Abstract
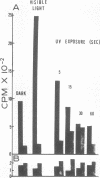
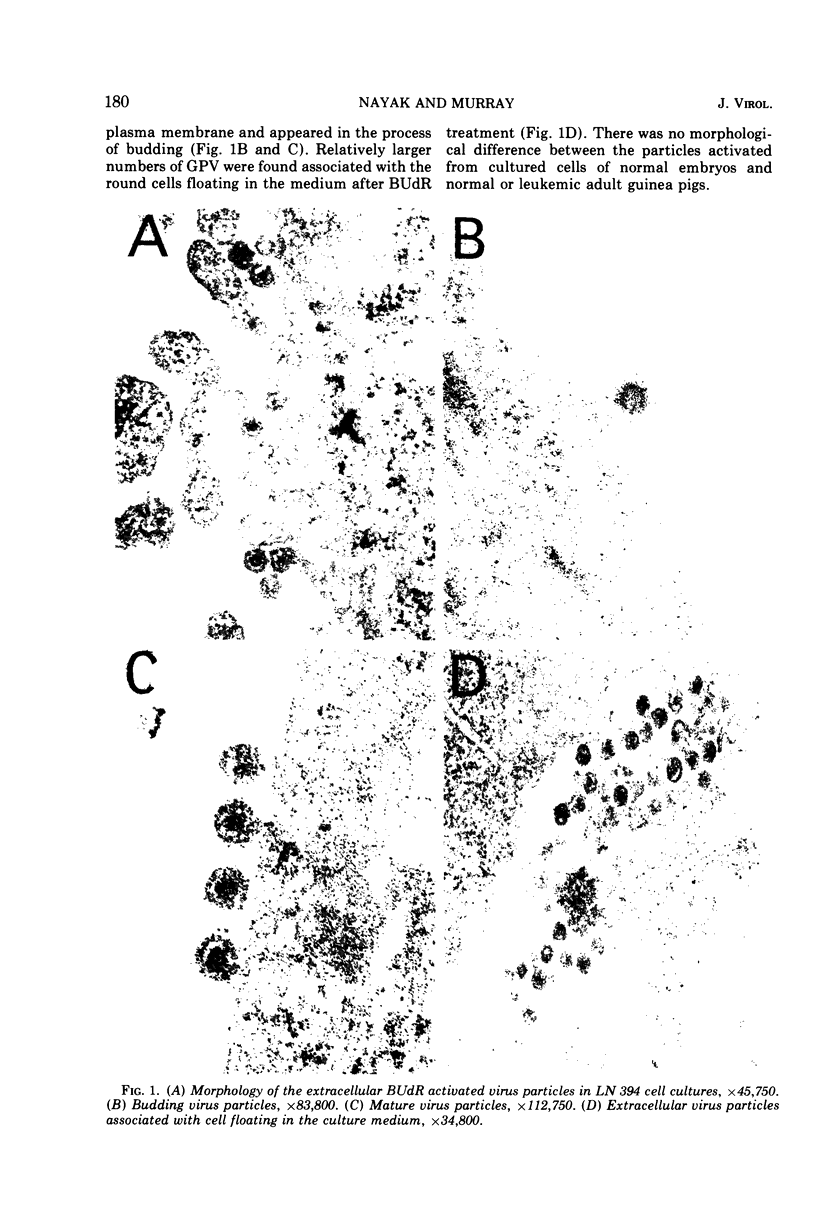
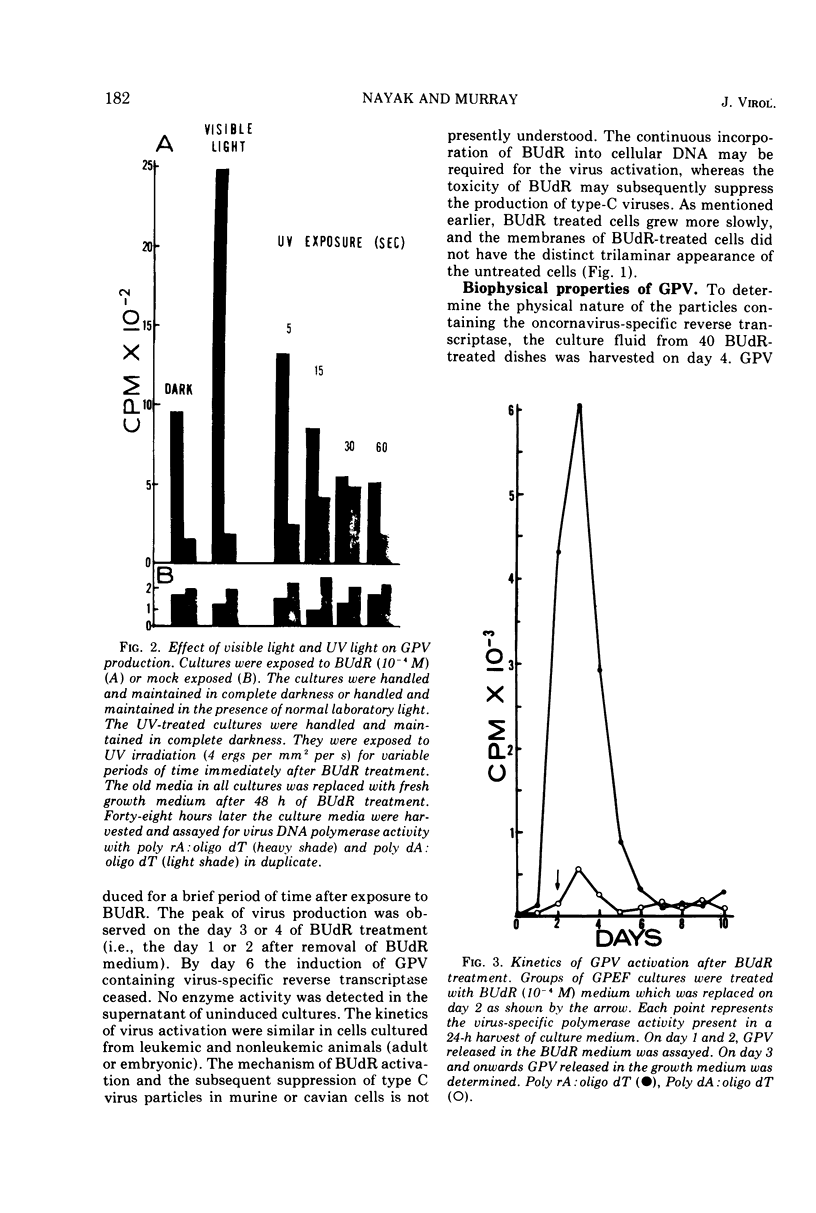
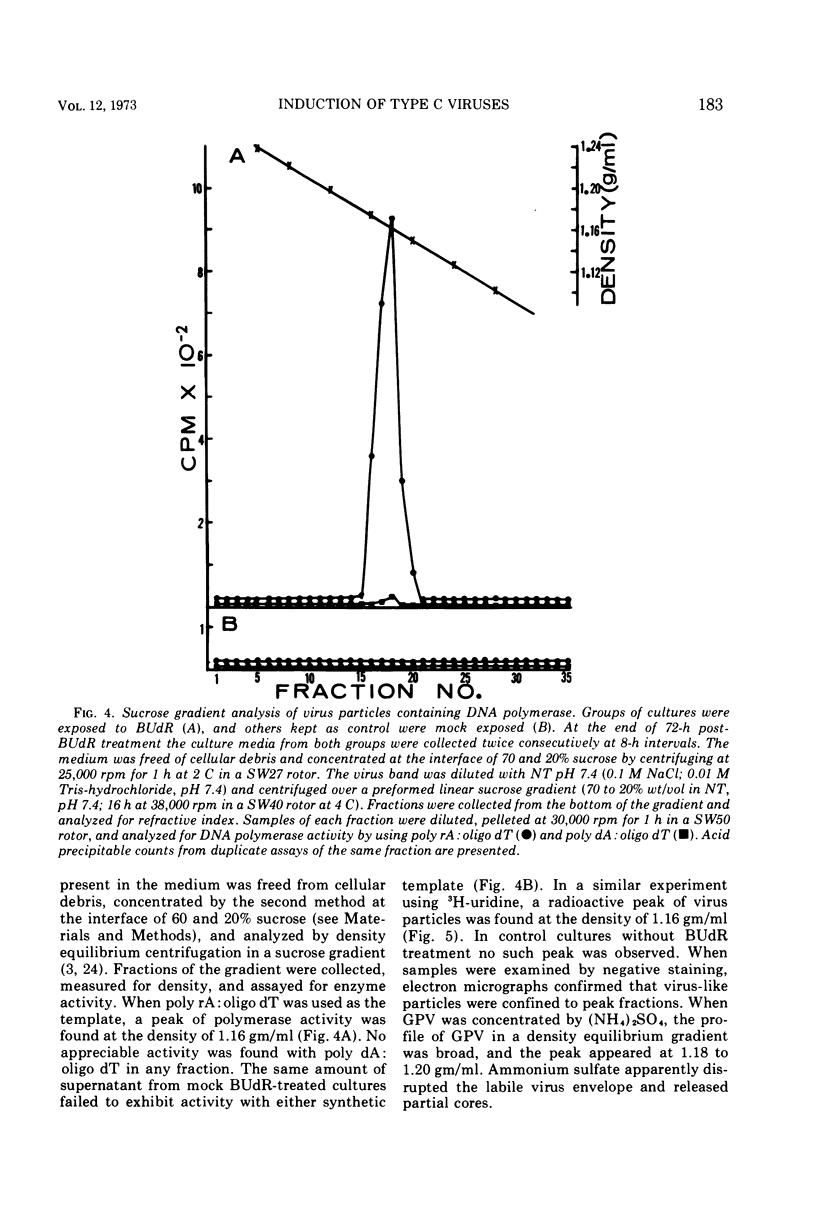
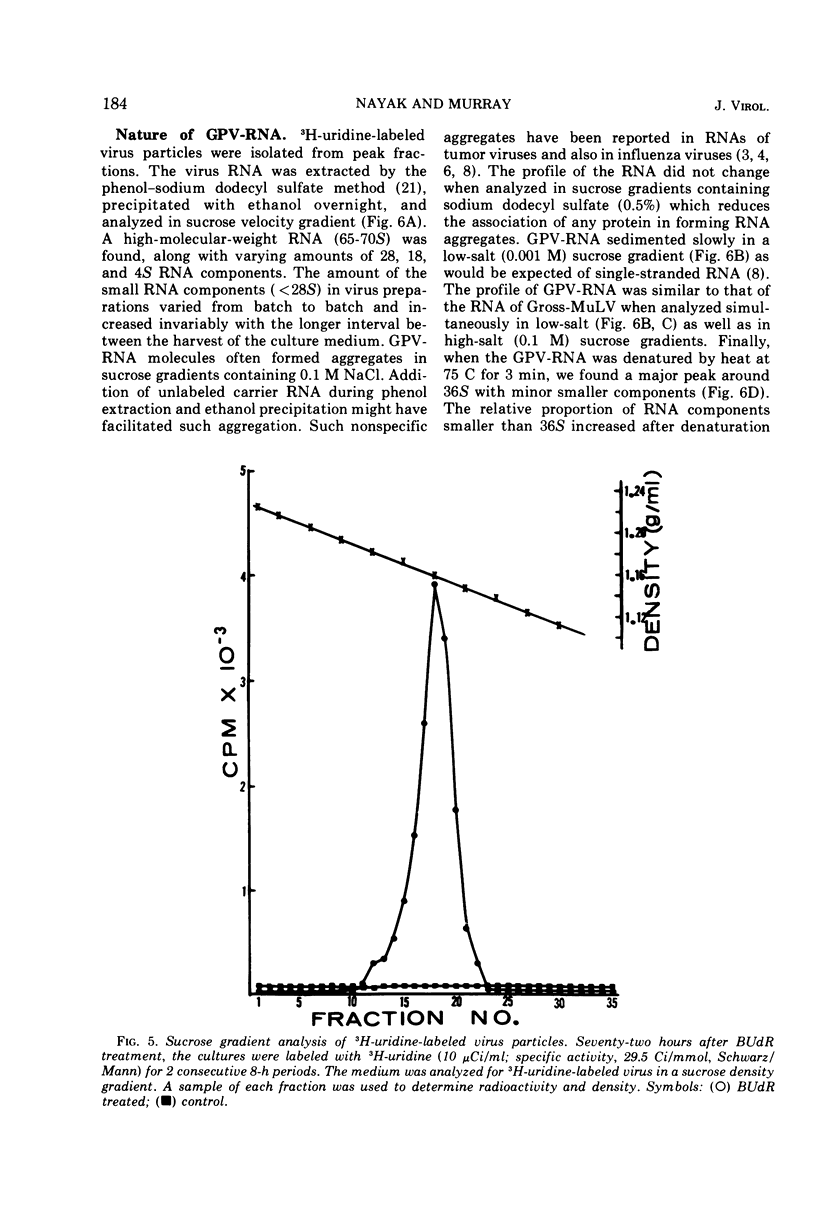
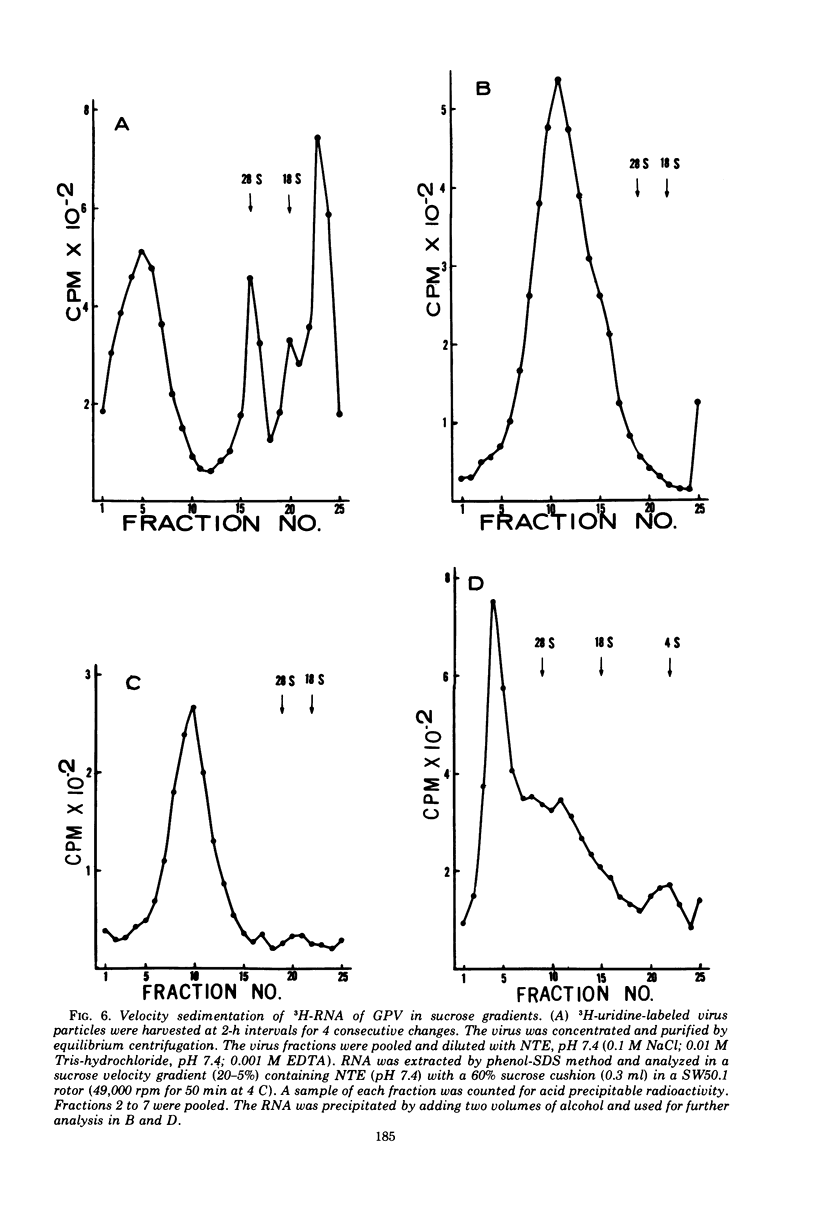
Particles morphologically resembling type C viruses were activated by bromodeoxyuridine (BUdR; 10−4 M) treatment of cultured guinea pig cells. Virus particles were isolated from the cells of normal and leukemic strain 2 and random-bred guinea pigs (adult and embryonic). Immature virus particles with electron-lucent cores were found in the cytoplasmic matrix. The mature particles with electron-dense cores were found outside the cells and some appeared in the process of budding from the plasma membrane. The peak of virus production was observed within 4 days of BUdR treatment. When compared to the amount of virus produced in darkness, visible light enhanced virus production, whereas exposure of BUdR-treated cells to UV light either had no effect or inhibited virus production. Virus particles had a density of 1.16 gm/ml, possessed an oncornavirus-specific reverse transcriptase, and contained a large-molecular-weight RNA (65-70S) which dissociated into 36S subunits after heat denaturation. The BUdR-activated virus particles, therefore, possessed the morphological, biophysical, and biochemical characteristics of type C oncornaviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Barry R. D., Davies P. The sedimentation of influenza virus and its RNA in sucrose density gradients. J Gen Virol. 1968 Jan;2(1):59–69. doi: 10.1099/0022-1317-2-1-59. [DOI] [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Deusberg P. H., Robinson W. S. On the structure and replication of influenza virus. J Mol Biol. 1967 May 14;25(3):383–405. doi: 10.1016/0022-2836(67)90193-3. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Fox S. M., De Harven E., Biedler J. L., Sanders F. K. Virus-like agents from patients with Hodgkin's disease. Nature. 1971 Sep 10;233(5315):104–108. doi: 10.1038/233104a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Feldman D. G., Gross L. Electron microscopic study of the guinea pig leukemia virus. Cancer Res. 1970 Nov;30(11):2702–2711. [PubMed] [Google Scholar]
- Fogel M. Induction of virus synthesis in polyoma-transformed cells by DNA antimetabolites and by irradiation after pretreatment with 5-bromodeoxyuridine. Virology. 1972 Jul;49(1):12–22. doi: 10.1016/s0042-6822(72)80003-5. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Morton D. L., Schidlovsky G., Trahan E. Cross-reacting tumor-specific transplantation antigens in methylcholanthrene-induced guinea pig sarcomas. J Natl Cancer Inst. 1971 Apr;46(4):693–700. [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Levinson W. E., Bishop J. M., Quintrell N., Jackson J., Fanshier L. Synthesis of RNA in normal and Rous sarcoma virus-infected cells: effect of bromodeoxyuridine. Virology. 1970 Sep;42(1):221–224. doi: 10.1016/0042-6822(70)90256-4. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Ma B. I., Swartzendruber D. C., Murphy W. H. Detection of virus-like particles in germinal centers of normal guinea pigs. Proc Soc Exp Biol Med. 1969 Feb;130(2):586–590. doi: 10.3181/00379727-130-33613. [DOI] [PubMed] [Google Scholar]
- Nadel E., Banfield W., Burstein S., Tousimis A. J. Virus particles associated with strain 2 guinea pig leukemia (L2C/N-B). J Natl Cancer Inst. 1967 Jun;38(6):979–981. [PubMed] [Google Scholar]
- Nayak D. P. Activation of guinea pig herpesvirus antigen in leukemic lymphoblasts of guinea pig. J Virol. 1972 Nov;10(5):933–936. doi: 10.1128/jvi.10.5.933-936.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Baluda M. A. Ribonucleic acid synthesis in cells infected with influenza virus. J Virol. 1968 Feb;2(2):99–109. doi: 10.1128/jvi.2.2.99-109.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P. Isolation and characterization of a herpesvirus from leukemic guinea pigs. J Virol. 1971 Oct;8(4):579–588. doi: 10.1128/jvi.8.4.579-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opler S. R. Observations on a new virus associated with guinea pig leukemia: preliminary note. J Natl Cancer Inst. 1967 May;38(5):797–800. [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Silagi S., Beju D., Wrathall J., Deharven E. Tumorigenicity, immunogenicity, and virus production in mouse melanoma cells treated with 5-bromodeoxyuridine. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3443–3447. doi: 10.1073/pnas.69.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Genetic factors influencing C-type RNA virus induction. J Exp Med. 1972 Jul 1;136(1):175–184. doi: 10.1084/jem.136.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. E., Kasnic G., Jr, Draycott C., Ben T. Activation of viruses in human tumors by 5-iododeoxyuridine and dimethyl sulfoxide. Science. 1972 Jan 14;175(4018):198–199. doi: 10.1126/science.175.4018.198. [DOI] [PubMed] [Google Scholar]
- Stewart S. E., Kasnic G., Jr, Draycott C., Feller W., Golden A., Mitchell E. Activation in vitro, by 5-iododeoxyuridine, of a latent virus resembling C-type virus in a human sarcoma cell line. J Natl Cancer Inst. 1972 Jan;48(1):273–277. [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]
- Wepsic H. T., Zbar B., Whang-Peng J., Borsos T., Rapp H. J. Guinea pig leukemia L2C: characterization of chromosomes and attempts to demonstrate antigenicity. J Natl Cancer Inst. 1970 Jul;45(1):99–105. [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]