Abstract
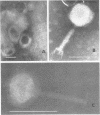
A phage, SBX-1, and its bacterial host, Xanthomonas sp. 1, were isolated consistently from roots, internal portions of stems, and leaves of soybean plants. Phage titer in leaves was highly variable. It was very low in seedlings, reached a maximum of 104 PFU/ml of sap after 11 weeks of plant growth and again dropped to very low levels. We isolated SBX-1 from plants of all 45 varieties studied, but not consistently from some. Plants of some varieties also carried Xanthomonas sp. 2, which was resistant to infection by SBX-1. The SBX-1 particle has a polyhedral head containing DNA of density 1.709. It has an edge-to-edge diameter of 80 nm and a tail length of 112 nm. The tail has a base plate and spikes. This is the first report of the extensive and continuous occurrence of a phage and its host bacterium in plants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENSTARK A., GOLDBERG S. S., BERNSTEIN L. B. Lysogenicity in Xanthomonas pruni. J Gen Microbiol. 1955 Jun;12(3):402–405. doi: 10.1099/00221287-12-3-402. [DOI] [PubMed] [Google Scholar]
- Ghei O. K., Eisenstark A., To C. M., Consigli R. A. Structure and composition of Xanthomonas pruni bacteriophage. J Gen Virol. 1968 Jul;3(1):133–136. doi: 10.1099/0022-1317-3-1-133. [DOI] [PubMed] [Google Scholar]
- PATTEE P. A., BALDWIN J. N. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J Bacteriol. 1961 Dec;82:875–881. doi: 10.1128/jb.82.6.875-881.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. Algal virus: isolation. Science. 1963 May 10;140(3567):679–680. doi: 10.1126/science.140.3567.679. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER I. R., DIENER T. O., SAFFERMAN R. S. BLUE-GREEN ALGAL VIRUS LPP-1: PURIFICATION AND PARTIAL CHARACTERIZATION. Science. 1964 May 29;144(3622):1127–1130. doi: 10.1126/science.144.3622.1127. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Diener T. O., Desjardins P. R., Morris M. E. Isolation and characterization of AS-1, a phycovirus infecting the blue-green algae, Anacystis nidulans and Synechococcus cedrorum. Virology. 1972 Jan;47(1):105–113. doi: 10.1016/0042-6822(72)90243-7. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Schneider I. R., Steere R. L., Morris M. E., Diener T. O. Phycovirus SM-1: a virus infecting unicellular blue-green algae. Virology. 1969 Mar;37(3):386–395. doi: 10.1016/0042-6822(69)90222-0. [DOI] [PubMed] [Google Scholar]