Abstract
Background and Purpose
Hydrocephalus is an important complication of subarachnoid hemorrhage (SAH). We investigated the occurrence of acute hydrocephalus in a rat SAH model.
Methods
SAH was induced by endovascular perforation in adult male Sprague-Dawley rats (n=36). Sham rats (n=8) underwent the same procedure without perforation. MRI was performed 24 hours after SAH and the volume of the ventricular system and extent of T2* hypointensity lesions were measured. We defined hydrocephalus as ventricular volume greater than +3 standard deviations above the mean in sham animals. SAH grade was determined and brains were used for histology, immunohistochemistry, Perls’ staining and Western blot analysis. Ventricular wall damage was defined as percentage of ependymal surface disruption.
Results
All surviving rats (n=27) after SAH had ventricular enlargement (33.6±4.7 vs. 13.5±1.4 mm3 in sham animals, p<0.01). Ventricular volume correlated with SAH severity (r=0.48, p<0.05). 12 of 27 SAH rats demonstrated hydrocephalus and all had intraventricular blood accumulation. Rats with hydrocephalus had more severe ventricular wall damage (7.4±1.2%) than the sham animals (0.6±0.2%, p<0.01) and rats without hydrocephalus (1.1±0.2%, p<0.01). Periventricular iron deposition was observed and HO-1 and Iba-1 expression were markedly increased in hydrocephalus rats.
Conclusions
SAH causes ventricular enlargement in a rat endovascular perforation model, with hydrocephalus occurring in 44% of animals at 24 hours. Rats with hydrocephalus had more severe SAH, intraventricular hemorrhage and greater ventricular wall damage.
Keywords: acute hydrocephalus, subarachnoid hemorrhage, iron, rats
Acute hydrocephalus is a common complication after aneurysmal subarachnoid hemorrhage (SAH). Hydrocephalus can result in increased intracranial pressure that may lead to decreased cerebral blood flow and clinical deterioration.1, 2 Hydrocephalus portends a worse prognosis in patients with aneurysmal SAH.3 Few studies have investigated acute hydrocephalus after SAH in animal models. Here we report a rat model of acute hydrocephalus after SAH induced by endovascular perforation that may prove useful for developing potential therapies.
Materials and Methods
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. 44 adult male Sprague-Dawley rats were used in this study. SAH induction (n=36) was performed using an endovascular perforation technique.4, 5 Sham rats (n=8) underwent the same procedure without perforation. 24 hours after SAH MRI was performed in a 7.0-T Varian MR scanner (Varian Inc.) with acquisition of T2 fast spin-echo and T2* gradient-echo sequences using a field of view of 35×35 mm, matrix of 256×128 mm and 25 coronal slices (0.5-mm-thick). Ventricular volume was measured from the frontal horn of lateral ventricle to the foramen of Luschka by combining ventricular area over all slices and multiplying by section thickness. Hydrocephalus was defined as ventricular volume over +3 standard deviations(SD) of the mean in sham animals. Volume of hemorrhage on MRI was calculated from hypointensity signal on T2* gradient-echo sequence.6
After MRI, animals were euthanized and their brains were examined. The extent of SAH was assessed using a modified grading system.5, 7 The basal brain including brainstem was divided into six segments. Each segment was assigned a grade from 0 to 3 depending on the amount of SAH as follows; Grade 0:no SAH; Grade 1:minimal SAH; Grade 2:moderate SAH with recognizable arteries; Grade 3:SAH covering the cerebral arteries. Brains were then embedded and sectioned in 18μm slices. Coronal sections at the level of the optic nerve, chiasm and optic tract were stained with hematoxylin and eosin. Ependymal surface disruption (length of ependymal disruption divided by the total ventricular surface perimeter per section) was determined as a percentage. All image analysis was performed using ImageJ software by a blinded observer.
Immunohistochemical staining was performed using avidin-biotin complex. Brain iron deposition was assessed using Perls’ staining8. We found that light blue Perls positive cells can be detected using the Perls’ reaction without DAB enhancement close to the ventricular wall in the hydrocephalic-SAH group. We (and others) have found that the method of Perls’ reaction without DAB enhancement is not very sensitive. The reaction has to be done in a glass container rather than a plastic container.
Western blot analysis was performed as previously described.8 A 3-mm thick coronal brain slice was cut approximately 5-mm from the frontal pole and the periventricular tissues including the hippocampi were sampled (sham animals=3, SAH with hydrocephalus=4, SAH without hydrocephalus=4). We used polyclonal anti-rat HO-1 IgG (StressGen), and polyclonal anti-rat Iba-1 IgG(Abcam) at 1:2000 dilution.
Values are presented as the means±SEM. Data were analyzed with Student’s t-test, analysis of variance and Spearman rank correlation test. Statistical significance was set at p<0.05.
Results
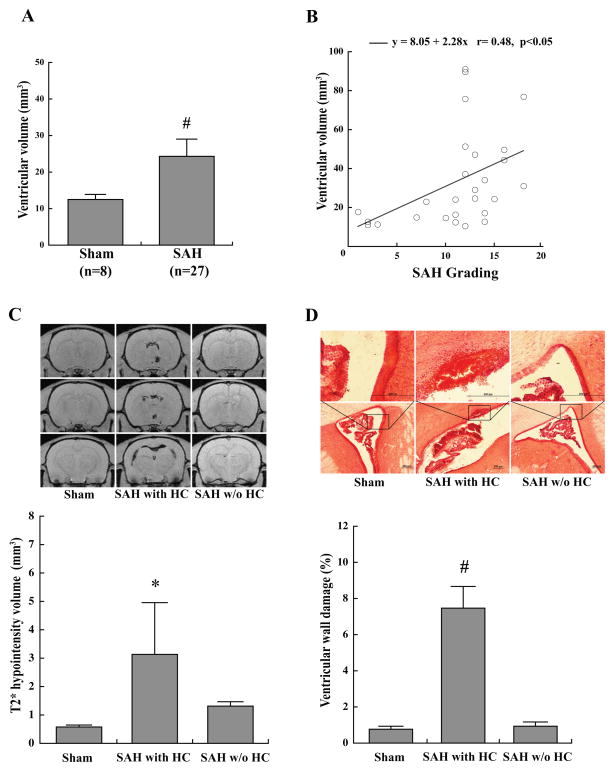
The mortality in this study was 25%(9 of 36 rats) at 24 hours after endovascular perforation. No sham animals died(n=8). Endovascular perforation induced SAH in all cases (n=27) and results in ventricular enlargement at 24 hours (33.6±4.7 mm3) compared with sham controls (13.5±1.4 mm3, n=8, p<0.01; Fig. 1, 2A). The ventricular volume in SAH animals correlated with SAH grade (r=0.48, p<0.05; Fig. 2B). Hydrocephalus was defined as ventricular volume greater than +3SD above the mean in sham animals and was present in 44% (12/27) of SAH animals. All animals with hydrocephalus (n=12) had intra-ventricular hemorrhage confirmed by MRI and histology. Rats with hydrocephalus had larger T2* hypointensity volume (4.9±1.8 mm3, n=12) than the sham animals (0.6±0.1 mm3, n=8, p<0.05) and SAH animals without hydrocephalus (1.3±0.2 mm3, n=15, p<0.05; Fig. 2C). The percentage of ventricular wall damage was greater in SAH animals with hydrocephalus (7.4±1.2%, n=8) compared to those without hydrocephalus (1.1±0.2%, n=11, p<0.01) and sham controls (n=5) (0.6±0.2%, n=5, p<0.01; Fig. 2D). Using Perls’ staining, iron-positive cells were found in ependyma and subependyma of hydrocephalic SAH rats (Fig. 3A). Periventricular HO-1 immunoreactivity was also observed in hydrocephalic SAH animals. By Western blotting, HO-1 levels were increased in rats with hydrocephalus (4526±312 pixels) compared to sham controls (1370±191 pixels in the sham animals, n=3–4, p<0.01; Fig. 3B). Periventricular Iba-1 immunoreactivity was observed in hydrocephalic SAH animals. By Western blotting, Iba-1 levels were increased in rats with hydrocephalus (4119±384 pixels) compared to sham controls (1911±348 pixels, p<0.05) and those without hydrocephalus (1889±307 pixels, n=3–4, p<0.05; Fig. 3C).
Fig. 1.

Coronal T2 and T2* images, photomicrographs and hematoxylin and eosin sections 24 hours after endovascular perforation(A) or sham procedure(B). HE: hematoxylin and eosin staining.
Fig. 2.
Ventricular volume 24 hours after endovascular perforation or sham procedure (A). Correlation of ventricular volume and SAH grade at 24 hours (B). Coronal T2* images of sham and SAH animals with or without hydrocephalus at 24 hours. Rats with hydrocephalus have a larger hypointensity volume than the sham animals or rats without hydrocephalus (C). Hematoxylin and eosin staining of sham and SAH animals with or without hydrocephalus. Note the presence of intraventricular hemorrhage in the hydrocephalic rat. Boxes show intact ependyma (sham, SAH without hydrocephalus) and disrupted ependyma with intraventricular hemorrhage (SAH with hydrocephalus). SAH animals with hydrocephalus have more ventricular wall damage compared to sham or SAH animals without hydrocephalus (D). *p<0.05 and #p<0.01. Values are mean±SEM. Scale bar=200μm. SAH-subarachnoid hemorrhage; HC-hydrocephalus.
Fig. 3.
(A) Perls’ reaction (with or without 3,3′Diaminobenzidine, DAB, enhancement) showed iron-positive cells (brackets or arrowheads) in ependyma and subependyma in hydrocephalic SAH rats but not sham and non-hydrocephalic rats. The inserts in A: show DAB staining alone which only showed erythrocytes on the ependymal surface. Periventricular HO-1 immunoreactivity of sham and SAH animals with or without hydrocephalus. Western blot analysis demonstrated higher HO-1 levels in SAH animals with hydrocephalus compared with sham controls (#p<0.01; n=3–4) (B). Periventricular Iba-1 immunoreactivity of sham and SAH animals with or without hydrocephalus. Western blot analysis demonstrated higher Iba-1 levels in hydrocephalic SAH rats compared with SAH animals without hydrocephalus and sham controls (*p<0.05; n=3–4) (C). Scale bars=20 or 200μm. Values are mean±SEM. SAH-subarachnoid hemorrhage; HC-hydrocephalus.
Discussion
We have demonstrated a rat model of SAH induced by endovascular perforation that results in hydrocephalus in 44% of animals at 24 hours. Hydrocephalus is associated with SAH grade, intraventricular hemorrhage, and ventricular wall damage. Periventricular iron deposition was observed. HO-1 (a stress marker, also called heat shock protein 32) and Iba-1 (a marker of microglia and macrophages) expression was increased in animals with hydrocephalus, suggesting that hydrocephalus may exacerbate SAH-induced brain injury. Clinical studies have shown that amount of blood in the subarachnoid space and the presence of intraventricular hemorrhage increase the risk of acute hydrocephalus.9, 10 Similar to these reports, ventricular volume was correlated with the severity of SAH and all animals with hydrocephalus had intraventricular blood in our study. Our results suggest that the amount of subarachnoid blood or presence of intraventricular blood may cause obstruction of CSF flow. However, ependymal deficits and periventricular iron deposition were also observed in the animals with hydrocephalus. Ependymal damage and iron deposition may lead to increased periventricular brain injury, which may play a role in hydrocephalus. High periventricular accumulation of iron in the hydrocephalic SAH rats may indicate a role of iron in producing hydrocephalus because our previous study has shown that iron causes hydrocephalus in a rat intraventricular hemorrhage model6.
Conclusions
Induction of SAH by endovascular perforation in rats results in a 44% rate of hydrocephalus at 24 hours. Intraventricular hemorrhage, damage to the ventricular wall, periventricular iron deposition and tissue stress were observed in this rat model of acute hydrocephalus after SAH. This model should prove useful in examining potential therapeutics, including iron chelators and anti-inflammatory agents, for SAH-induced hydrocephalus.
Acknowledgments
Sources of Funding
This study was supported by grants NS-039866, NS-057539, NS-073595, NS-079157 and NS-007222.
Footnotes
Disclosures
None
References
- 1.Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJ, van der Schaaf IC, Rinkel GJ. Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. Am J Neuroradiol. 2010;31:67–70. doi: 10.3174/ajnr.A1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989;20:747–753. doi: 10.1161/01.str.20.6.747. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2010;30:1793–1803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42:465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 9.Demirgil BT, Tugcu B, Postalci L, Guclu G, Dalgic A, Oral Z. Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Minim Invasive Neurosurg. 2003;46:344–348. doi: 10.1055/s-2003-812500. [DOI] [PubMed] [Google Scholar]
- 10.Jartti P, Karttunen A, Jartti A, Ukkola V, Sajanti J, Pyhtinen J. Factors related to acute hydrocephalus after subarachnoid hemorrhage. Acta Radiol. 2004;45:333–339. doi: 10.1080/02841850410004274. [DOI] [PubMed] [Google Scholar]