Abstract
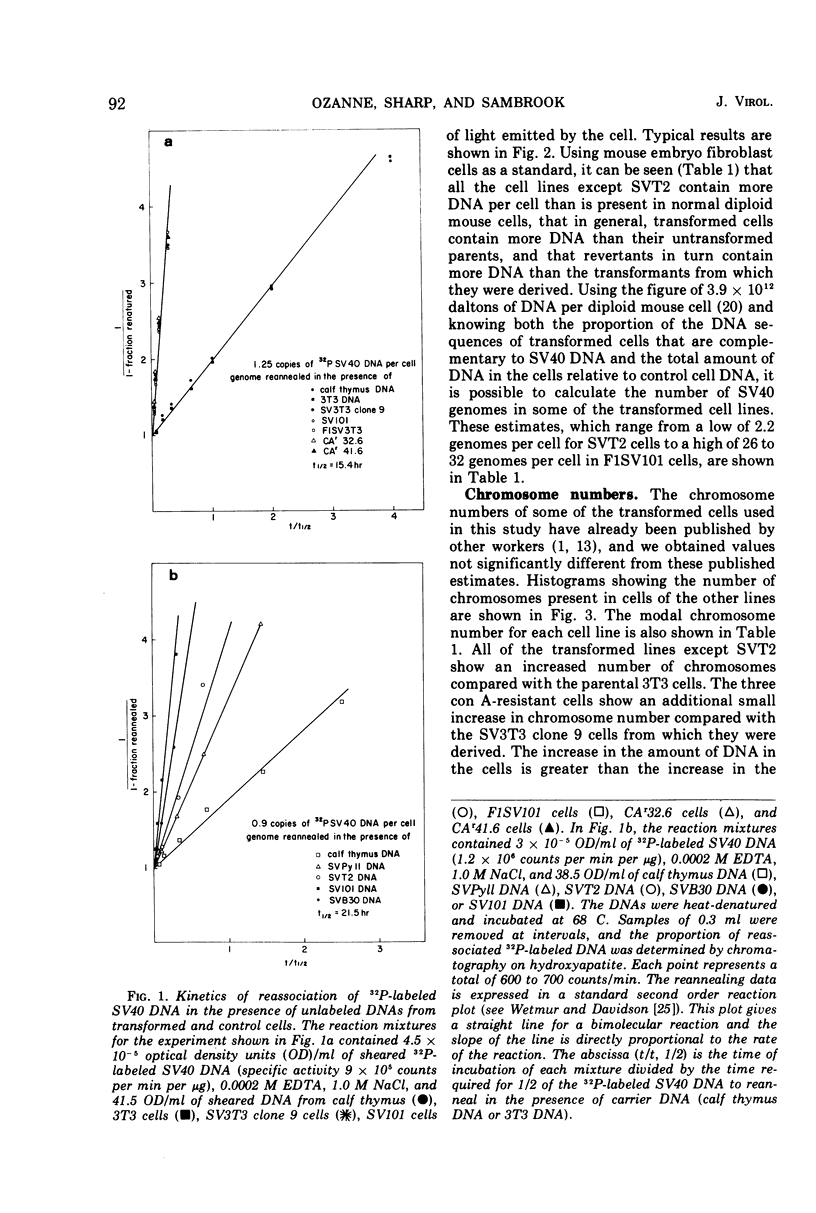
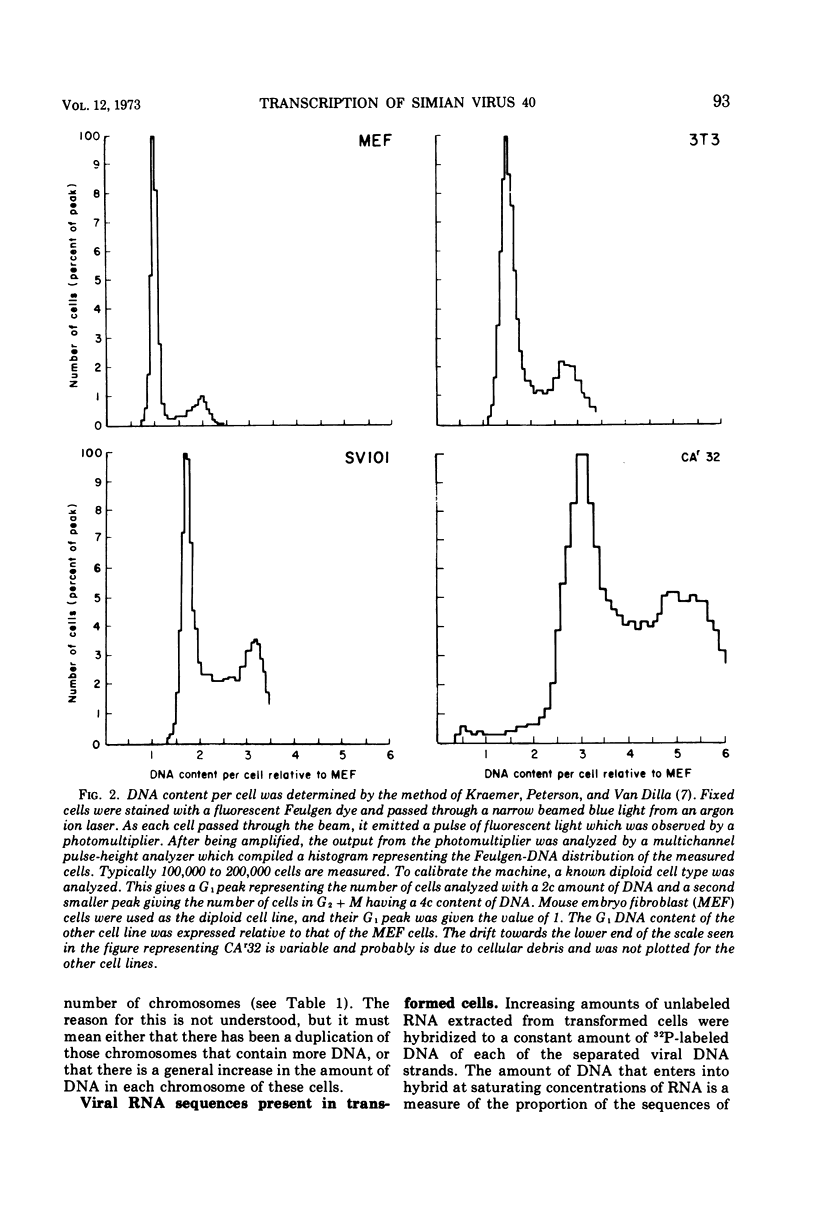
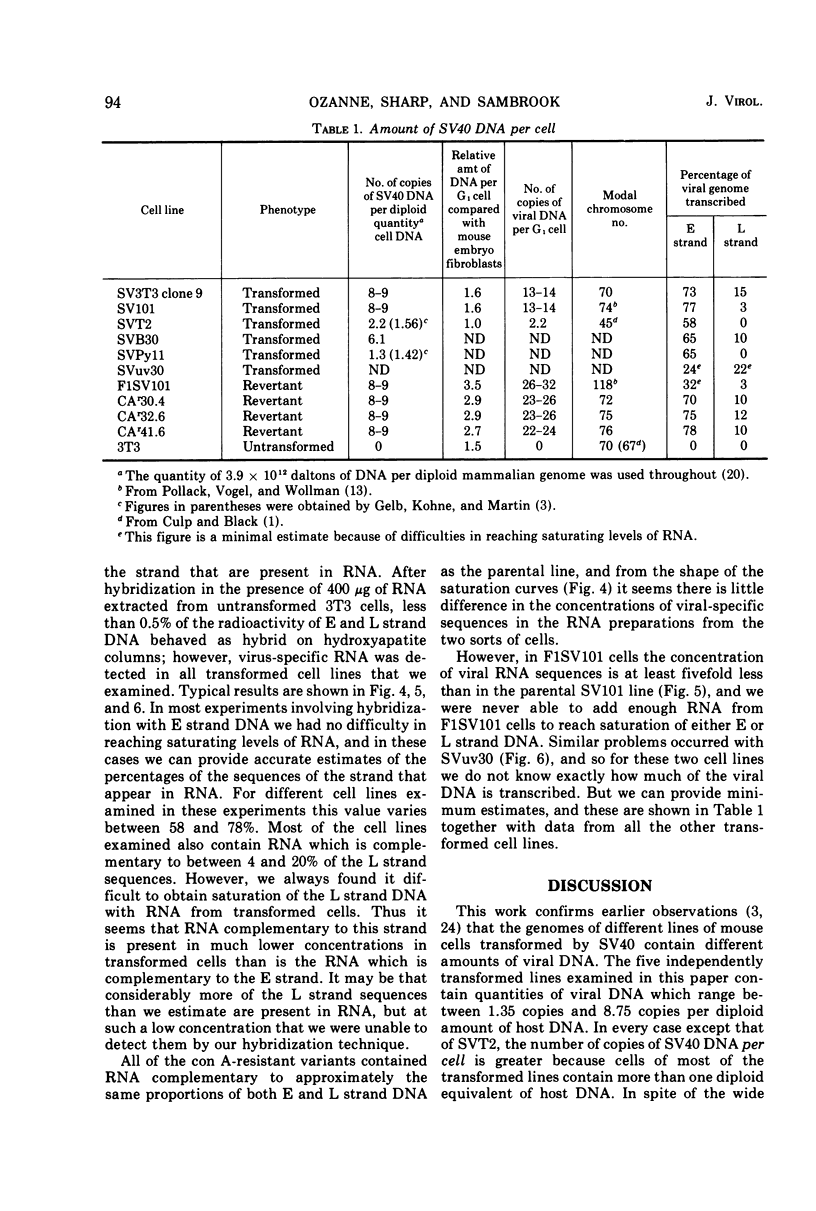
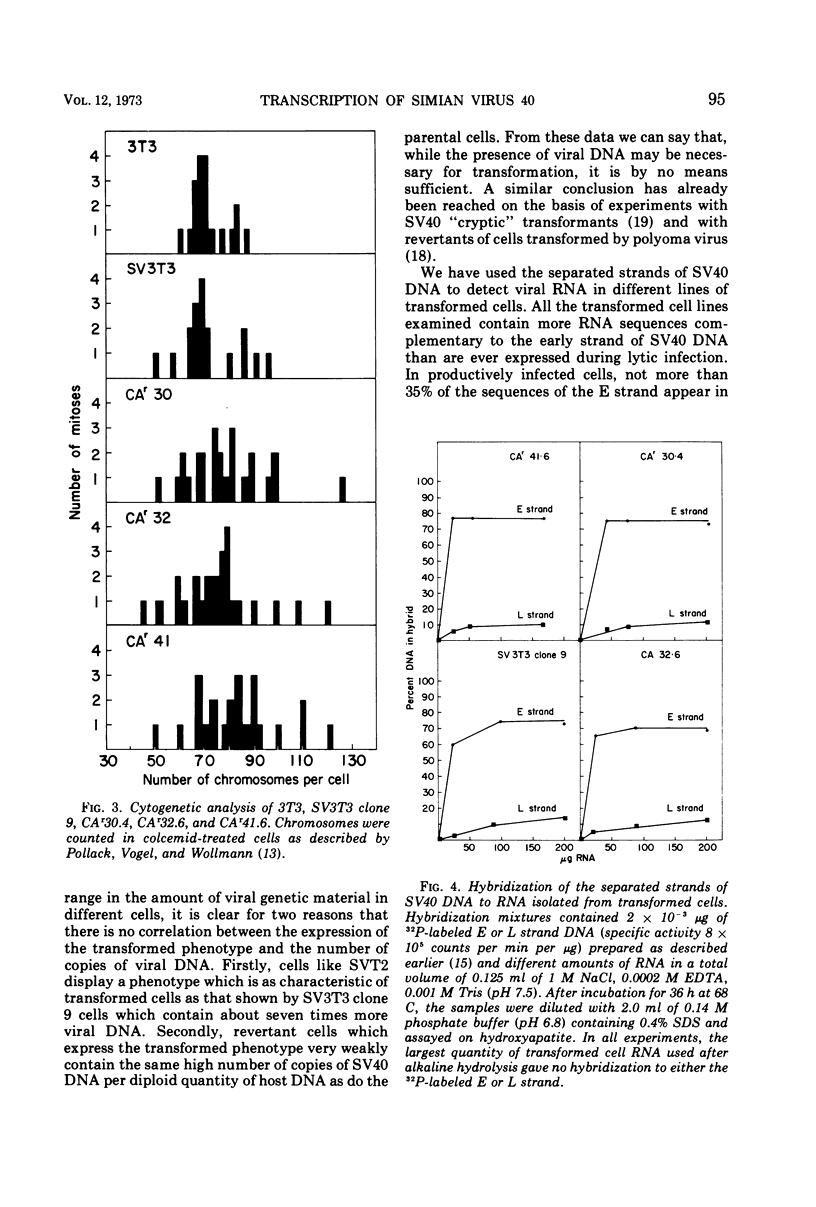
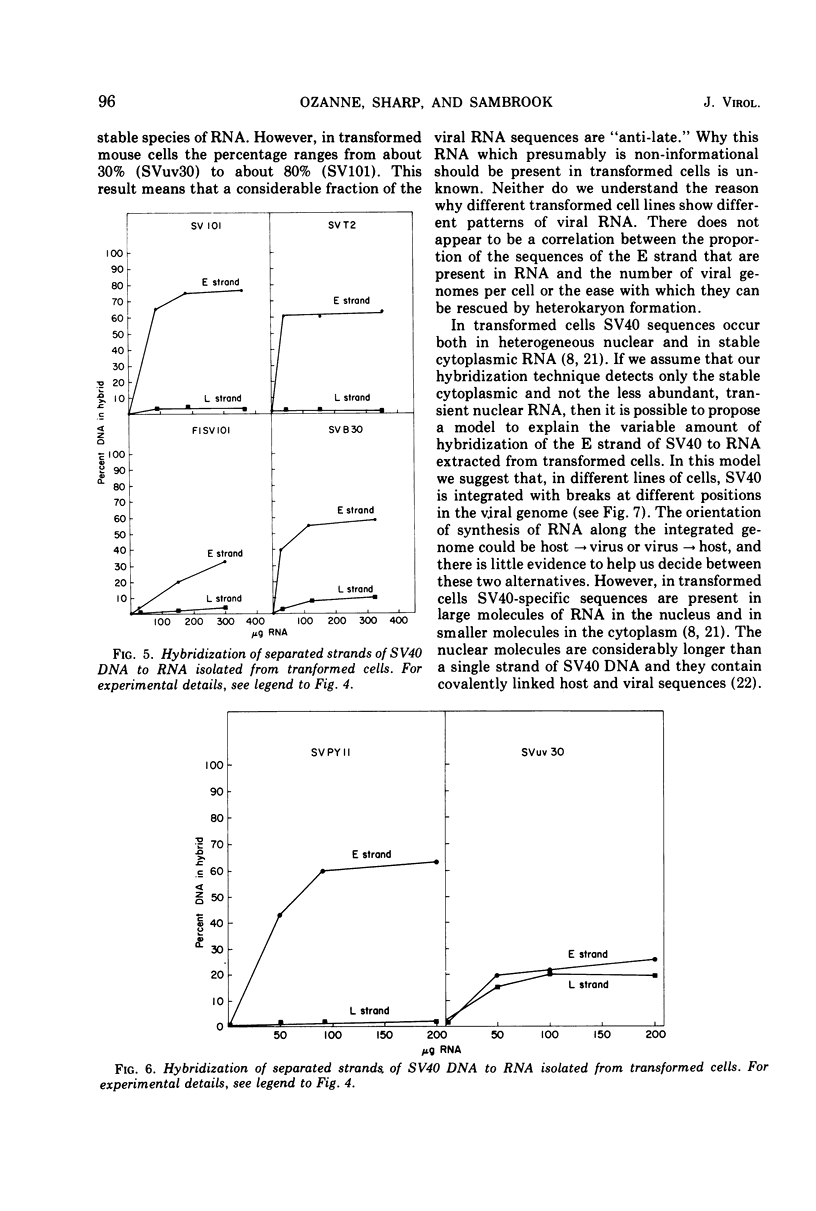
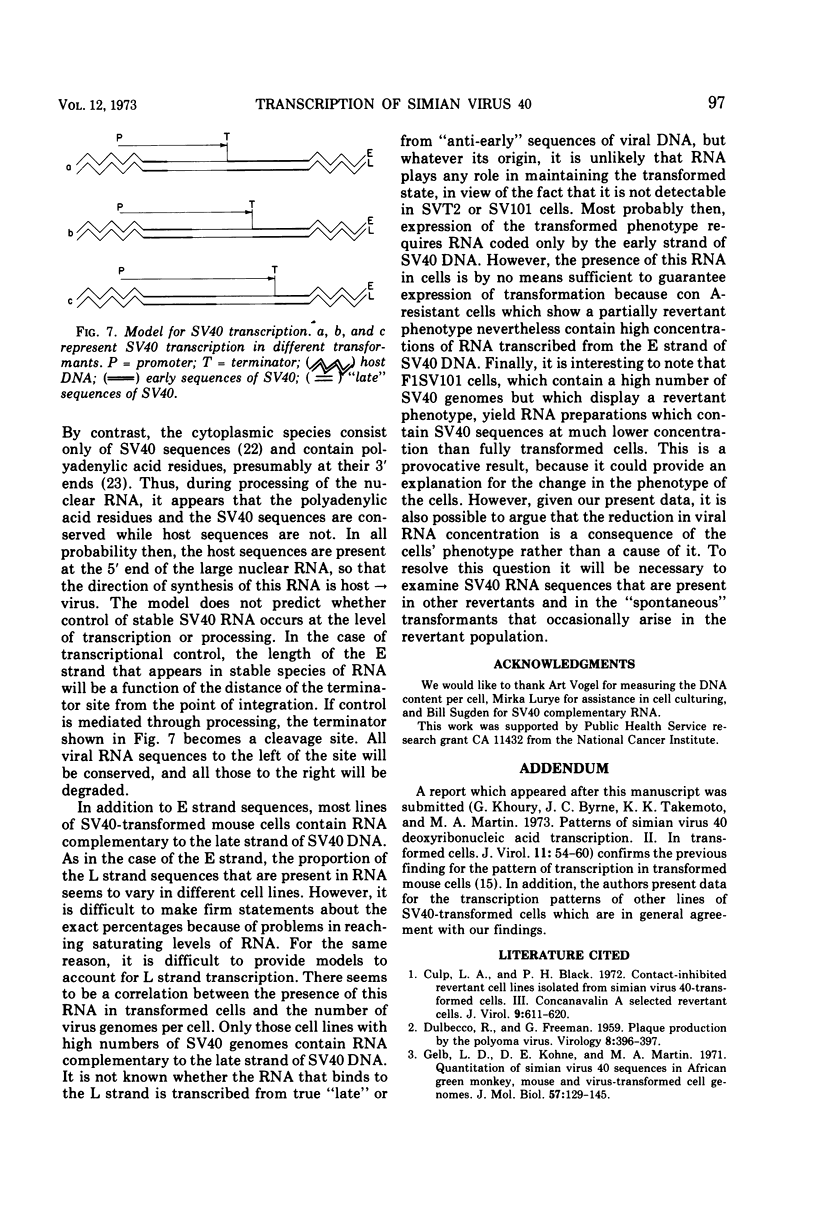
The amount of simian virus 40 (SV40) DNA present in various SV40-transformed mouse cell lines and “revertants” isolated from them was determined. The number of viral DNA copies in the different cell lines ranged from 1.35 to 8.75 copies per diploid quantity of mouse cell DNA and from 2.2 to 14 copies per cell. The revertants had the same number of viral DNA copies per diploid quantity of mouse cell DNA as their parental cell lines. (However, they showed an increased number of viral DNA copies per cell due to their increased amount of DNA.) By using separated strands of SV40 DNA, the extent of each DNA strand transcribed into stable RNA species was determined for the transformed and “revertant” cell lines. From 30 to 80% of the “early” strand and from 0 to 20% of the “late” strand was present as stable RNA species in the cell lines tested. There was no alteration in the pattern of the stable viral RNA species present in three concanavalin A-selected revertants, whereas in a fluorodeoxyuridine-selected revertant there appeared to be less viral-specific RNA present in the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from simian virus 40-transformed cells. 3. Concanavalin A-selected revertant cells. J Virol. 1972 Apr;9(4):611–620. doi: 10.1128/jvi.9.4.611-620.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Patterns of simian virus 40 deoxyribonucleic acid transcription. II. In transformed cells. J Virol. 1973 Jan;11(1):54–60. doi: 10.1128/jvi.11.1.54-60.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M., Petersen D. F., Van Dilla M. A. DNA constancy in heteroploidy and the stem line theory of tumors. Science. 1971 Nov 12;174(4010):714–717. doi: 10.1126/science.174.4010.714. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Darnell J. E. SV40-specific RNA in the nucleus and polyribosomes of transformed cells. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1089–1096. doi: 10.1073/pnas.65.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B. Variants of simian virus 40-transformed 3T3 cells that are resistant to concanavalin A. J Virol. 1973 Jul;12(1):79–89. doi: 10.1128/jvi.12.1.79-89.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Transformation by polyoma virus and simian virus 40. Adv Cancer Res. 1972;16:141–180. [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Rabinowitz Z., Sachs L. Virus deoxyribonucleic acid sequences in subdiploid and subtetraploid revertants of polyoma-transformed cells. J Virol. 1972 Sep;10(3):456–461. doi: 10.1128/jvi.10.3.456-461.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Gelb L. D., Martin M. A. Detection and quantitation of simian virus 40 genetic material in abortively transformed BALB-3T3 clones (mice-diploid cells-virus equivalents). Proc Natl Acad Sci U S A. 1972 Jan;69(1):152–156. doi: 10.1073/pnas.69.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Poly A associated with SV40 messenger RNA. Nat New Biol. 1972 Jul 26;238(82):111–113. doi: 10.1038/newbio238111a0. [DOI] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


