Abstract
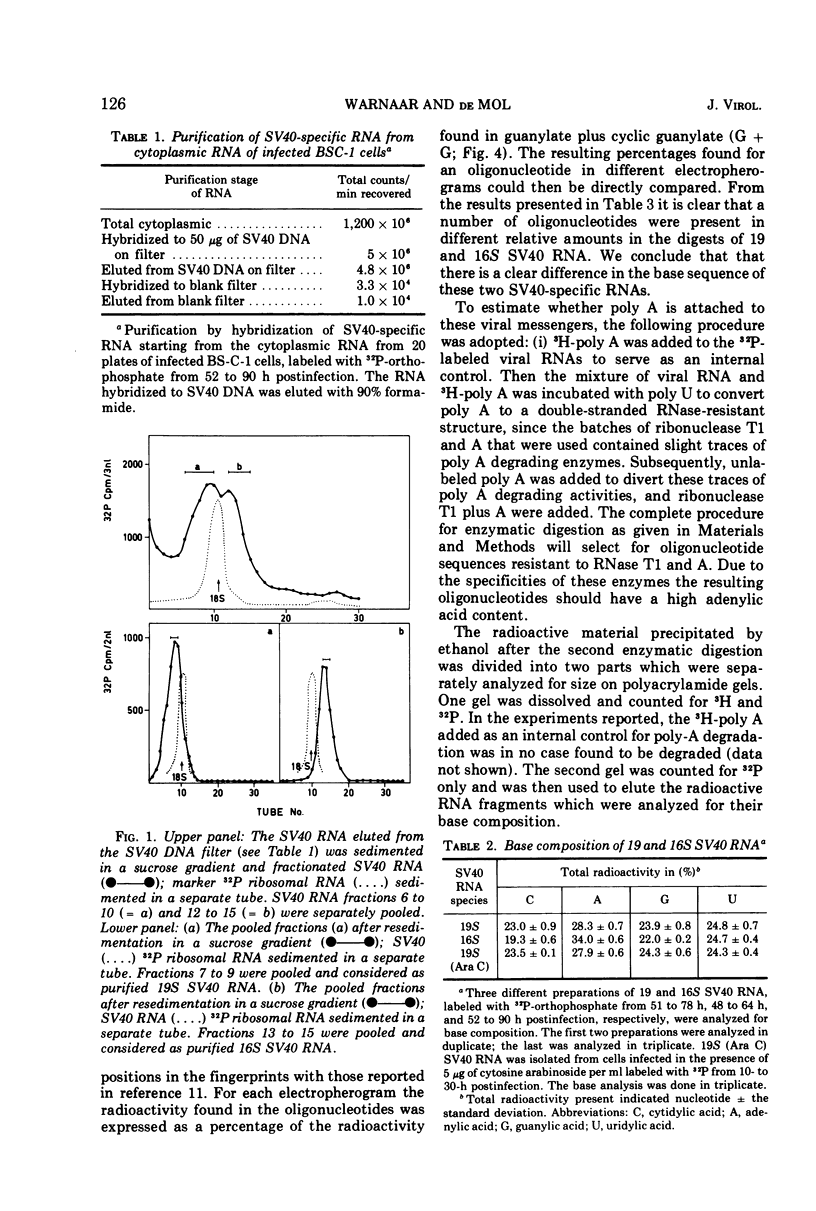
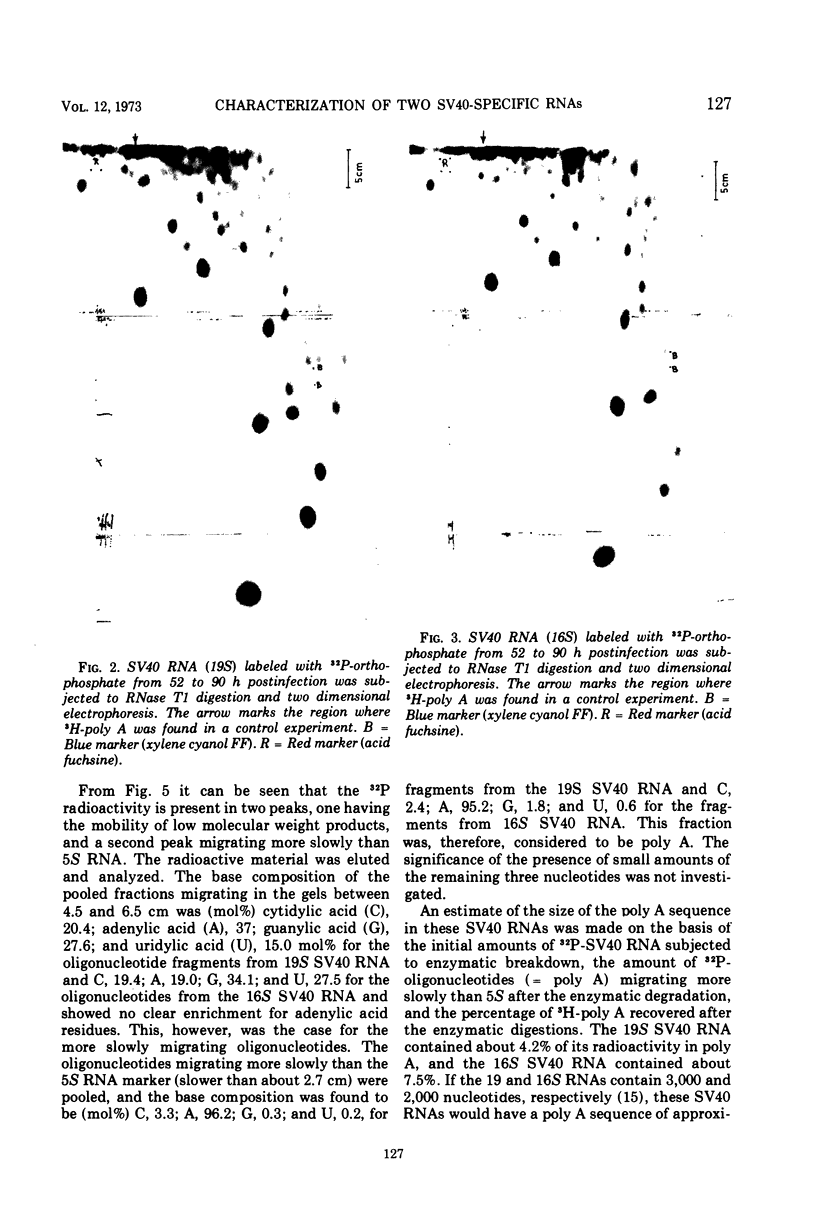
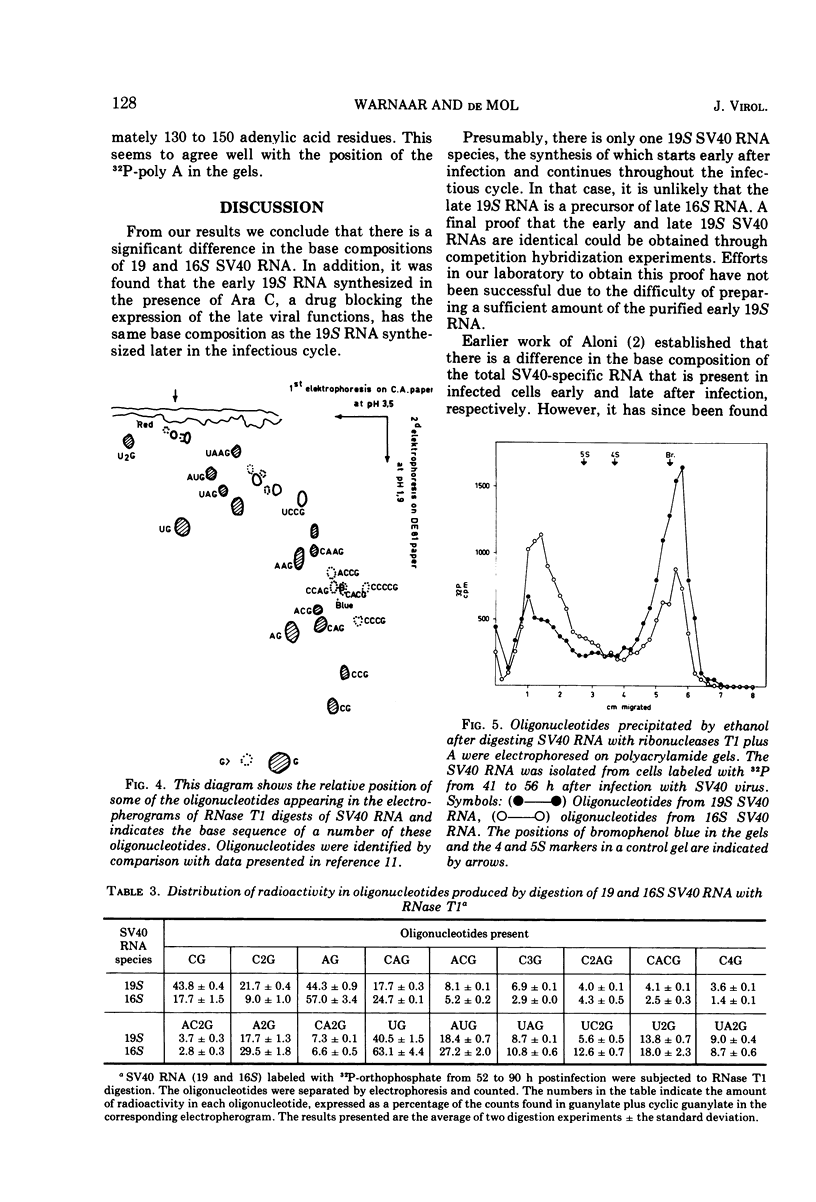
Two discrete simian virus 40 (SV40) RNA species sedimenting at 19 and 16S, respectively, that are present in infected BS-C-1 cells were characterized with respect to the base composition and the ribonuclease T1 fingerprints. The base composition of the 19S SV40 RNA was found to be cytidylic acid (C), 23.0; adenylic acid (A), 28.3; guanylic acid (G), 23.9; and uridylic acid (U), 24.8; that of the 16S SV40 RNA was C, 19.3; A, 34.0; G, 22.0; and U, 24.7 mol%. Analysis of the ribonuclease T1 fingerprints indicated a difference in the base sequence of the 19 and 16S SV40 RNA. The presence of long sequences of adenylic acid residues (poly A) in these viral RNAs was confirmed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Extensive symmetrical transcription of Simian Virus 40 DNA in virus-yielding cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2404–2409. doi: 10.1073/pnas.69.9.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Choules G. L., Zimm B. H. An acrylamide gel soluble in scintillation fluids: its application to electrophoresis at neutral and low pH. Anal Biochem. 1965 Nov;13(2):336–344. doi: 10.1016/0003-2697(65)90202-2. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Winocour E. Covalently linked cell and SV40-specific sequences in an RNA from productively infected cells. Virology. 1972 Nov;50(2):558–566. doi: 10.1016/0042-6822(72)90407-2. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Sauer G. Apparent differences in transcriptional control in cells productively infected and transformed by SV40. Nat New Biol. 1971 Jun 2;231(22):135–138. doi: 10.1038/newbio231135a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Poly A associated with SV40 messenger RNA. Nat New Biol. 1972 Jul 26;238(82):111–113. doi: 10.1038/newbio238111a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]