Abstract
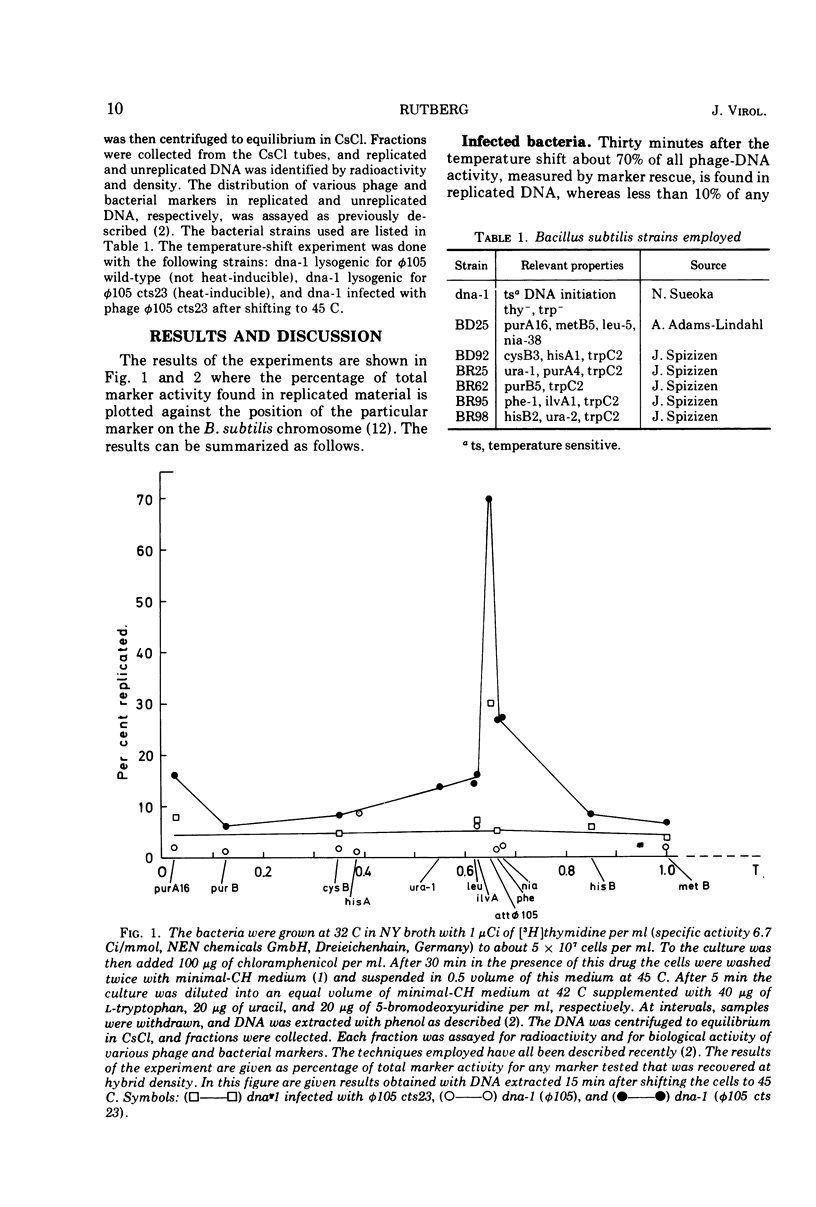
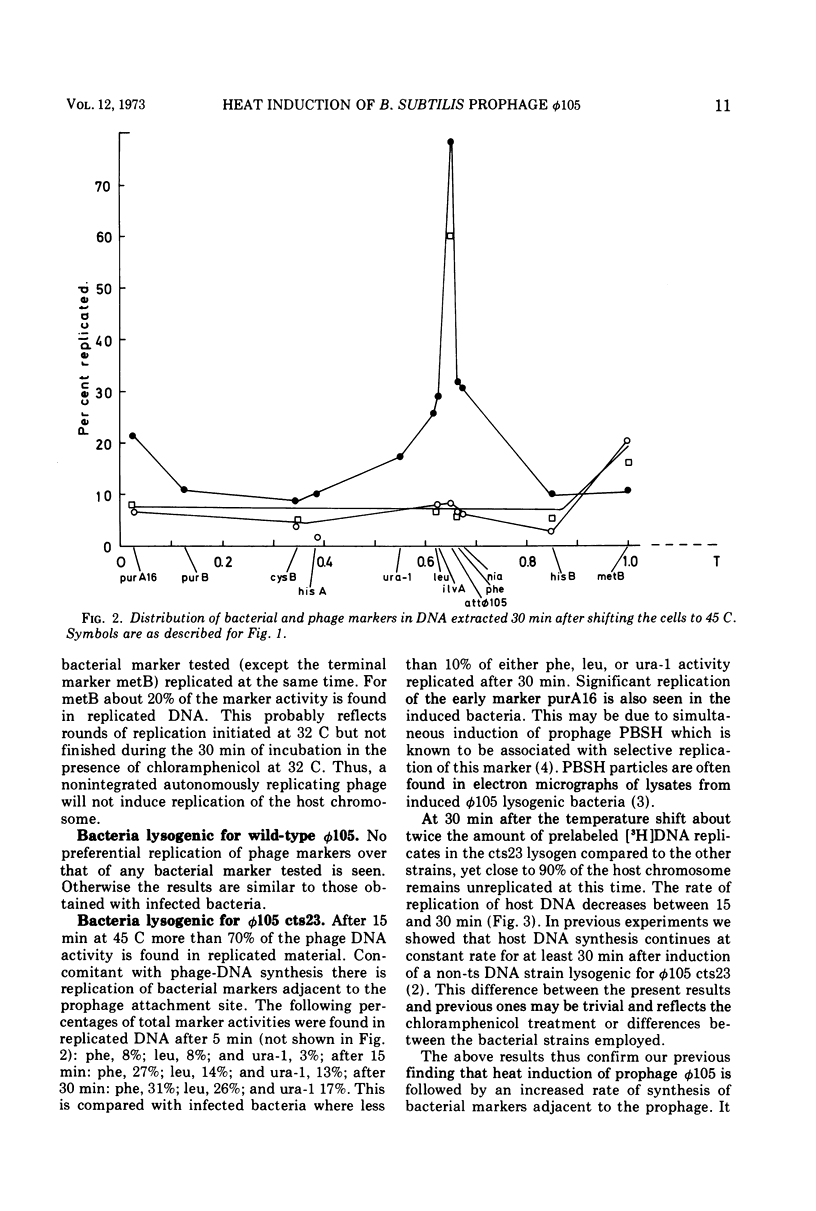
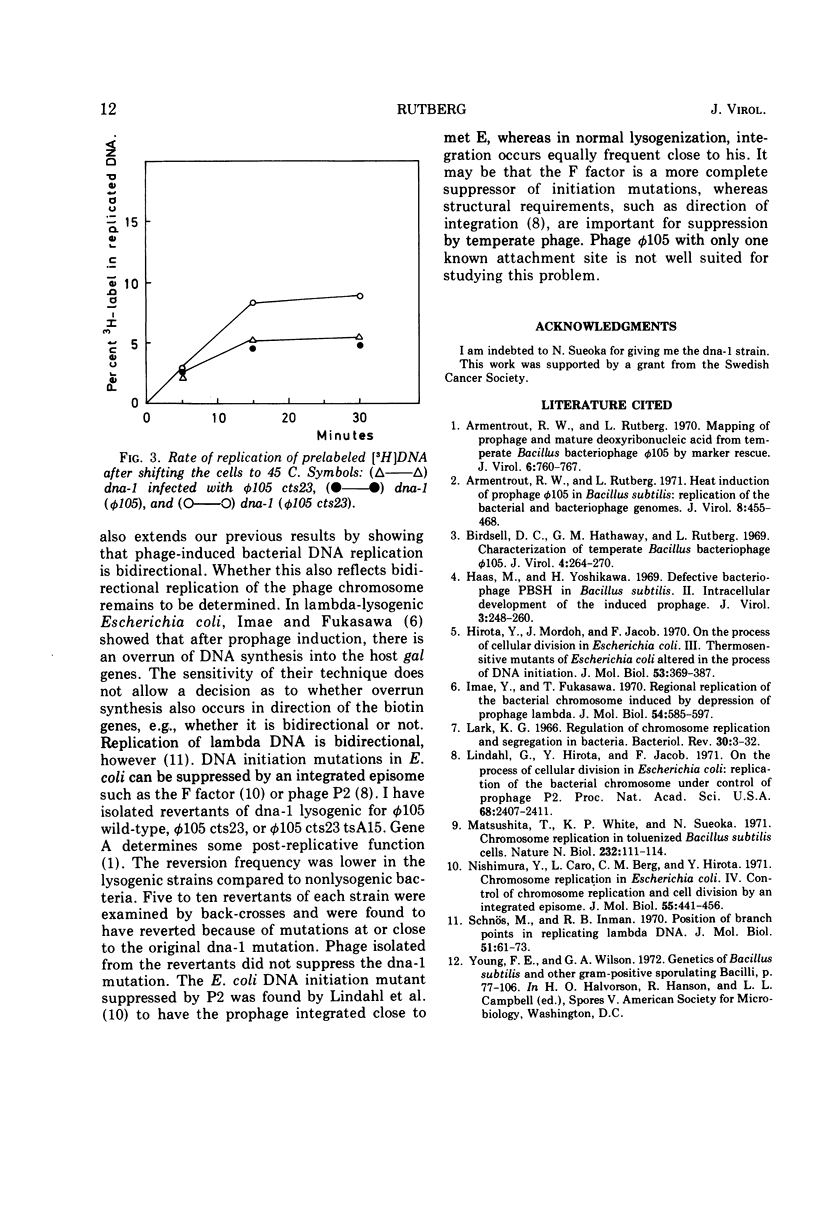
A mutant of Bacillus subtilis, dna-1, which cannot initiate new rounds of DNA replication (obtained from N. Sueoka) was lysogenized with wild-type φ 105 and with the heat-inducible mutant φ 105 cts23. Bacteria were incubated at the permissive temperature in the presence of chloramphenicol and then shifted to the nonpermissive temperature where induction of φ 105 cts23 occurs. DNA made after the shift was labeled with a density label, and the distribution of bacterial and phage markers in replicated and unreplicated DNA was determined. Similar experiments were performed with nonlysogenic dna-1 infected with phage φ 105 cts23 after the temperature shift. The results show that after induction of φ 105 cts23 prophage, bacterial markers on either side of the prophage replicate at an increased rate compared to more distant markers. No selective stimulation of bacterial DNA synthesis was observed on infection or after shifting bacteria lysogenic for noninducible phage to the higher temperature. Attempts to suppress the initiation mutation dna-1 by phage φ 105 were unsuccessful.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentrout R. W., Rutberg L. Heat induction of prophage phi 105 in Bacillus subtilis: replication of the bacterial and bacteriophage genomes. J Virol. 1971 Oct;8(4):455–468. doi: 10.1128/jvi.8.4.455-468.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentrout R. W., Rutberg L. Mapping of prophage and mature deoxyribonucleic acid from temperate Bacillus bacteriophage phi 105 by marker rescue. J Virol. 1970 Dec;6(6):760–767. doi: 10.1128/jvi.6.6.760-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Hathaway G. M., Rutberg L. Characterization of Temperate Bacillus Bacteriophage phi105. J Virol. 1969 Sep;4(3):264–270. doi: 10.1128/jvi.4.3.264-270.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. II. Intracellular development of the induced prophage. J Virol. 1969 Feb;3(2):248–260. doi: 10.1128/jvi.3.2.248-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Imae Y., Fukasawa T. Regional replication of the bacterial chromosome induced by derepression of prophage lambda. J Mol Biol. 1970 Dec 28;54(3):585–597. doi: 10.1016/0022-2836(70)90129-4. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli: replication of the bacterial chromosome under control of prophage P2. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2407–2411. doi: 10.1073/pnas.68.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T., White K. P., Sueoka N. Chromosom replication in toluenized Bacillus subtilis cells. Nat New Biol. 1971 Jul 28;232(30):111–114. doi: 10.1038/newbio232111a0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]


