 39), it was recently reported that the primary response was diminished and the differentiation of memory precursors was impaired (38). An infection with LCMV Armstrong is a valuable model for studying acute infections, and CD70 upregulation on DC is higher during LCMV infection than any other model studied, such as infection with Vaccinia Virus, VSV, and Listeria Monocytogenes (39). Moreover, the pathology related to chronic strains of LCMV can be diminished in CD27-deficient animals or animals treated with blocking antibody to CD70 (38, 40), suggesting a prominent role of CD27-CD70 interaction in the infection with this virus. To assess the role of CD70-mediated signals in an acute infection with LCMV, as well as to dissect both timing and location of CD27-CD70-delivered costimulation during the primary and secondary CD8 T cell responses, we generated CD70-deficient mice and examined their response to LCMV.
Materials and Methods
Mice
C57BL/6 (B6) mice were obtained from Frederick Cancer Research Facility (Frederick, MD). β-actin flpe transgenic and β-actin cre transgenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). P14 TCR Tg mice (P14) expressing a TCR specific for an LCMV epitope GP33–41 (GP33) were purchased from Taconic (Hudson, NY) (41), backcrossed onto the B6 background, and bred at our animal facility. To obtain the P14.CD45.1 congenic strain, P14 mice were bred with C57BL/6.CD45.1 mice, purchased from The Jackson Laboratory. All animal studies were approved by the NCI Animal Care and Use Committee.
Generation of CD70−/− mice
CD70 conditional knockout mice were generated by genetic recombineering, as described (42) (http://ncifrederick.cancer.gov). The 4.3 kB region including exons 1–2 from the BAC clone RP23-312G18 (BACPAC Resource Center) was subcloned in the targeting vector pLMJ235. A loxP-neo -loxP cassette was inserted in an EcoRI site upstream of exon 1 and subsequently excised by Cre induction in EL350 cells. The FRT-Neor-FRT-loxP cassette was inserted downstream of exon 2. The vector was linearized with the restriction enzyme NotI and electroporated into strain 129 × B6-derived mouse embryonic stem cells in Mouse Cancer Genetics Program in NCI-Frederick (Frederick, MD). The genotype of recombinant mice was confirmed by Southern blot and PCR analysis. Recombinant CD70 mice were first crossed to β-actin-Flpe Tg mice to cut out the Neo cassette. Tissue-wide deletion of the loxP-flanked segments was achieved by crossing the mice that carried the recombinant allele with mice expressing Cre under the control of the β-actin promoter. These mice are designated as CD70−/−. CD70−/− mice were subsequently backcrossed onto the B6 background for at least nine generations, and up to 14 in some experiments.
Reagents
Antibodies to CD3ε, CD4, CD40, CD44, CD62L, CD69, CD70, TCRβ, B220, IFN-γ, TNF-α, IL-2, NK1.1, CD19, CD11b, 2.4G2 (Fc block) as well as GolgiStop and BD fixation and permeabilization buffers were obtained from BD Biosciences. Antibodies against CD8, CD45.1, CD127, CD11c, CD11a, CD49d, Eomes, PD-1, and MHC Class II, the annexin V staining kit and nuclear fixation and permeabilization buffers were purchased from eBioscience. Antibodies against T-bet and KLRG1 were purchased from Santa Cruz Biotechnology and SouthernBiotech, respectively. GP33–41 H-2Db (GP33), GP276–286 H-2Db (GP276), and NP396–404 H-2Db tetramers (NP396) were obtained from the NIH Tetramer facility at Emory University, Atlanta, GA. GP33–41 and GP61–80 peptides were purchased from Peptide 2.0 Inc. Power SYBR Green was obtained from Applied Bioscience. LPS and polyI:C were purchased from Sigma-Aldrich. LIVE/DEAD Fixable Dead Cell Stain was obtained from Life Technologies.
Viral growth, LCMV infection, generation of memory cells, and adoptive transfers
LCMV Armstrong 53b was grown in our laboratory in baby hamster kidney cells and viral titers were determined as described (43). B6 and CD70−/− mice were infected i.p. with 2 × 105 plaque-forming units (PFU) of LCMV Armstrong. To prepare LCMV memory cells for adoptive transfer, 5 × 105 P14.CD45.1 cells prepared from lymph nodes were injected i.v. one day prior to LCMV infection. Four to eight weeks following LCMV infection P14 memory cells were sorted based on CD45.1 and CD8 double positivity on FACSAria cell sorter. Five × 104 sorted cells were injected into naïve B6 or CD70−/− hosts, which were subsequently infected with LCMV several hours after the cell transfer.
Flow cytometry and cell stimulation
For detection of intracellular cytokines, 3 × 106 splenocytes were stimulated with 0.3 μg/ml GP33–41 or 1 μg/ml GP61–80 for 4–6 hr or left untreated. Cells were treated with BD GolgiStop for the last 3–4 hr of incubation and then stained with antibodies to surface markers, followed by fixation. The antibody 2.4G2 was added to the permeabilization buffer to block Fc-receptor binding prior to the addition of anti-cytokine antibodies. Detection of transcription factors, following surface staining, was performed after fixation and nuclear permeabilization with eBioscience buffers. Flow cytometry was done with a BDLSRFortessa cytometer using BD FACSDiva software (BD Biosciences). Annexin V staining was performed according to the manufacturer's instructions. All flow cytometry data analysis was performed with FlowJo software (Tree Star).
PCR and quantitative RT-PCR (qRT-PCR)
To test WT and CD70−/− mice, two separate PCR reactions were performed using the following primers: WT Forward: TCGTATAATGTATGCTATACGAAGTT, WT Reverse: CTTGCTTTAACTCTCTGTCTATATTTCAGC, CD70−/− Forward: GCACACAGCTGAGTTACAGCTG, CD70−/− Reverse: GCTTACACATCACTAGTGCTCAGATG. Real-time PCR for CD70 was performed with SYBR Green using 7500 Real Time PCR System by Applied Bioscience (Carlsbad, CA), with the following primers: CD70-Forward: TGCTGTTGGTTTCATTGTAGCG CD70-Reverse: ATCCTGGAGTTGTGGTCAAGGG. Viral loads were determined by measuring viral RNA by RT-PCR in spleen lysates with nucleoprotein (NP) primers as described (44). Housekeeping ribosomal 18S RNA was amplified to normalize RNA content of the lysate and obtain dCT value.
Statistical analysis
Statistical analysis was done by using a Student's two-tailed unpaired t test with GraphPad Prism software.
Results
Generation of CD70-deficient mice
A construct in which the first two of the three exons of the CD70 gene (encoding the intracellular, transmembrane and part of the extracellular domain) were flanked by loxP sites was introduced into embryonic stem cells by homologous recombination (Fig. 1A). Mice carrying the recombinant allele were crossed with mice expressing Cre-recombinase under the control of the β-actin promoter to achieve deletion of CD70. As the residual exon 3 has no open reading frame that could generate a truncated protein, such a deletion is expected to generate a complete CD70 knockout. The progeny were screened for the recombinant allele by PCR, one reaction amplifying the CD70 WT allele, the other the CD70-deleted allele (Fig. 1B), which confirmed the efficiency of deletion. CD70 expression is upregulated in dendritic cells (DC) during the early phases of infection and in vitro by stimulation with Toll-like receptor (TLR) agonists. To verify that the CD70 mRNA was absent in CD70-deficient mice, bone marrow-derived dendritic cells (BMDC) from WT or CD70-deficient mice were stimulated with LPS. Whereas WT cells expressed low levels of CD70 mRNA that was upregulated by activation, CD70-deficient cells expressed no detectable CD70 mRNA (Fig. 1C). Moreover, when splenocytes were exposed to stimuli that increase CD70 expression, CD70 protein was detected on the surface of DC from WT but not CD70−/− mice, confirming that the latter cannot produce CD70 (Fig. 1D).
Figure 1.
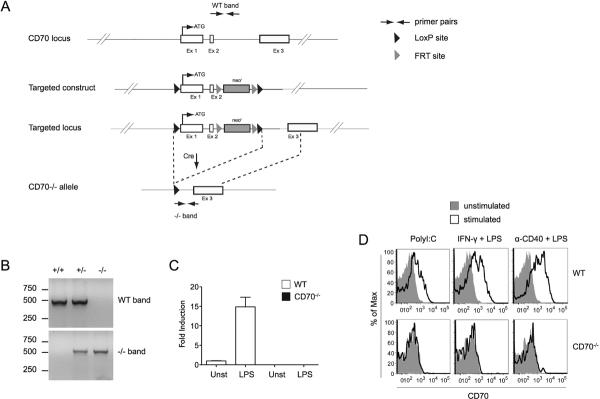
Generation of CD70-deficient mice. (A) To generate CD70−/− mice, homologous recombination was used to insert a targeting construct into the endogenous CD70 locus. The recombinant allele contained two LoxP sites flanking exons 1 and 2. Upon Cre-mediated excision of exons 1 and 2, a CD70-deleted allele was obtained. Neo Cassette, PCR primers for screening, and loxP and FRT recombination sites are indicated in the inserted legend. Note that the map is not drawn to scale. (B) CD70−/− mice were distinguished from WT littermates by two PCR reactions, one amplifying the WT band (top panel), the other amplifying the CD70-deleted allele (bottom panel). The respective primer binding sites are shown schematically in A. (C) BMDC from WT or CD70−/− mice were stimulated with LPS for 6 hr and CD70 expression was detected by qRT-PCR. Data are shown as fold induction over unstimulated WT BMDC (fold induction = 1). Mean ± SEM for three independent experiments is shown. (D) Splenocytes from WT or CD70−/− mice were stimulated with TLR agonists and other indicated stimuli that elicit DC maturation. Upregulation of CD70 was evaluated by surface staining of gated conventional DC (CD11chiMHCIIhi) from spleen. One representative experiment of three is shown.
CD70−/− mice were born at the expected Mendelian ratios and developed normally. Their thymic development was undistinguishable from WT counterparts in both subset ratios and numbers (Fig. 2A and data not shown). Early thymocyte progression through the DN1–DN4 developmental stages, as monitored by CD44 and CD25 expression, was also normal (Fig. 2B). Peripheral lymphoid organs of CD70−/− mice contained normal numbers and frequencies of T cells, B cells, and conventional DC (cDC) (Fig. 2C and data not shown). Within the T cell compartment, CD4 to CD8 ratios were normal (Fig. 2C). Moreover, as all CD70 transgenic mice had signs of overt T cell activation, we determined if fewer activated/memory cells could be found in CD70−/− mice. We found normal levels of naturally-arising activated/memory T cells (i.e. CD44hi cells in unmanipulated mice) in both CD4 and CD8 compartments (Fig. 2D). Therefore, mice lacking CD70 exhibited normal lymphoid tissue development, as was found for mice lacking its receptor, CD27 (16).
Figure 2.
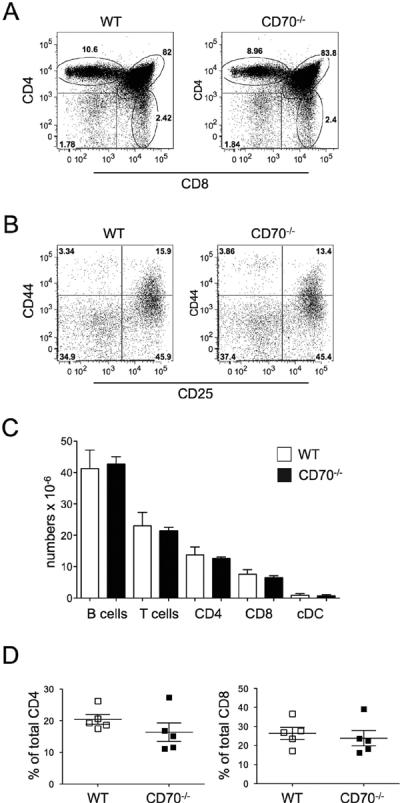
CD70-deficient mice have normal steady-state immune compartments. (A–B) The frequencies of thymocyte subsets stained for CD4 and CD8 are shown in WT or CD70−/− mice (A), CD25 and CD44 staining is shown for gated CD4−CD8− (DN) Lin− cells (Lin− = CD3ε−CD11c−CD11b−CD19−γδ−) (B). One representative experiment of four is shown. (C) Absolute numbers of different cell subsets in the spleens of WT and CD70−/− mice are depicted. Mean ± SEM of nine mice is shown. (D) Percentage of CD44hi cells out of total CD4 or CD8 T cells in the spleen of five individual WT or CD70−/− mice is shown. The horizontal bars represent the mean ± SEM. The differences were not statistically significant.
Impaired primary CD8 T cell response and delayed viral clearance in CD70-deficient mice
Several reports have demonstrated that CD27−/− mice or the mice treated with a blocking antibody against CD70 have diminished primary responses to a number of pathogens, including Influenza, Vaccinia Virus, Vesicular Stomatitis Virus, and Listeria Monocytogenes (16, 29, 39). The response to LCMV was initially thought to be independent of CD27–CD70 interactions (29, 39), although a recent report with transient anti-CD70 blockage has questioned this (38). As antibody-mediated blockage can have unpredictable results, CD70−/− mice were used to address this question. WT and CD70−/− mice were infected with the LCMV Armstrong strain, which elicits massive CD8 T cell expansion with as many as 95% of the cells being LCMV-specific and having an effector phenotype on days 7–8, the peak of the CD8 T cell immune response (45, 46). As expected, on day 8 spleens were enlarged in LCMV-infected WT mice (Fig. 3A), which was mainly attributed to a prominent increase in CD8 T cells (Fig. 3B). CD8 T cells in CD70−/− mice, on the other hand, expanded much less, resulting in approximately half as many CD8 T cells. In accordance with previous findings (45), there were very few naive CD8 T cells in WT LCMV-infected animals, with approximately 95% having an activated/effector phenotype (CD44hiCD62Llo) (Fig. 3C). In contrast, a much larger fraction of CD8 T cells in CD70−/− mice were naïve (CD44lo-intCD62Lhi) or had a TCM phenotype (CD44hiCD62Lhi) (Fig. 3C), which is unusual for LCMV infection. Notably, a prominent difference was seen in the number of effector CD8 T cells (Fig. 3D), whereas the absolute numbers of naïve and TCM CD8 T cells were not different between the strains (Fig. 3E and F). Unlike CD8 T cells, CD4 T cells are dispensable for clearance of acute LCMV infection and expand much less than CD8 T cells (47). However, it was recently reported that as many as 50% of CD4 cells at the peak of LCMV infection are LCMV-specific and that they can be distinguished by expression of the trafficking molecules CD11a and CD49d (48). No difference in frequency or absolute numbers of activated CD11ahiCD49D+ CD4 T cells was observed between the strains (Fig. 3G and 3H), arguing for the importance of CD70-mediated costimulatory stimuli for optimal CD8 but not CD4 T cell expansion.
Figure 3.
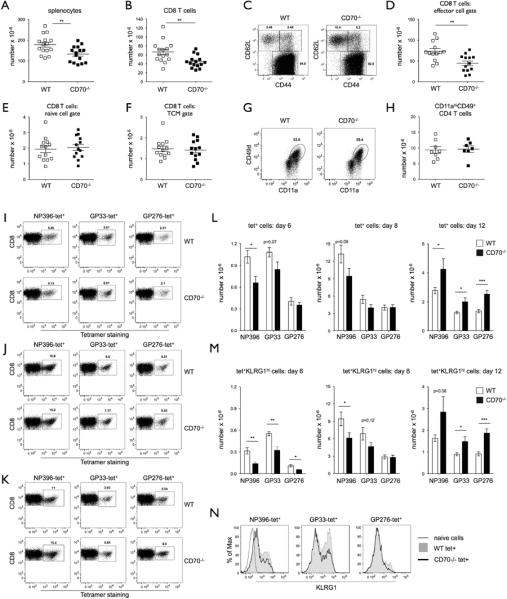
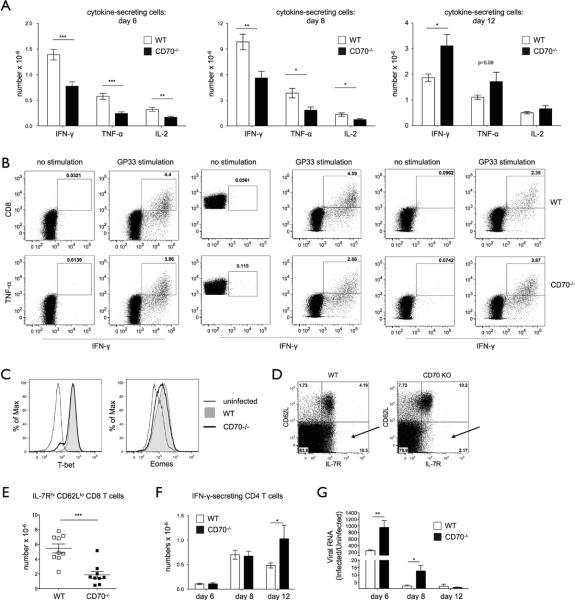
CD70-deficient mice have an impaired primary response to LCMV. (A–B) WT or CD70−/− mice were infected with LCMV Armstrong and analyzed on day 8, the the peak of the response. Absolute numbers of total cells (A) or CD8 T cells (B) in the spleens of individual mice are depicted, with mean ± SEM. (C–F) A representative plot (C) and absolute numbers (D–F) of effector (CD44hiCD62Llo), naïve (CD44lo-intCD62Lhi), and TCM (CD44hiCD62LhiCMhi) CD8 T cells in spleens from individual WT or CD70−/− mice on day 8 of LCMV infection. (G–H) A representative plot of activated CD4 T cells (CD11ahiCD49+) (G) and their absolute numbers from individual mice (H) in spleens from LCMV-infected WT or CD70−/− mice on day 8 of LCMV infection. (I–K) A representative plot of NP396, GP33, and GP276 tetramer-positive CD8 T cells on day 6 (I), 8 (J), and 12 (K) of LCMV infection (note the Y axis scale difference between days). (L–M) Absolute numbers of NP396-, GP33-, and GP276-tetramer positive (L) or KLRG1hi tetramer-positive CD8 T cells (M) on day 6, 8, and 12 of LCMV infection (7–8 mice per time point of each genotype). (N) Expression of KLRG1 on NP396-, GP33-, and GP276-specific CD8 T cells on day 6 of LCMV infection is shown from one representative WT and CD70−/− mouse. Values from the individual mice with mean ± SEM are depicted. * p value < 0.05, ** p value <0.001, *** p value < 0.005.
To understand the breadth and kinetics of the CD8 expansion defect in CD70−/− mice, antigen-specific CD8 T cells against two major immunodominant (NP396 and GP33) and one subdominant (GP276) LCMV epitopes were assessed by tetramer staining over time. A similar immunodominance hierarchy (NP396>GP33>GP276) and clone frequencies were found in WT and CD70−/− mice at the early phase of infection (day 6) (Fig. 3I), the peak of the response (Fig. 3J), and during the contraction phase (day 12) (Fig. 3K). Although the frequencies were similar, lower absolute numbers of tetramer+ cells were found in CD70−/− mice at days 6–8 of infection (Fig. 3L), and the difference between the strains was even larger if KLRG1hi antigen-specific cells were compared (Fig. 3M). KLRG1 has been proposed to be a marker of terminally differentiated CD8 T cells (termed short lived effector cells) that received higher level of stimulation than their KLRG1-negative counterparts (49). A slightly higher expression of KLRG1 was observed in WT compared to CD70−/−-antigen specific cells, suggesting that the former received a higher level of stimulation (Fig. 3N). Notably, the differences were more pronounced in cells specific for the immunodominant antigens NP396 and GP33. Interestingly, during the contraction phase at day 12 (note the Y axis scale difference between days), relatively more antigen-specific cells were found in CD70−/− than WT mice, raising the possibility that contraction was delayed due to the persistence of virus.
The lack of CD70-mediated costimulation during priming could have several consequences, such as less effective precursor recruitment, inefficient priming, and/or generation of dysfunctional cells. To discern between these possibilities, we analyzed the consequence of CD70 expression in the conditioning of T cell effector functions. At days 6 and 8 post infection CD70−/− mice exhibited at least a two-fold decrease in the numbers of CD8 T cells that secreted IFN-γ, TNF-α, or IL-2 when challenged with an LCMV-epitope (Fig. 4A). In contrast, a higher number of cytokine-secreting cells was observed at day 12. The frequency of cytokine-secreting cells in the CD8 T cell compartment was only slightly lower in CD70−/− mice (Fig. 4B and Supplemental Fig. 1A and B), arguing that the large majority of the difference in their absolute numbers was due to a difference in effector CD8 T cells. Nevertheless, as the difference between numbers of GP33-tetramer+ cells at days 6 and 8 (Fig. 3M) was less impressive than the difference in the numbers of cytokine secreting cells (Fig. 4A), a slightly lower capacity to secrete cytokines in CD70x−/− cells probably also contributed. It is also possible that some antigen-specific cells were unaccounted for due to TCR-downregulation (50).T-bet and Eomes, transcription factors that govern the CD8 T cell cytotoxic program, and the majority of surface markers of differentiation, such as CD122 (β component of IL-2 and IL-15 cytokine receptors), CD25 (β component of IL-2 receptor), and Ly6C, were indistinguishable between the strains at the peak of the response (Fig. 4C and data not shown). However, a difference was observed in the fraction of effector (CD62Llo) CD8 T cells that were positive for IL-7 receptor (IL-7R). IL-7R is transiently downregulated during T cell activation and is re-expressed during the memory transition (51). IL-7Rhi effector cells have higher self-renewal potential than IL-7Rlo fraction and are thus considered to be memory cell precursors (51). As much as 10% of effector cells in WT mice were IL7Rhi, while this population was largely absent in CD70−/− mice (Fig. 4D, arrows). This resulted in absolute numbers of IL-7Rhi effector cells being several-fold lower in CD70−/− mice (Fig. 4E). CD4 T cells challenged with antigen did not show any difference in IFN-γ, TNF-α, or IL-2 secretion at days 6–8 (Fig. 4F and Supplemental Fig. 2). However, like CD8 T cells, CD4 T cells from CD70−/− mice secreted more cytokines than their WT counterparts at day 12. To determine if the observed lack of antigen-specific CD8 T cells had functional consequences, LCMV clearance from the spleen was measured, and viral clearance was found to be substantially delayed in CD70−/− mice (Fig. 4G). These results indicate that CD27-CD70 interactions are important for CD8 T cell expansion and efficient clearance of LCMV, and suggest that the delayed viral clearance slows the contraction of CD8 and CD4 T cells.
Figure 4.
CD70-deficient mice have impaired CD8 T cell effector function and delayed viral clearance. (A–B) Splenocytes were restimulated in vitro with GP33 peptide on the indicated days after LCMV infection and evaluated for cytokine production by intracellular staining. Absolute numbers (A) or a representative staining (B) of IFN-γ, TNF-α, and IL-2-producing CD8 T cells from 8–16 individual mice per strain and per time point are represented, with mean ± SEM. (C) T-bet and Eomes expression on gated CD8 cells of uninfected WT (thin black line), and LCMV-infected WT (filled grey histogram) or CD70−/− (thick black line). (D–E) A representative gating of IL-7RhiCD62Llo CD8 T cells (D) and their absolute numbers from individual mice (E) in spleens from day 8 LCMV-infected WT or CD70−/− mice are shown. (F) Absolute numbers of IFN-γ-producing CD4 T cells from LCMV-infected mice on day 6, 8, and 12, with mean ± SEM. (G) Viral RNA from splenocytes of infected WT or CD70−/− mice on the indicated days (5–11 mice per time point of each genotype) was analyzed by qRT-PCR. Values from the individual mice with mean ± SEM are depicted. * p value < 0.05, ** p value <0.001, *** p value < 0.005.
Efficient memory CD8 T cell generation in CD70-deficient mice
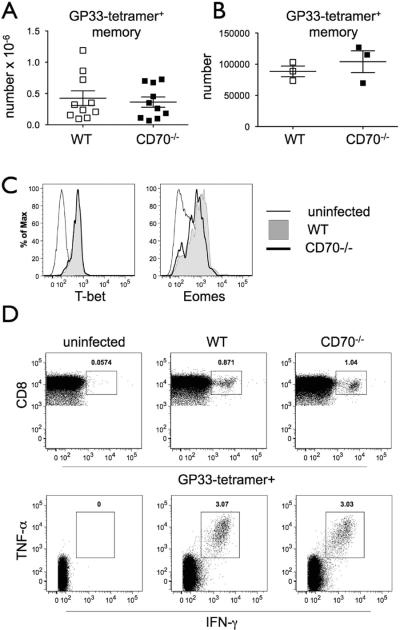
The suboptimal expansion of CD8 T cells at the peak of the LCMV response, as well as the 5-fold lower frequency of IL-7Rhi CD8 effector T cells, could have resulted in a reduction in CD8 memory T cells (49, 51). However, we found no difference in the numbers of LCMV-specific cells in WT and CD70−/− mice at either early or late memory phases (1–2.5 months or 8 months post infection, respectively) (Fig. 5A and B). The expression of T-bet and Eomes transcription factors was also indistinguishable in memory CD8 T cells between those strains (Fig. 5C). The function of memory cells was tested by in vitro re-stimulation with LCMV GP33 peptide. No differences between WT and CD70-deficient memory cells were found in the percentage of CD8 T cells that were GP33-tetramer+ and secreted the cytotoxic cytokines IFN-γ and TNF-α (Fig. 5D). Therefore, both the number and function of LCMV-specific cells were normal in the memory phase of the immune response in CD70−/− mice, as was previously found for CD27−/− mice in a similar acute infection model with low dose of LCMV WE (29).
Figure 5.
CD70-deficient mice have a normal memory response to LCMV infection. (A–B) WT or CD70−/− mice were infected with LCMV Armstrong and analyzed one to 2.5 (A) or 8 months (B) later. Absolute numbers of GP33-tetramer+ CD8 T cells in the spleens of individual mice are shown with mean ± SEM. (C) T-bet and Eomes expression is shown on gated GP33-tetramer+ CD8 cells from uninfected (thin black line), and LCMV-infected WT (grey filled histogram) or CD70−/− (thick black line) mice. (D) Splenocytes of infected mice were restimulated in vitro with GP33 peptide and IFN-γ and TNF-α production was detected by intracellular staining. One representative plot is shown from a total of eight mice. No statistical differences were found.
CD70-mediated costimulation is not required for secondary responses
Whereas the numbers of steady-state memory T cells in CD27−/− mice were reported to be mildly decreased or unperturbed, depending on the immunization model (28, 29, 52), a marked defect was observed in their capability to mount a recall response in vivo (28). This was primarily ascribed to the need of CD27 engagement during the priming phase of the response (17, 31). However, the experiments performed in CD27−/− mice could not distinguish between the importance of CD27-CD70 interactions during the priming phase and the recall response itself, as such interactions were precluded in both occasions. To discriminate between these possibilities, WT P14 memory T cells, specific for the LCMV peptide GP33 in the context of H-2Db, were adoptively transferred to WT or CD70-deficient recipient mice, and rechallenged them with LCMV (Fig. 6A). The memory P14 cells in both recipients expanded swiftly, and those in CD70−/− mice actually accumulated to modestly higher absolute numbers (Fig. 6B). At the same time, the number of endogenous GP33-tetramer+ cells in CD70−/− mice (Fig. 6C), as well as the total number of activated endogenous cells (Fig. 6D), was more than twofold lower than in WT mice, similar to our findings with endogenous primary responses in the absence of adoptive transfer (Fig. 3). It was proposed that cells undergoing recall responses in the absence CD27-CD70 interactions fare poorly due to the lack of prosurvival molecules (17). However, we found no difference between adoptively transferred memory cells expanding in WT or CD70−/− mice in regard to their susceptibility to apoptosis as measured by annexin staining, KLRG-1 expression, and the expression of a marker of T cell activation and exhaustion, PD-1 (Fig. 6E). It is also of note that endogenous responses in CD70−/− mice were not aided by the expanding population of WT P14 memory cells (i.e. CD70-sufficient T cells), arguing against the possibility that CD8 T cells expressing normal endogenous levels of CD70 provide costimulatory signals to each other. In addition, although viral clearance was delayed in CD70−/− mice (Fig. 3), viral clearance in WT and CD70−/− hosts that received P14 memory cells was comparable (data not shown). Thus, WT P14 memory cells were fully functional in CD70−/− hosts, arguing that CD70 is dispensable during the course of the recall response.
Figure 6.
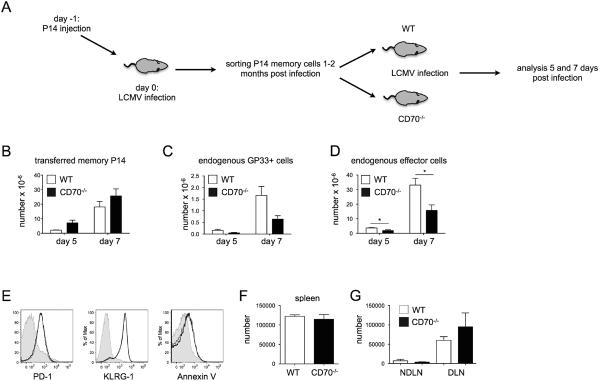
CD70 is dispensable for recall responses. (A) The experimental scheme is depicted. CD45.1+P14 TCR Tg transgenic CD8 T cells were adoptively transferred to a WT recipient (CD45.2+), which was then infected with LCMV. After 1–2 months memory P14 cells were sorted and adoptively transferred into WT or CD70−/− recipients. The recipient mice were subsequently infected with LCMV and the recall response was evaluated five or seven days later. (B–D) Absolute numbers of adoptively transferred memory P14 CD8 T cells (B), GP33-tetramer+ cells (C) and endogenous effector CD8 T cells (D) in WT or CD70−/− mice after five or seven days of infection with LCMV are depicted. Mean ± SEM of three-five mice of each strain per time point is shown. (E) PD-1, KLRG1 and Annexin V surface levels on CD8 T cells of uninfected (filled histogram), P14-Tg cells in WT (thin black line), or P14-Tg cells in CD70−/− (black thick line) splenocytes is depicted. (F–G) WT or CD70−/− mice were infected with LCMV and 8 months later rechallenged in vivo with GP33 peptide by footpad administration. Absolute numbers of GP33-tetramer+ T cells in draining and non-draining lymph nodes (F) and in spleen (G). Mean ± SEM of three mice is shown.
The finding that CD70 was not needed during rechallenge of WT memory cells in CD70−/− hosts suggested that the difference in recall responses reported in CD27−/− mice might be ascribed to differences in CD8 T cell conditioning during the course of the primary response itself, as previously suggested (17, 31). To test this, WT and CD70−/− mice were infected with LCMV to generate memory response, and 10 months later were rechallenged with GP33 peptide by footpad administration. At day 7 of the recall response, both WT and CD70−/− mice had a small number of GP33-tetramer+ cells in non-draining lymph nodes that had no signs of overt activation, i.e. representing steady-state memory cells (Fig. 6F, and the data not shown). In addition, the number and phenotype of GP33-tetramer+ CD8 T cells in the spleen were indistinguishable between WT and CD70−/− mice (Fig. 6F, and the data not shown). As expected, GP33-specific cells in the draining lymph nodes of WT mice expanded to the secondary challenge (Fig. 6G). Unexpectedly, however, GP33-tetramer+ CD8 T cells expanded to an equal degree. These results demonstrate that in contrast to CD27−/− animals during influenza infection (28), CD70−/− mice mount a normal recall response to LCMV.
Discussion
Although CD27-CD70 costimulatory pathway is thought to be essential for various immune responses, there are some discrepancies between data generated in CD27−/− mice and studies using neutralizing antibodies against CD70, raising the possibility that these two experimental approaches might not be entirely complementary. The generation of the genetic model for CD70 deletion reported in this study allowed us to address some of the issues that were unresolved in previous experimental settings. Off-target effects, such as non-specific binding, antibody complex formation and/or phagocytic cell activation, as well as incomplete blocking are possible artifacts of the neutralizing/blocking antibody approach. To our knowledge, reverse signaling by blocking anti-CD70 has not been assessed, but remains a possibility as several anti-CD70 antibodies were shown to directly affect B and T cells function (53, 54). Perhaps even more importantly, CD70 could have functions independent of its only known receptor, CD27. It is notable that in DC CD70 localizes with MHC Class II antigen-presenting machinery in endosomal vesicles, is delivered to the immune synapse together with MHC class II upon cognate antigen recognition, and that both molecules even utilize the same invariant chain (Ii) chaperone for this process (55, 56). It is thus conceivable that the presence or lack of CD70 would exert structural differences in DC:T interactions or mitigate the DC function in some unknown fashion. Moreover, because crosstalk between CD70 and CD27 results in downregulation of surface CD70 (29 and our unpublished results), it is possible that the higher levels of unliganded CD70 in CD27−/− animals could affect the immune response. It is of particular interest to determine if other putative binding partners for CD70 exist, and whether their function could be mitigated by the lack of CD27.
CD27-CD70 interactions have been implicated at several steps of CD8 T cell differentiation during an immune response. Initial studies demonstrated impaired primary CD8 T cell expansion in CD27−/− mice during Influenza infection (16, 28), although this was recently disputed (52). Another report showed that the virulence of Vaccinia species directly correlated with the engagement of OX40 and CD27, and consequently with the magnitude of the primary antigen-specific CD8 T cell response (6). Our data with CD70−/− mice demonstrate the need for costimulation during priming for both optimal CD8 effector T cell generation during LCMV infection and viral clearance. It was suggested that anti-CD70 treatment at the late phase of the primary CD8 T cell response to Influenza increases the sensitivity of antigen-specific CD8 T cells to Fas-mediated apoptosis (12). As the CD8 effector T cells of CD70−/− mice had similar Bcl-xL and Bcl-2 staining to WT mice (our unpublished observation), it is likely that lack of expansion rather than enhanced death contributed to their diminished numbers.
Memory CD8 T cells are distinguished from their naïve precursors quantitatively and qualitatively, and are marked by their capacity for mounting rapid and protective recall responses (57). It is thought that the major traits necessary for recall are “imprinted” during early phases of the immune response, when antigen dose and costimulatory and cytokine makeup determine whether responding CD8 T cells will become short-lived effectors or memory precursors (58), although a requirement for fine-tuning memory responses during their course has also been recently suggested (59). Importantly, the lack of CD4 cell help during priming has been linked to generation of dysfunctional CD8 T memory cells that die during rechallenge (60). Several procedures that modulate or mimic CD70-mediated stimuli or regulate its expression were shown to be able to bypass the need for CD4 help. Treatment with agonistic anti-CD27 or anti-CD40 antibodies (the latter causing robust CD70 upregulation on DC), or stimulation by soluble CD70 allow for CD4-independent CD8 T cell responses (30, 61, 62). Several studies using CD27−/− mice reported diminished formation of memory CD8 T cells and/or their diminished capacity for secondary expansion in different immunization settings, further establishing a role for CD27 in recall responses (6, 28, 63). It was therefore expected that memory CD8 T cells would be perturbed following LCMV infection. However, although anti-CD70 treatment resulted in a seemingly lesser frequency of memory CD8 T cell precursors (IL-7R+ effector cells), no differences were found during the memory stage, prompting the authors to suggest that their antibody blocking regimen was not sufficient or that the long-term antibody block would be necessary to perturb the memory response (38). We addressed this issue with a genetic approach in CD70−/− mice, finding that neither steady state CD8 T cell memory numbers nor recall functions were impaired. As in the study by Penaloza-MacMaster et al., lack of IL-7R+ effector cells was found in CD70−/− mice, but as it clearly did not correlate to memory cell numbers it is likely that IL-7R downregulation was a consequence of the persistence of virus. Indeed, when the virus was cleared, at day 12, the same frequency of memory precursor cells was found in both WT and CD70−/− mice (data not shown). It is of note that it was recently reported that in CD27-deficient mice there was no difference in the memory response to Influenza, contradicting an earlier study with another strain of Influenza (28, 52). It is thus conceivable that the cytokine environment during different infections, such as type I IFN and IL-12, dominant during LCMV and Vaccinia infections respectively, could greatly mitigate the need for CD70-mediated costimulation. In support of this, the generation of memory precursors in Vaccinia infection (64) was substantially more efficient in IL-12−/− CD27−/− than in CD27−/− mice.
Due to their swift recall responses in vitro, it was long thought that memory CD8 T cells are largely costimulation-independent (65). This notion was recently challenged as in vivo recall responses were found to be severely diminished in CD28−/− mice, and the need for such costimulation inversely correlated with the antigen dose during the primary response (59, 66, 67). CD27-CD70 engagement greatly complements CD28-mediated stimuli during primary responses, but its role during rechallenge remains controversial. Arguing against the role of CD70-mediated costimulation in secondary responses was the finding that anti-CD70 blocking did not prevent adoptively transferred EAE (68). On the other hand, anti-CD70 treatment abrogated the ability of lymph-node resident CD8α+ DC to allow both naïve and memory CD8 T cell proliferation (69). Adoptive transfer of WT memory cells into CD70−/− mice allowed us to test the requirement for CD70 during the course of rechallenge in vivo. LCMV-memory T cells in CD70−/− and WT hosts had equivalent capacities for expansion when rechallenged with antigen. While it is conceivable that some other models of rechallenge could perhaps have a different result, we find it unlikely as the endogenous memory recall response to peptide/CFA rechallenge following LCMV infection was also normal. Therefore, our data strongly suggest that CD70 is dispensable for recall responses.
CD70 has been established as a prominent factor in generating productive immune responses in several vaccination strategies (6, 70). Moreover, manipulation of CD70-CD27 axis can break tolerance and enhance T cell-mediated tumor control (33, 71) as well as allograft rejection (72, 73). Because CD27-CD70 interactions have both beneficial and deleterious effects, to therapeutically exploit this costimulatory pathway a thorough understanding of the underlying biology is of considerable importance. CD70−/− mice should be a useful tool in further explorations of how this receptor-ligand pair regulates adaptive and, perhaps, innate immune functions.
Supplementary Material
Acknowledgements
We thank Lino Tessarollo and Eileen Southon (Mouse Cancer Genetics Program, NCI Frederick) for their assistance during generation of CD70−/− mice, Paul R. Mittelstadt and Maria Letizia Giardino Torchia for helpful discussions, Ehydel Castro for assistance with animal experiments, and Bei Dong and Moomal Shaikh for technical assistance. We thank Rafi Ahmed for providing LCMV Armstrong, and the NIH Tetramer facility at Emory University for their generous supply of tetramers.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 2.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1:231–240. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 5.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 6.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, Schoenberger SP, Croft M. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J. Clin. Invest. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 8.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 9.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 10.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 11.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 12.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J. Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 14.Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J. Immunol. 2005;174:3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 15.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J. Immunol. 2008;181:1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 18.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 21.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J. Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Laouar A, Haridas V, Vargas D, Zhinan X, Chaplin D, van Lier RA, Manjunath N. CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nat. Immunol. 2005;6:698–706. doi: 10.1038/ni1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobata T, Agematsu K, Kameoka J, Schlossman SF, Morimoto C. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J. Immunol. 1994;153:5422–5432. [PubMed] [Google Scholar]
- 24.Gravestein LA, Nieland JD, Kruisbeek AM, Borst J. Novel mAbs reveal potent co-stimulatory activity of murine CD27. Int. Immunol. 1995;7:551–557. doi: 10.1093/intimm/7.4.551. [DOI] [PubMed] [Google Scholar]
- 25.Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J. Immunol. 1997;159:4959–4965. [PubMed] [Google Scholar]
- 26.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins NA, Gilbert DJ, Copeland NG, Muto T, Yagita H, Okumura K. Characterization of murine CD70 by molecular cloning and mAb. Int. Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J. Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 29.Matter M, Mumprecht S, Pinschewer DD, Pavelic V, Yagita H, Krautwald S, Borst J, Ochsenbein AF. Virus-induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur. J. Immunol. 2005;35:3229–3239. doi: 10.1002/eji.200535179. [DOI] [PubMed] [Google Scholar]
- 30.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J. Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 31.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27–CD70 interactions. Nat Commun. 2012;3:948. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 33.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 34.van Gisbergen KP, van Olffen RW, van Beek J, van der Sluijs KF, Arens R, Nolte MA, van Lier RA. Protective CD8 T cell memory is impaired during chronic CD70-driven costimulation. J. Immunol. 2009;182:5352–5362. doi: 10.4049/jimmunol.0802809. [DOI] [PubMed] [Google Scholar]
- 35.Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Lethal T cell immunodeficiency induced by chronic costimulation via CD27–CD70 interactions. Nat. Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 36.Wolthers KC, Otto SA, Lens SM, Kolbach DN, van Lier RA, Miedema F, Meyaard L. Increased expression of CD80, CD86 and CD70 on T cells from HIV-infected individuals upon activation in vitro: regulation by CD4+ T cells. Eur. J. Immunol. 1996;26:1700–1706. doi: 10.1002/eji.1830260806. [DOI] [PubMed] [Google Scholar]
- 37.Brugnoni D, Airo P, Marino R, Notarangelo LD, van Lier RA, Cattaneo R. CD70 expression on T-cell subpopulations: study of normal individuals and patients with chronic immune activation. Immunol. Lett. 1997;55:99–104. doi: 10.1016/s0165-2478(96)02693-4. [DOI] [PubMed] [Google Scholar]
- 38.Penaloza-MacMaster P, Ur Rasheed A, Iyer SS, Yagita H, Blazar BR, Ahmed R. Opposing effects of CD70 costimulation during acute and chronic lymphocytic choriomeningitis virus infection of mice. J. Virol. 2011;85:6168–6174. doi: 10.1128/JVI.02205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur. J. Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 40.Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J. Exp. Med. 2006 doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 42.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutko FJ, Oldstone MB. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 1983;64:1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- 44.McCausland MM, Crotty S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. J. Virol. Methods. 2008;147:167–176. doi: 10.1016/j.jviromet.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J. Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, von Herrath MG, Buchmeier MJ, Grey H, Sette A. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J. Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDermott DS, Varga SM. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J. Immunol. 2011;187:5568–5576. doi: 10.4049/jimmunol.1102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munitic I, Decaluwe H, Evaristo C, Lemos S, Wlodarczyk M, Worth A, Le Bon A, Selin LK, Riviere Y, Di Santo JP, Borrow P, Rocha B. Epitope specificity and relative clonal abundance do not affect CD8 differentiation patterns during lymphocytic choriomeningitis virus infection. J. Virol. 2009;83:11795–11807. doi: 10.1128/JVI.01402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 52.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, Ten Brinke A, Tak PP, Eldering E, Nolte MA, van Lier RA. The Costimulatory Molecule CD27 Maintains Clonally Diverse CD8(+) T Cell Responses of Low Antigen Affinity to Protect against Viral Variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 53.Arens R, Nolte MA, Tesselaar K, Heemskerk B, Reedquist KA, van Lier RA, van Oers MH. Signaling through CD70 regulates B cell activation and IgG production. J. Immunol. 2004;173:3901–3908. doi: 10.4049/jimmunol.173.6.3901. [DOI] [PubMed] [Google Scholar]
- 54.Garcia P, De Heredia AB, Bellon T, Carpio E, Llano M, Caparros E, Aparicio P, Lopez-Botet M. Signalling via CD70, a member of the TNF family, regulates T cell functions. J. Leukoc. Biol. 2004;76:263–270. doi: 10.1189/jlb.1003508. [DOI] [PubMed] [Google Scholar]
- 55.Keller AM, Groothuis TA, Veraar EA, Marsman M, de Buy Wenniger LM, Janssen H, Neefjes J, Borst J. Costimulatory ligand CD70 is delivered to the immunological synapse by shared intracellular trafficking with MHC class II molecules. Proc. Natl. Acad. Sci. U S A. 2007;104:5989–5994. doi: 10.1073/pnas.0700946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zwart W, Peperzak V, de Vries E, Keller AM, van der Horst G, Veraar EA, Geumann U, Janssen H, Janssen L, Naik SH, Neefjes J, Borst J. The invariant chain transports TNF family member CD70 to MHC class II compartments in dendritic cells. J. Cell Sci. 2010;123:3817–3827. doi: 10.1242/jcs.068510. [DOI] [PubMed] [Google Scholar]
- 57.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 58.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin. Immunol. 2009;21:69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowley TF, Al-Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J. Immunol. 2004;172:6039–6046. doi: 10.4049/jimmunol.172.10.6039. [DOI] [PubMed] [Google Scholar]
- 62.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J. Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 63.Yamada A, Salama AD, Sho M, Najafian N, Ito T, Forman JP, Kewalramani R, Sandner S, Harada H, Clarkson MR, Mandelbrot DA, Sharpe AH, Oshima H, Yagita H, Chalasani G, Lakkis FG, Auchincloss HJ, Sayegh MH. CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo. J. Immunol. 2005;174:1357–1364. doi: 10.4049/jimmunol.174.3.1357. [DOI] [PubMed] [Google Scholar]
- 64.Dong H, Franklin NA, Roberts DJ, Yagita H, Glennie MJ, Bullock TN. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. J. Immunol. 2012;188:3829–3838. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 66.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86–CD28 costimulation and restoration by IL-2 during the recall response. J. Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, Larsen CP, Ford ML. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J. Immunol. 2011;186:2033–2041. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakajima A, Oshima H, Nohara C, Morimoto S, Yoshino S, Kobata T, Yagita H, Okumura K. Involvement of CD70–CD27 interactions in the induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2000;109:188–196. doi: 10.1016/s0165-5728(00)00324-6. [DOI] [PubMed] [Google Scholar]
- 69.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat. Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 70.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J. Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 71.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, Bullock TN. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33:769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaura K, Boenisch O, Watanabe T, Ueno T, Vanguri V, Yang J, Tanaka K, Guleria I, Borst J, Zhai Y, Kupiec-Weglinski JW, Najafian N. Differential requirement of CD27 costimulatory signaling for naive versus alloantigen-primed effector/memory CD8+ T cells. Am J Transplant. 2010;10:1210–1220. doi: 10.1111/j.1600-6143.2010.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dai H, Chen J, Shao W, Wang F, Xu S, Peng Y, Lin Y, Xia J, Ekberg H, Wang X, Qi Z. Blockade of CD27/CD70 pathway to reduce the generation of memory T cells and markedly prolong the survival of heart allografts in presensitized mice. Transpl. Immunol. 2011 doi: 10.1016/j.trim.2011.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.