Abstract
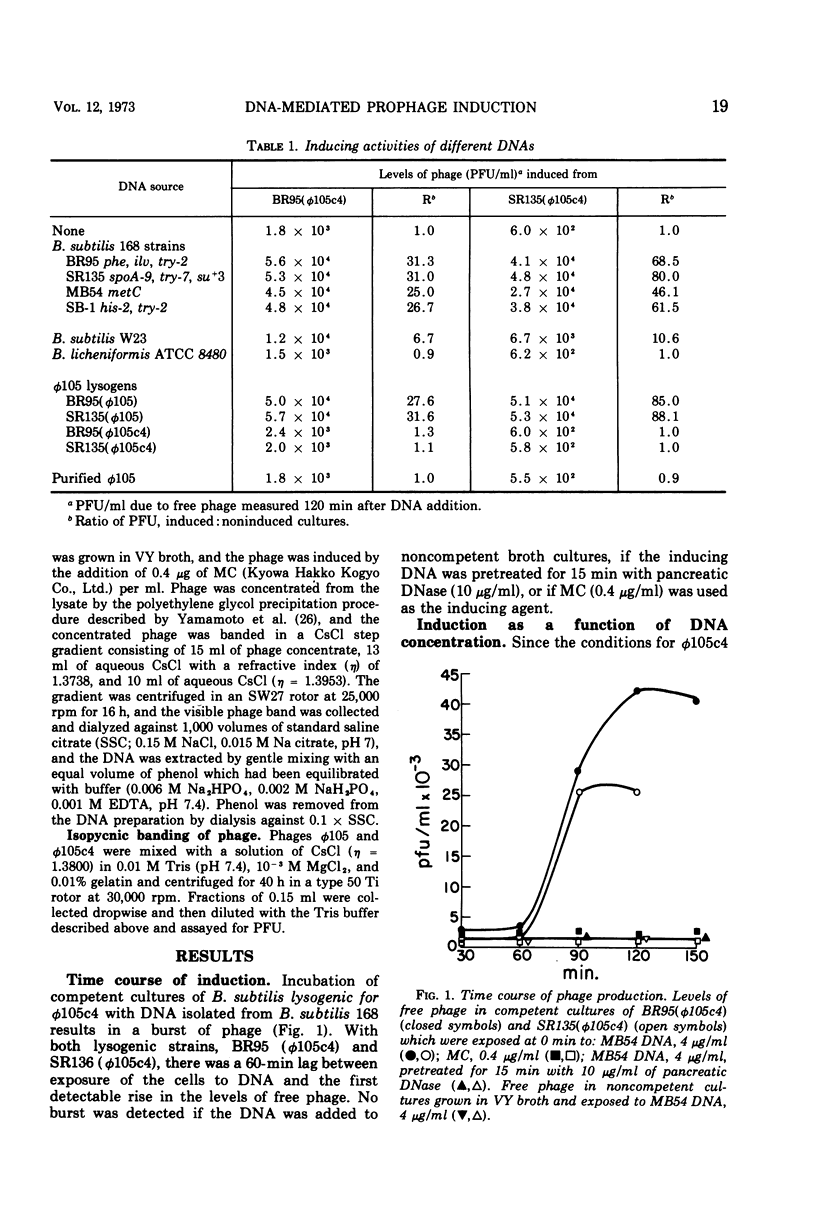
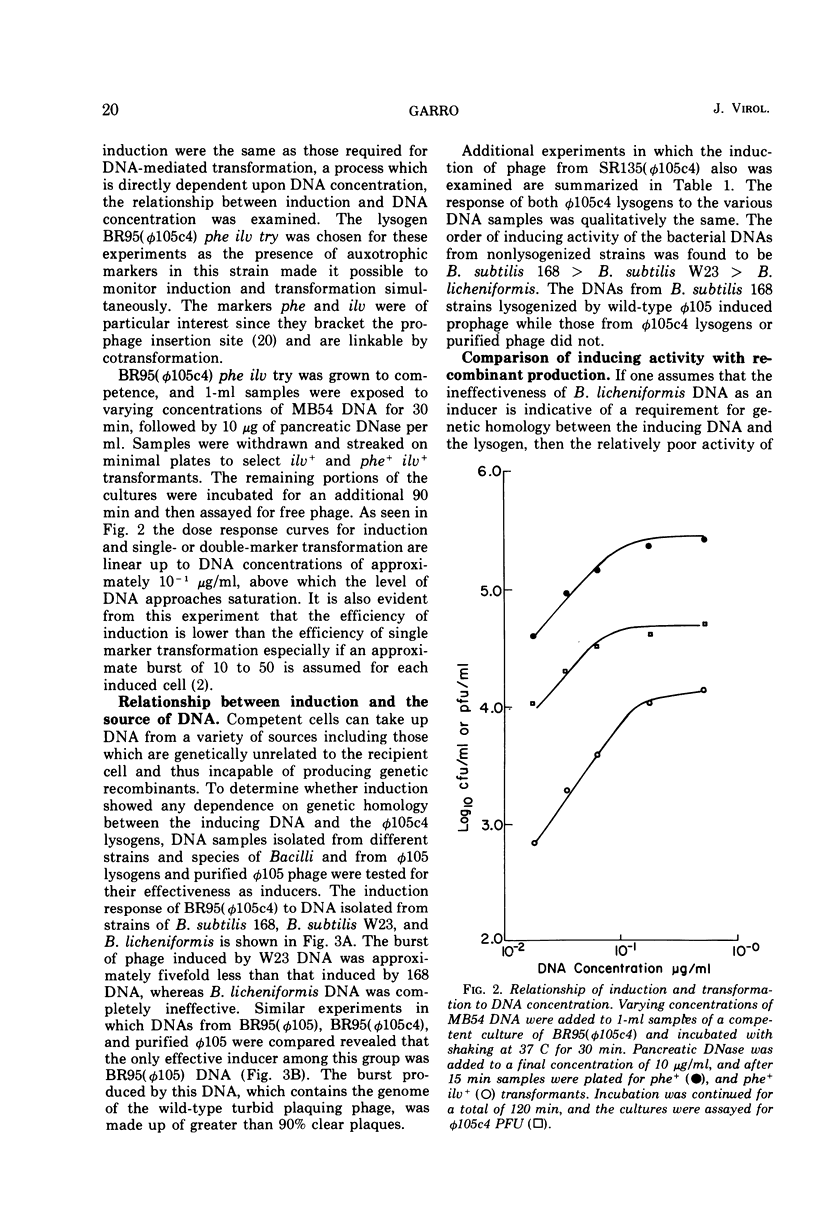
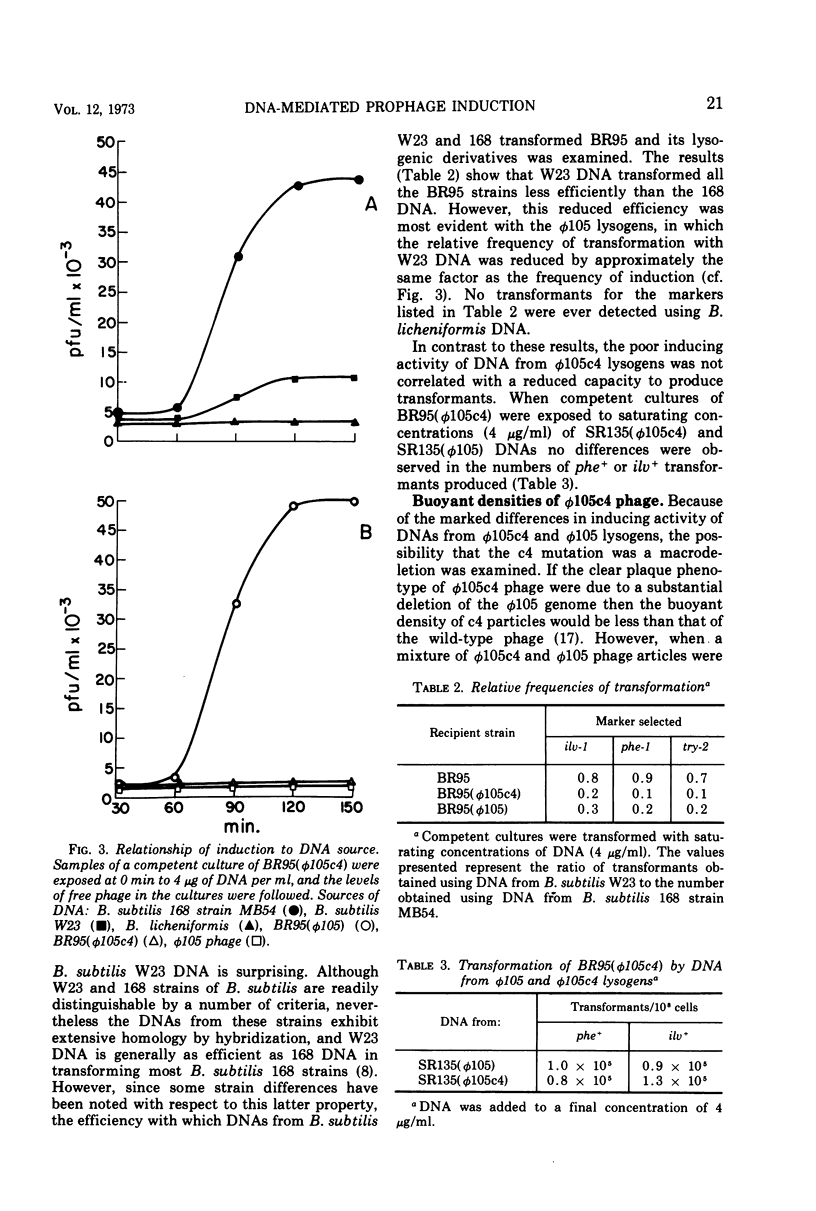
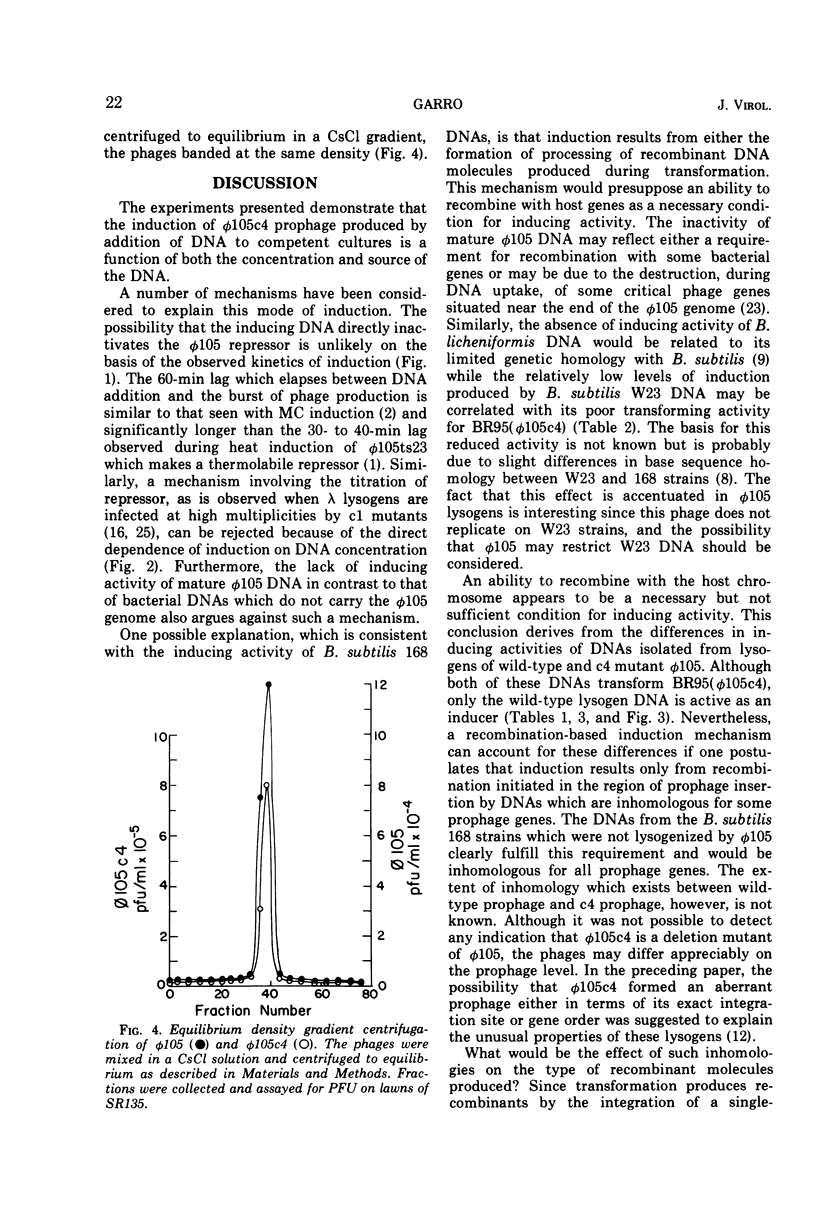
Prophage was induced when strains of Bacillus subtilis 168 lysogenic for φ105c4 were grown to competence and exposed to specific bacterial DNAs. The time course of phage production was similar to that observed for mitomycin C induction of wild-type prophage. Induction was directly dependent upon DNA concentration up to levels which were saturating for the transformation of bacterial auxotrophic markers. The extent of induction varied with the source of DNA. The burst of phage induced by DNA isolated from a W23 strain of B. subtilis was fivefold less than that induced by DNA from B. subtilis 168 strains, while B. licheniformis DNA was completely inactive. This order of inducing activity was correlated with the ability of the respective DNAs to transform auxotrophic markers carried by one of the φ105c4 lysogens. Differences in inducing activity also were observed for different forms of φ105 DNA. The DNAs isolated from φ105 phage particles and φ105c4 lysogens were inactive, whereas DNA from cells lysogenized by wild-type φ105 induced a burst of phage. When tested for transforming activity, however, both φ105c4 and φ105 lysogen DNAs were equally effective. An induction mechanism which involves recombination at the prophage insertion site is proposed to explain these differences.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentrout R. W., Rutberg L. Heat induction of prophage phi 105 in Bacillus subtilis: replication of the bacterial and bacteriophage genomes. J Virol. 1971 Oct;8(4):455–468. doi: 10.1128/jvi.8.4.455-468.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Hathaway G. M., Rutberg L. Characterization of Temperate Bacillus Bacteriophage phi105. J Virol. 1969 Sep;4(3):264–270. doi: 10.1128/jvi.4.3.264-270.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Kreneva R. A., Kushev V. V. Correction of molecular heterozygotes in the course of transformation. Mol Gen Genet. 1968;102(3):257–268. doi: 10.1007/BF00385983. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R. The regulation of phage development. Annu Rev Microbiol. 1970;24:241–296. doi: 10.1146/annurev.mi.24.100170.001325. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H. Lysogeny: viral repression and site-specific recombination. Annu Rev Biochem. 1971;40:827–854. doi: 10.1146/annurev.bi.40.070171.004143. [DOI] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J. Isolation and properties of Bacillus subtilis strains lysogenized by a clear plaque mutant of bacteriophage phi 105. J Virol. 1973 Jul;12(1):13–17. doi: 10.1128/jvi.12.1.13-17.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodgal S. H., Postel E. H. On the mechanism of integration following transformation with single-stranded DNA of Hemophilus influenzae. J Mol Biol. 1967 Sep 14;28(2):261–273. doi: 10.1016/s0022-2836(67)80008-1. [DOI] [PubMed] [Google Scholar]
- Guerrini F., Fox M. S. Effects of DNA repair in transformation-heterozygotes of pneumococcus. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1116–1123. doi: 10.1073/pnas.59.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., SUSSMAN R., MONOD J. [On the nature of the repressor ensuring the immunity of lysogenic bacteria]. C R Hebd Seances Acad Sci. 1962 Jun 13;254:4214–4216. [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., WEIGLE J. A mutation affecting the DNA content of bacteriophage lambda and its lysogenizing properties. J Mol Biol. 1961 Aug;3:399–408. doi: 10.1016/s0022-2836(61)80053-3. [DOI] [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. M., Rutberg L. Linked transformation of bacterial and prophage markers in Bacillus subtilis 168 lysogenic for bacteriophage phi 105. J Bacteriol. 1969 Jun;98(3):874–877. doi: 10.1128/jb.98.3.874-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Hopkins N. The operators controlled by the lambda phage repressor. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1282–1287. doi: 10.1073/pnas.60.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Rutberg L. Growth of bacteriophage phi 105 and its deoxyribonucleic acid in radiation-sensitive mutants of Bacillus subtilis. J Virol. 1971 Dec;8(6):919–921. doi: 10.1128/jvi.8.6.919-921.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W. Low-frequency rescue of a genetic marker in deoxyribonucleic acid from Bacillus bacteriophage phi 105 by superinfecting bacteriophage. J Virol. 1970 Dec;6(6):768–771. doi: 10.1128/jvi.6.6.768-771.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. One way to do experiments on gene conversion? Transfection with heteroduplex SPP1 DNA. Mol Gen Genet. 1970;109(1):84–106. doi: 10.1007/BF00334048. [DOI] [PubMed] [Google Scholar]
- Wiesmeyer H. Prophage repression as a model for the study of gene regulation. I. Titration of the lambda repressor. J Bacteriol. 1966 Jan;91(1):89–94. doi: 10.1128/jb.91.1.89-94.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


