Abstract
Background
The present study examined the prospective relationships between subjective fatigue, cognitive function, and everyday functioning.
Methods
A cohort study with secondary data analysis was conducted using data from 2,781 community-dwelling older adults without dementia who were enrolled to participate in the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) randomized intervention trial. Measures included demographic and health information at baseline, and annual assessments of subjective fatigue, cognitive function (i.e., speed of processing, memory, and reasoning), and everyday functioning (i.e., everyday speed and everyday problem-solving) over 5 years.
Results
Four distinct classes of subjective fatigue were identified using growth mixture modeling: one group complaining fatigue “some of the time” at baseline but “most of the time” at 5-year follow-up (increased fatigue), one complaining fatigue “a good bit of the time” constantly over time (persistent fatigue), one complaining fatigue “most of the time” at baseline but “some of the time” at 5-year follow-up (decreased fatigue), and the fourth complaining fatigue “some of the time” constantly over time (persistent energy). All domains of cognitive function and everyday functioning declined significantly over five years; and the decline rates, but not the baseline levels, differed by the latent class of subjective fatigue. Except for the decreased fatigue class, there were different degrees of significant associations between the decline rates of subjective fatigue and all domains of cognitive function and everyday functioning in other classes of subjective fatigue.
Conclusion
Future interventions should address subjective fatigue when managing cognitive and functional abilities in community-dwelling older adults.
Keywords: subjective fatigue, cognition, everyday functioning, ACTIVE
Introduction
Having a good memory and being cognitively alert are two ways that older adults value themselves as aging well (Laditka et al., 2009). These cognitive resources are also related to reduced health care cost, decreased morbidity and mortality, and increased functional independence in old age (Thies and Bleiler, 2011). Emerging perspectives suggest the likelihood that interventions to prevent cognitive decline or improve cognitive function will include non-pharmacological approaches (Fotuhi et al., 2009). In this paper, the potential modifiable factor, subjective fatigue, was examined for its influence on cognitive and functional performance in a community-based sample of older adults.
Subjective fatigue, the feeling of being tired or having difficulty in initiating activities (Lou, 2009), is the most common symptom in old age and is experienced by over 20% of non-disabled, community-dwelling older adults (Reyes-Gibby et al., 2003; Wijeratne et al., 2007; Yu et al., 2009); in fact, the feeling of fatigue increases with age that over half of older adults aged 70+ years report experiencing subjective fatigue in their daily activities (Avlund, 2010). Subjective fatigue is associated with declines in physical functioning, disability, and risk of hospitalization (Eldadah, 2011). Recent cross-sectional studies also found that fatigue is associated with brain functional change (e.g., hypo-metabolism, brain atrophy, abnormal activity of prefrontal cortex and frontal basal ganglia), compromise cognitive abilities (Andreasen et al., 2011; Chaudhuri and Behan, 2004; Holtzer and Foley, 2009; Holtzer et al., 2011; Marrie et al., 2005) and everyday functioning demanding on cognitive abilities (e.g., instrumental activities of daily living) (Vestergaard et al., 2009). However, relatively few longitudinal studies have examined the prospective relationship between subjective fatigue and cognitive function or cognitively demanding everyday activities. In Verdelho et al.'s study (Verdelho et al., 2004), they found that using a single item from the Montgomery and Asberg Depression Rating Scale, subjective fatigue was more frequently reported in patients with dementia than their healthy counterparts at 3-year follow-up. In contrast, in Boyle et al.'s study (Boyle et al., 2011), using two items from the Center Epidemiologic Studies Depression Scale, they found that subjective fatigue was not associated with cognitive decline at 12 years follow-up. The inconsistency of results between the two studies may be explained by the timeline of measuring subjective fatigue. Subjective fatigue is often considered a relatively acute state (Eldadah, 2011); therefore, continuously measuring subjective fatigue over time is recommended to comprehensively understand its association with cognitive outcomes.
Furthermore, subjective fatigue has long been considered a criterion for a major depressive episode (American Psychiatric Association, 2000), a component of frailty (Walston et al., 2006), or having a circularity with cardiovascular disease risk factors (CVDRFs) (Kaltsas et al., 2011; Melamed et al., 2006). Depression (Huang et al., 2011), grip strength which is another component of frailty (Boyle et al., 2011), and CVDRFs (Lin et al., 2012) have been consistently related to cognitive abilities. However, subjective fatigue may have its own casual relation with cognitive abilities through both pathophysiological and behavior mechanisms. First, the energy homeostasis at vascular, especially endothelium function, is considered an objective assessment of subjective fatigue (Ohno et al., 2011). Increased subjective fatigue, possibly reflecting as vascular pathology (e.g., endothelial dysfunction, atherosclerosis, cytokines), may contribute to the cognitive decline in old age (Alexander et al., 2011; Panza et al., 2011). Next, subjective fatigue may indirectly influence cognitive abilities through interfering with initiating and sustaining in self-motivated daily activities (e.g., exercise, social activities, mental activities) that is potentially neuroprotective (Kelley et al., 2003). Regardless, to claim the role of subjective fatigue in predicting cognitive abilities will need a clarification of the complexity between subjective fatigue, cognitive function, depression, frailty, and CVDRFs.
In this study, the hypothesis that the trajectory of subjective fatigue in old age was related to the decline of cognitive function and everyday functioning was tested. We used data from a cohort of 2,781 community-dwelling adults without dementia at baseline aged 65 – 94 over 5 years. Cognitive function and cognitively demanding everyday activities were measured by a series of laboratory-based or ecologically validated neuropsychological or functional assessments over five years, capturing abilities related to speed of processing, memory, and reasoning in laboratory settings, as well as speed of processing and problem solving in everyday life. Subjective fatigue in this study was defined as the perception of energy imbalance, which does not simply result from sleep problems or physical exertion (Alexander et al., 2011), measured using the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) vitality subscale, a well-established measurement for the state of fatigue related to energy or vitality (O'Connor, 2004). The specific aims of this study were to examine: 1) the trajectory of subjective fatigue over time; and 2) the influence of subjective fatigue on cognitive function and everyday functioning over time when controlling for depression, grip strength, CVDRFs and other relevant confounding factors.
Methods
Participants
A secondary data analysis was performed using data from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial, an on-going prospective dataset (Ball et al., 2002; Willis et al., 2006). The ACTIVE trial is a randomized controlled trial designed to evaluate three types of cognitive training interventions (memory, speed of processing, and reasoning) on cognitive and functional abilities. A subset of participants in the three training groups also attended four booster training sessions 11 months and 35 months after the original training sessions. There were 2,832 community-dwelling older adults (≥ 65 years old at baseline) without dementia (as screened using Mini Mental State Examination ≥ 23) who participated in the study. The exclusion criteria included: 1) self-reported diagnosis of Alzheimer's disease, 2) substantial decline in basic activities of daily living function, 3) certain life-threatening medical conditions (e.g., cancer), 4) recent cognitive training, 5) being unavailable during the testing and training period of study, and 6) severe sensory loss or communicative problems. Participants were recruited from 6 metropolitan areas in the United States including the University of Alabama at Birmingham, Wayne State University, the Hebrew Rehabilitation Center for the Aged, the Johns Hopkins University School of Medicine, Indiana University, and Pennsylvania State University. The recruitment strategies for each site differed and details on these and other aspects of the ACTIVE trial are available elsewhere (Jobe et al., 2001). An analytic sample of 2,802 was randomized to one of the three cognitive training groups or a no-contact control group. Institution specific institutional review boards approved the ACTIVE protocol and consent was obtained for each participant prior to participation. The retention rate at 5-year follow-up was 67% in the ACTIVE trial; participants who were older, male, and less educated, and had more health problems and lower cognitive function were less likely to be retained at 5 years (Willis et al., 2006). The analytic sample of the present study was 2,781 participants who had at least two waves of data on subjective fatigue.
Measurement
Subjective Fatigue
Subjective fatigue was measured using the Vitality subscale from the SF-36 at baseline, 1-, 2-, 3-, and 5-year follow-up (Ware Jr and Sherbourne, 1992). The Vitality subscale included 4 items measuring the recalled frequency of feeling of fatigue (i.e., feeling pep, energetic, worn out, and tired) over the past month (O'Connor, 2004). Participants responded to each item using a Likert scale from 1 “all of the time” to 6 “none of the time.” The sum score was calculated with higher scores indicating lower levels of subjective fatigue and higher levels of energy. The internal consistency reliability (Cronbach's α) of the Vitality subscale in previous large sample studies ranged from 0.85 to 0.87, and test-retest reliability was 0.80 over 2 weeks in patients with heart disease (Ware, 2000). The Vitality subscale is one of the most commonly used measurements for subjective fatigue (O'Connor, 2004). The internal consistency of the four items in this study were 0.84 – 0.86 across visits.
Cognitive Function and Everyday Functioning
Cognitive function and everyday functioning were measured using 11 neuropsychological or everyday functional tests belonging to 5 domains at baseline, 1-, 2-, 3-, and 5-year follow-up. Cognitive function included three domains: speed of processing measured using the Useful Field of View (Owsley et al., 1991); memory measured using Hopkins Verbal Learning Test (Brandt, 1991), Auditory Verbal Learning Test (Rey, 1942), and Rivermead Behavioral Memory Test (Wilson et al., 1985); and reasoning measured using Word Series (Gonda and Schaie, 1985), Letter Series (Thurstone and Thurstone, 1949), and Letter Sets (Ekstrom et al., 1976). Everyday functioning that demands on cognitive abilities included two domains: everyday speed measured using Complex Reaction Time (Ball et al., 2000) and Timed Instrumental Activities of Daily Living (Owsley et al., 2002); and everyday problem-solving measured using Everyday Problem Test (Willis and Marsiske, 1993) and Observed Tasks of Daily Living (Diehl et al., 1995). Five separate composite scores for the three domains of cognitive function and two domains of everyday functioning were developed using the mean and standard deviation of the original ACTIVE sample (n = 2,802) in the following procedure: for tests belonging to the same domain, Z-transformation was firstly performed on the raw score of each test, and then the mean score (composite score) of Z scores of those tests was calculated. Higher composite scores indicated poorer levels of speed of processing and everyday speed but higher levels of memory, reasoning, and everyday problem-solving. The purpose of using the original analytic sample from ACTIVE (n = 2,802) instead of the analytic sample of the present study (n = 2,781) was to compare the cognitive function and everyday functioning between the subgroup excluded from the present study who did not have at least two waves of data on subjective fatigue with the participants included in the study. The subgroup excluded from the current study (n = 21) had significantly poorer performances on speed of processing, memory, everyday speed of processing, and everyday problem-solving (data was not shown).
Demographic and Health Information Related Covariates
Data on age, sex, race, and years of education were collected. The following health variables, i.e., depression, grip strength, and history of CVDRFs, that may confound with subjective fatigue in predicting cognitive or everyday functioning were included as covariates. Level of depression was measured using 11 items from the Center for Epidemiological Studies Depression (CES-D) scale, excluding one item that is often representative of fatigue (“I couldn't get going”) (Radloff, 1977). Mean score of the 11 items was calculated. Grip strength was included as a measure of general physical robustness and was assessed using a dynamometer (Lafayette Instruments, Layfayette, Ind., USA). Participants were allowed to make their maximal effort with the dominant hand as instructed in the trial. One minute of rest was taken between two trials. The mean of the scores from the two trials were computed. Higher scores indicated greater grip strength. History of CVDRFs included heart disease, congestive heart failure (CHF), stroke, hypertension, diabetes, and high cholesterol, all collected using a single question “Has a doctor or a nurse ever told you that you have (the health condition)?” Smoking was identified by a single question: “Do you smoke now?” Objectively measured height and weight were used in calculating body-mass index (BMI), and obesity was identified using BMI ≥ 30 kg/m2. A total number of CVDRFs was calculated. All demographic and health information were collected at baseline.
Data Analysis
Growth mixture modeling (GMM) from Mplus 6 version was used to determine the number of classes of trajectory in subjective fatigue over 5 years. The purpose of using GMM was to find the smallest number of classes of respondents with similar trajectory of change in subjective fatigue. A series of models were tested beginning with a one-class model and moving to a five-class model. The optimal number of classes was decided based on the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and adjusted BIC (Nylund et al., 2007). The AIC, BIC, and adjusted BIC are commonly used fit indices, in which lower values indicate a more parsimonious model. Each class should have more than 1% of the total sample (Jung and Wickrama, 2008). For each distinctive class, the model was described with the shape of the trajectory (i.e., intercept and slope) and the number of respondents belonging to the class.
After deciding the latent class (number = 4 in this study), remaining analyses were performed in IBM SPSS 19.0. Analysis of variance (ANOVA) was applied to compare the continuous variables by the class of subjective fatigue, and chi-square test was applied to compare the categorical variables by the class. Linear mixed-effects (LME) modeling was applied to assess the longitudinal relationships of visit and the latent class of subjective fatigue with cognitive function and everyday functioning adjusted for covariates (West et al., 2007).
Two separate sets of models were applied:
- When taking the latent class of subjective fatigue as the predictor:
- When taking the time-dependent subjective fatigue (baseline, 1-, 2-, 3-, and 5-year follow-up) as the predictor within each latent class of subjective fatigue:
In these models, visit refers to the baseline, 1-, 2-, 3-, and 5-year follow-up. All βs were the coefficients for fixed-effects; γ1 and γ2 were the coefficients for the random-effects and ε is the error term; y referred to each domain of cognitive function or everyday functioning. The model fit is fitted by restricted maximum likelihood estimation. Age, sex, race, education, recruitment site, assignment of intervention group, participation in booster sessions, depression, grip strength, and a total number of CVDRFs were included as covariates.
All tests were 2-tailed and p < 0.05 were considered as significant differences in all analyses except for the Bonferroni's correction p < 0.0125.
Results
Latent Class of Change of Subjective Fatigue Over 5 Years
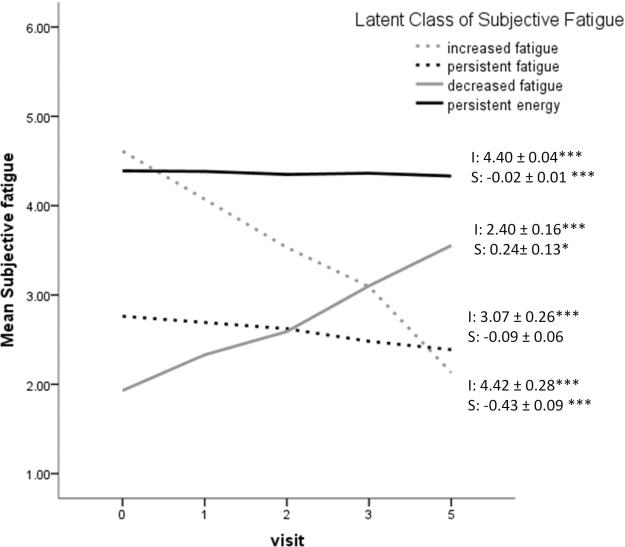
Table 1 summarizes the series of model fit statistics, indicating the 4-class model was the best solution. Figure 1 displays the four classes. Class 1, comprising 58 participants (2.1%), was characterized by a high initial level of energy (mean intercept = 4.42, p < 0.001) that declined substantially over time (mean slope = −0.43, p < 0.001). We labeled this class as increased fatigue class. Class 2, comprising 456 participants (16.4%), was characterized by a constant low level of energy (mean intercept = 3.07, p < 0.001) over time (mean slope = −0.09, p > 0.05). We labeled this as persistent fatigue class. Class 3, comprising 61 participants (2.2%), was characterized by a very low initial level of energy (mean intercept = 2.40, p < 0.001) that increased over time (mean slope = 0.24, p < 0.05). We labeled this as decreased fatigue class. Class 4, comprising 2,206 participants (79.3%), was characterized by a relatively high initial level of energy (mean intercept = 4.40, p < 0.001) that slightly decreased over time (mean slope = −0.02, p < 0.001). We labeled this as persistent energy class.
Table 1.
Growth Mixture Model Fit Statistics for 1-, 2-, 3-, 4-, and 5-Class Models of Trajectory of Subjective Fatigue
| Model | Latent class | N | AIC | BIC | Adjusted BIC |
|---|---|---|---|---|---|
| 1-class | 1 | 2,781 | - | - | - |
|
| |||||
| 2-class | 1 | 557 | 22895.17 | 22960.41 | 22925.46 |
| 2 | 2,224 | ||||
|
| |||||
| 3-class | 1 | 170 | 22785.22 | 22868.25 | 22823.77 |
| 2 | 2,259 | ||||
| 3 | 352 | ||||
|
| |||||
| 4-class | 1 | 58 | 22743.11 | 22843.93 | 22789.91 |
| 2 | 456 | ||||
| 3 | 61 | ||||
| 4 | 2,206 | ||||
|
| |||||
| 5- class | 1 | 303 | 22746.36 | 22854.97 | 22791.42 |
| 2 | 10 | ||||
| 3 | 44 | ||||
| 4 | 185 | ||||
| 5 | 2,239 | ||||
Note. AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion
Figure 1.
Graphical representation of subjective fatigue over time by the latent class. Note. I: intercept; S: slope. *p < 0.05; *** p < 0.001. Higher scores in subjective fatigue indicated higher levels of energy.
Baseline Demographic and Health Variables by the Latent Class of Subjective Fatigue
Table 2 displays the demographic and health variables at baseline by the latent class of subjective fatigue. Participants in the persistent fatigue class were significantly older than those in the persistent energy class. Participants with increased fatigue (who had lowest levels of subjective fatigue at baseline) had significantly the lowest levels of depression, while participants with decreased fatigue (who had highest levels of subjective fatigue at baseline) had the highest levels of depression. Participants with persistent energy had significantly the highest levels of grip strength than other groups. Increased fatigue and persistent energy classes had similar numbers of CVDRFs, which were significantly fewer than the persistent fatigue class. In terms of individual CVDRFs, persistent fatigue had significantly higher percentages of participants with the presence of heart disease, CHF, stroke, and obesity than the persistent energy class. There were no significantly differences for other classes.
Table 2.
Raw Scores for Baseline Demographic and Health Information Characteristics at Baseline by the Latent Class of Subjective Fatigue (N = 2,781)
| Increased fatigue N = 58 | Persistent fatigue N = 456 | Decreased fatigue N = 61 | Persistent energy N = 2,206 | F or X2 test | |
|---|---|---|---|---|---|
| Age, mean (SD) | 74.09 (5.29)a,b | 74.71 (6.22)a | 74.15 (6.84)a,b | 73.35 (5.77)b | 7.04*** |
| Male, n (%) | 14 (24.1%) | 91 (20.0%) | 14 (23.0%) | 550 (24.9%) | 5.16 |
| Caucasian, n (%) | 48 (82.8%)a | 344 (75.4%)b | 54 (88.5%)a | 1567 (71.0%)c | 15.21** |
| Years of education, mean (SD) | 13.54 (3.06)a,b | 13.01 (2.62)a | 13.52 (2.63)a,b | 13.64 (2.70)b | 6.86*** |
| Depression, mean (SD) | 0.31 (0.30)a | 0.74 (0.52)b | 0.87 (0.56)c | 0.37 (0.38)d | 127.85*** |
| Grip strength, mean (SD) | 23.71 (10.08)a,b | 22.84 (7.86)a | 21.90 (7.76)a | 24.42 (8.31)b | 5.04** |
| CVDRFs | |||||
| • Total number, mean (SD) | 1.72 (1.25)a | 2.24 (1.46)b | 2.15 (1.44)a,b | 1.73 (1.24)a | 21 44*** |
| • History of heart disease, n (%) | 9 (15.8%)a,b | 119 (26.4%)b | 14 (23.0%)a,b | 279 (12.8%)a | 56.93*** |
| • History of CHF, n (%) | 2 (3.4%)a,b | 48 (10.7%)b | 5 (8.3%)a,b | 82 (3.7%)a | 39.59*** |
| • History of stroke, n (%) | 4 (7.0%)a,b | 48 (10.6%)b | 12 (19.7%)b | 129 (5.9%)a | 28.29*** |
| • History of hypertension, n (%) | 31 (53.4%) | 258 (57.0%) | 33 (54.1%) | 1099 (50.1%) | 7.37 |
| • History of diabetes, n (%) | 4 (6.9%) | 68 (14.9%) | 10 (16.4%) | 274 (12.4%) | 4.59 |
| • History of high cholesterol, n (%) | 25 (45.5%) | 223 (49.8%) | 30 (49.2%) | 943 (43.5%) | 6.45 |
| • Smoking, n (%) | 1 (3.7%) | 42 (18.8%) | 4 (14.3%) | 160 (16.0%) | 4.27 |
| • Obesity, n (%) | 24 (41.4%)a,b | 215 (47.1%)b | 23 (37.7%)a,b | 846 (38.3%)a | 12.38** |
Note. Each subscript letter denotes a subset of class whose column proportions do not differ significantly from each other at the 0.0125 level (Bonferroni's correct).
p < .01;
p < .001.
CVDRFs = cardiovascular diseases and risk factors; CHF = congestive heart failure.
Latent Class of Subjective Fatigue and Cognitive Function and Everyday Functioning over Time
Table 3 shows the LME models of each domain of cognitive function and everyday functioning across visits by latent class of subjective fatigue, controlling for age, sex, education, race, recruitment site, assignment of intervention group, participation in booster sessions, depression, grip strength, and history of CVDRFs. Persistent energy class was considered a referent group. All domains of cognitive function and everyday functioning declined significantly over time, and the average declines per visit ranged from 0.0249 (everyday problem solving) to 0.0352 (reasoning) units.
Table 3.
Relationships between Latent Class of Subjective Fatigue and Cognitive Function and Everyday Functioning (Parameter Estimate, β ± SE)a
| Speed of processingb | Memory | Reasoning | Everyday speedb | Everyday problem-solving | |
|---|---|---|---|---|---|
|
|
|||||
| Time | .0263 ± .0032*** | −.0309 ± .0032*** | −.0352 ± .0025*** | .0346 ± .0043*** | −.0249 ± .0032*** |
| Subjective fatigue | |||||
| • Increased fatigue | .0572 ±.0896 | .0576 ±.0946 | −.0340 ± .0982 | −.1969 ± .0933 | .1230 ± .0948 |
| • Persistent fatigue | −.0238 ± .0386 | .0595 ± .0406 | .0437 ±.0420 | −.0031 ± .0404 | .1560 ± .0408 |
| • Decreased fatigue | −.0934 ± .0942 | .1273 ± .0994 | .1246 ± .1031 | −.0649 ± .0983 | .3112 ± .0997** |
| • Persistent energy (referent) | 0 | 0 | 0 | 0 | 0 |
| Subjective fatigue × Time | |||||
| • Increased fatigue × Time | .0337 ± .0179 | −.0319 ± .0175* | −.0471 ± .0141** | .1050 ± .0235*** | −.0722 ± .0178*** |
| • Persistent fatigue × Time | .0108 ± .0086 | −.0173 ± .0082* | −.0106 ± .0066 | .0118 ± .0111 | −.0095 ± .0082 |
| • Decreased fatigue × Time | .0043 ± .0194 | .0150 ± .0189 | −.0137 ± .0152 | −.0290 ± .0255 | −.0149 ± .0194 |
| • Persistent energy × Time (referent) | 0 | 0 | 0 | 0 | 0 |
Note.
controlling for age, race, sex, education, recruitment site, assignment of intervention group, participation in booster sessions, depression, grip strength, and the total number of CVDRFs (estimates not shown).
Higher scores indicate lower abilities.
p < 0.05;
p < 0.01;
p < 0.001.
Baseline levels of cognitive function and everyday functioning were similar across latent class of subjective fatigue, except that the decreased fatigue class had significantly higher baseline level of everyday problem-solving than the persistent energy class did.
In terms of changes of cognitive function and everyday functioning over time, the increased fatigue class declined significantly faster in memory (0.0319 unit/visit), reasoning (0.0471 unit/visit), everyday speed (0.1050 unit/visit), and everyday problem solving (0.0722 unit/visit) than the persistent energy class. The persistent fatigue class declined significantly faster in memory (0.0173 unit/visit) than the persistent energy class.
Associations of Changes of Subjective Fatigue and Cognitive Function and Everyday Functioning
Table 4 and Figure 2 shows the associations of annual rates of change in subjective fatigue with domains of cognitive function and everyday functioning within each latent class of subjective fatigue, after controlling age, sex, education, recruitment site, assignment of intervention group, participation in booster sessions, depression, grip strength, and total number of CVDRFs. There was no association between annual rates of changes in the decreased fatigue class. In the increased fatigue class, each 1 unit increase in fatigue per visit was significantly associated with 0.0666 – 0.2178 units decline in all domains of cognitive function and everyday functioning per visit. In the persistent fatigue class, each 1 unit increase in fatigue per visit was significantly associated with 0.0478 – 0.0730 units decline in memory, reasoning, and everyday speed per visit. In the persistent energy class, each 1 unit increase in fatigue per visit was significantly associated with 0.0127 – 0.0408 units decline in cognitive function and everyday speed per visit. In summary, domains of cognitive function and everyday functioning declined faster in the increased fatigue group than other groups.
Table 4.
Parameter Estimate (β ± SE) of the Relationships between the Changes of Subjective Fatigue and Cognitive Function and Everyday Functioning Over timea
| Class | Speed of processingb | Memory | Reasoning | Everyday speedb | Everyday problem-solving |
|---|---|---|---|---|---|
| Increased fatigue | −.0964 ± .0329** | .0666 ± .0309* | .1142± .0224*** | −.2178 ± .0746** | .1659 ± .0382*** |
| Persistent fatigue | −.0262 ± .0239 | .0478 ± .0199* | .0730 ± .0149*** | −.0712 ± .0250** | .0310 ± .0187 |
| Decreased fatigue | .0472 ± .0371 | .0083 ± .0356 | −.0537 ± .0335 | −.0081 ± .0487 | −.0383 ± .0419 |
| Persistent energy | −.0245 ± .0107* | .0399 ± .0108*** | .0174 ± .0084* | −.0408 ± .0110*** | .0128 ± .0107 |
Note.
controlling for age, race, sex, education, recruitment site, assignment of intervention group, participation in booster sessions, depression, grip strength, and the total number of CVDRFs (estimates not shown).
Higher scores indicate lower abilities.
p < 0.05;
p < 0.01;
p < 0.001.
Figure 2.
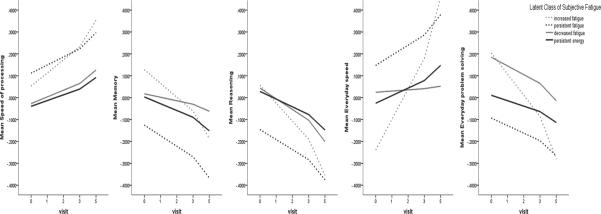
Graphical representation of the relationships between changes of subjective fatigue and cognitive function and everyday functioning over time by the latent class of subjective fatigue. Note. Higher scores in speed of processing and everyday speed indicated lower abilities.
Discussion
This study examined the longitudinal relationships between subjective fatigue and five domains of cognitive function or everyday functioning in a cohort of 2,781 community-dwelling older adults without baseline dementia. We identified four distinct trajectories of subjective fatigue over five years: one group with initial relatively high level of energy that declined substantially over time (increased fatigue class; i.e., complaining fatigue “some of the time” at baseline but “most of the time” at 5-year follow-up), one group with persistent fatigue (persistent fatigue class; i.e., complaining fatigue “a good bit of the time” constantly over time), one group with initial lower level of energy that increased over time (decreased fatigue class, i.e., complaining fatigue “most of the time” at baseline but “some of the time” at 5-year follow-up), and the fourth group with persistent high energy (persistent energy class; i.e., complaining fatigue “some of the time” constantly over time.). All domains of cognitive function and everyday functioning declined gradually but significantly over five years. The decline rates, but not the baseline levels of cognitive function and everyday functioning, differed by the latent class of subjective fatigue. Except for the decreased fatigue class, there were various degrees of significant associations between the decline rates of subjective fatigue and every domain of cognitive function and everyday functioning in each class.
Our study represents the first effort to determine the heterogeneous trajectories of subjective fatigue in old age. Subjective fatigue can be interpreted differently by individuals. To some older adults, subjective fatigue may be an acute state, while to others, subjective fatigue may actually persist or re-occur frequently enough to present as a chronic condition or part of aging process (Avlund, 2010). The use of GMM in longitudinal aging research was able to capture the inter-individual differences in intra-individual change of subjective fatigue over time (Hagenaars and McCutcheon, 2002). As found in this study, subjective fatigue in the majority of older adults can be described as the depletion of various amounts of energy constantly over time, from small (persistent energy class) to large (persistent fatigue class). The findings are consistent with the overall devastating experienced by older adults in general from previous studies (Yu et al., 2009). Differently, the other two classes (i.e., increased fatigue and decreased fatigue) represent two distinct trajectories of subjective fatigue with particular clinical interest. The increased fatigue class had highest level of energy at baseline but declined fastest in the energy level over time, while the increased fatigue class had lowest levels of depression and smallest number of CVDRFs at baseline. The decreased fatigue class had lowest energy level at baseline but increased in the energy level over time, while the decreased fatigue class had the highest levels of depression at baseline. In the literature two thirds of fatigue in old age cannot be explained by any health conditions (Walker et al., 1993), and the trajectories of subjective fatigue within this proportion can be complicated. The seemingly contradictory results of the increased fatigue and decreased fatigue classes with health factors in the present study suggested that the trajectory of subjective fatigue over time may be influenced by time-dependent or other unexplored confounding factors than the baseline demographic or health factors. It is equally important to explore any potential time-dependent protective factors that may interfere with baseline demographic or health factors in explaining the increase or decrease of energy level in the two class.
This study also represents the first effort to examine longitudinal relationships between subjective fatigue and cognitive and functional abilities in older adults. In this study, data from ACTIVE trial were examined longitudinally from baseline to year 5. According to the analysis of attrition rate in the original ACTIVE trial (Willis et al., 2006), participants who remained at 5-year follow-up may be overall healthier than those who dropped out from the study. Such data may be unbalanced because participants withdraw from the study for different reasons. LME models are thus developed to model the dependence with random-effects and to incorporate the heterogeneity among participants along with the fixed-effects for time trends and other covariates (West et al., 2007).
Our study found an independent causal relationship between subjective fatigue and decline rate in cognitive and functional abilities beyond the influence of depression, grip strength, and CVDRFs. Avlund reviewed the factors that may influence fatigue, and suggested that subjective fatigue may be seen not only as a self-reported indicator of frailty that results from decreased physiologic reserves, but also a state that can be influenced by other factors (e.g., social, mental and biological) throughout life. Fatigue itself may be an independent indicator of aging process (Avlund, 2010). Findings of the present study were consistent with that from a previous study using a similar measurement of subjective fatigue, that is, subjective fatigue is not necessarily a proxy for depression, a component of frailty, or a consequence of CVDRFs, in predicting cognitive function or everyday functioning, and may contribute independently to these deficits in old age (Vestergaard et al., 2009).
Along with other findings, our study suggests the importance of considering the long-term negative effect of subjective fatigue on cognitive and functional abilities, into developing interventions for older adults. Compared to other less modifiable factors that can influence cognitive plasticity (e.g., genetic influence, education, age), low energy or mental effort supply may be modifiable (Eldadah, 2011). As a direction for future research, clinical trials should test whether the strategies for reducing fatigue, such as acetyl L-carnitine, yoga and meditation, can help improve cognitive and functional abilities (Bower et al., 2011), and importantly, whether such improvement would be mediated by the change of these underlying mechanisms (e.g., vascular energy homeostasis and the engagement in potentially neuroprotective activities). Particularly, the attention should be paid to the group of older adults with increased fatigue over time who had much faster cognitive decline than any other groups, even after controlling for all potential confounding factors. In addition to examining other etiological factors that may potentially contribute to such increase in subjective fatigue, strategies to directly alleviate subjective fatigue should be initiated as early as possible.
Limitations should be considered when interpreting our findings. First, we only measured energy-based fatigue state. Other dimensions of fatigue should be measured to capture a more comprehensive understanding of the relationship between fatigue and cognitive function in community-dwelling older adults. For example, fatigability, the process of becoming tired or fatigued that results in difficulty maintaining activities at a desired level, is one such dimension that should be considered. Interestingly, this dimension of fatigue has been shown to be related to executive function (Holtzer et al., 2011). Second, although we controlled for depression, CVDRFs, and grip strength, other potential confounding factors of fatigue (e.g., sleepiness, lack of motivation, social participation, and beta-blockers), were not included in this examination. Third, two out of the four fatigue classes (i.e., increased fatigue and decreased fatigue) had relatively small numbers of participants. To avoid the potential over-exaction of the classes, reproducing these classes is needed in other cohort studies (Bauer and Curran, 2003). Finally, in spite of excluding patients with dementia at baseline, it was not clear whether any participants developed dementia at follow-up visits. To expand the findings from the present study, future studies should examine whether the difference in trajectories of subjective fatigue will predict the incidence of dementia.
Acknowledgements
The original ACTIVE study was supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01 NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), the University of Alabama at Birmingham (U01 AG14289), and the University of Florida (U01AG14276).
The authors wish to acknowledge the help from Dr. Giovanni Schifitto and Dr. James McMahon (University of Rochester Medical Center) for comments on original version of the manuscript.
Footnotes
Conflict of interest: Dr. Karlene Ball owns stock in the Visual Awareness Research Group (formerly Visual Awareness, Inc.), and Posit Science, Inc., the companies that market the Useful Field of View Test and speed of processing training software used in the ACTIVE study. Posit Science acquired Visual Awareness, and Dr. Ball continues to collaborate on the design and testing of these assessments and training programs as a member of the Posit Science Scientific Advisory Board.
The other authors report no personal or financial disclosures.
Author's role: F. Lin: Formulated the research question(s), designed the study, carried it out, analyzed the data and wrote the article.
D. Chen: Analyzed the data and assisted with writing the article.
D. Vance: Assisted with formulating the research question and writing the article.
K. Ball: Designed the parent study and collected the data for the parent study, assisted with formulating the research question in this study and writing the paper
M. Mapstone: Assisted with formulating the research question, designing the study, and writing the paper
References
- Alexander NB, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. Journal of the American Geriatrics Society. 2011;58:967–975. doi: 10.1111/j.1532-5415.2010.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IVTR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andreasen AK, Spliid PE, Andersen H, Jakobsen J. Fatigue and processing speed are related in multiple sclerosis. European Journal of Neurology. 2011;17:212–218. doi: 10.1111/j.1468-1331.2009.02776.x. [DOI] [PubMed] [Google Scholar]
- Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clinical and Experimental Research. 2010;22:100–115. doi: 10.1007/BF03324782. [DOI] [PubMed] [Google Scholar]
- Ball K, Beard B, Roenker D, Miller R, Griggs D. Increasing mobility and reducing accidents of older drivers. In: Schaie K, Pietrucha M, editors. Mobility and Transportation in the Elderly. Springer Publishing Co Inc; New York, NY: 2000. pp. 213–251. [Google Scholar]
- Ball K, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: implications for overextraction of latent trajectory classes. Psychological Methods. 2003;8:338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]
- Bower JE, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011 doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. Journal of the American Geriatrics Society. 2011;58:248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clinical Neuropsychologist. 1991 [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: observational assessment and cognitive correlates. Psychology and Aging. 1995;10:478–491. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, Derman D. Kit of Factor Referenced Cognitive Tests. Revised ed. Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Eldadah BA. Fatigue and fatigability in older adults. PM & R. 2011;2:406–413. doi: 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nature Reviews. Neurology. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Gonda J, Schaie K. Schaie-Thurstone Mental Abilities Test: Word Series Test. Consulting Psychologists Press; Palo Alto, Calif: 1985. [Google Scholar]
- Hagenaars JA, McCutcheon AL. Applied latent class analysis. Cambridge Univ Pr; 2002. [Google Scholar]
- Holtzer R, Foley F. The relationship between subjective reports of fatigue and executive control in multiple sclerosis. Journal of the Neurological Sciences. 2009;281:46–50. doi: 10.1016/j.jns.2009.02.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Shuman M, Mahoney JR, Lipton R, Verghese J. Cognitive fatigue defined in the context of attention networks. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition. 2011;18:108–128. doi: 10.1080/13825585.2010.517826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CQ, Wang ZR, Li YH, Xie YZ, Liu QX. Cognitive function and risk for depression in old age: a meta-analysis of published literature. International Psychogeriatrics. 2011;23:516–525. doi: 10.1017/S1041610210000049. [DOI] [PubMed] [Google Scholar]
- Jobe JB, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Kaltsas G, Vgontzas A, Chrousos G. Fatigue, Endocrinopathies, and Metabolic Disorders. PM&R. 2011;2:393–398. doi: 10.1016/j.pmrj.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kelley KW, et al. Cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Laditka SB, et al. Attitudes about aging well among a diverse group of older Americans: implications for promoting cognitive health. Gerontologist. 2009;49(Suppl 1):S30–39. doi: 10.1093/geront/gnp084. [DOI] [PubMed] [Google Scholar]
- Lin F, Friedman E, Quinn J, Chen D, Mapstone M. Effect of Leisure Activities on Inflammation and Cognitive Function in an Aging Sample. Archives of Gerontology and Geriatrics. 2012 doi: 10.1016/j.archger.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou JS. Physical and mental fatigue in Parkinson's disease: epidemiology, pathophysiology and treatment. Drugs Aging. 2009;26:195–208. doi: 10.2165/00002512-200926030-00002. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Fisher E, Miller DM, Lee J-C, Rudick RA. Association of fatigue and brain atrophy in multiple sclerosis. Journal of the Neurological Sciences. 2005;228:161–166. doi: 10.1016/j.jns.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Melamed S, Shirom A, Toker S, Berliner S, Shapira I. Burnout and risk of cardiovascular disease: evidence, possible causal paths, and promising research directions. Psychological Bulletin. 2006;132:327–353. doi: 10.1037/0033-2909.132.3.327. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14:535–569. [Google Scholar]
- O'Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. Journal of Psychosomatic Research. 2004;57:435–441. doi: 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Hashiguchi T, Maenosono R, et al. The diagnostic value of endothelial function as a potential sensor of fatigue in health. Vascular Health and Risk Management. 2011;6:135–144. doi: 10.2147/vhrm.s8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Ball K, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr., Ball K. Timed instrumental activities of daily living tasks: relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Panza F, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. Journal of Alzheimer's disease. 2011;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self Report Depression Scale for Research in the General. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. Archives de Psychologie. 1942;28 [Google Scholar]
- Reyes-Gibby CC, Mendoza TR, Wang S, Anderson KO, Cleeland CS. Pain and fatigue in community-dwelling adults. Pain Medicine. 2003;4:231–237. doi: 10.1046/j.1526-4637.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Thurstone L, Thurstone T. Examiner Manual for the SRA Primary Mental Abilities Test (Form 10–14) Science Research Associates; Chicago, III: 1949. [Google Scholar]
- Verdelho A, Henon H, Lebert F, Pasquier F, Leys D. Depressive symptoms after stroke and relationship with dementia: A three-year follow-up study. Neurology. 2004;62:905–911. doi: 10.1212/01.wnl.0000115107.66957.8c. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Katon WJ, Jemelka RP. Psychiatric disorders and medical care utilization among people in the general population who report fatigue. Journal of General Internal Medicine. 1993;8:436–440. doi: 10.1007/BF02599621. [DOI] [PubMed] [Google Scholar]
- Walston J. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. Journal of the American Geriatrics Society. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Ware J. SF-36 Health Survey manual and interpretation guide. QualityMetric; Lincoln, NE: 2000. [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical care. 1992:473–483. [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: a practical guide using statistical software. CRC Press; 2007. [Google Scholar]
- Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. Journal of Psychosomatic Research. 2007;62:153–158. doi: 10.1016/j.jpsychores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Willis S, Marsiske M. Manual for the Everyday Problems Test. Pennsylvania State University; University Park: 1993. [Google Scholar]
- Willis SL, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockbum J, Baddeley A. Reading. Thames Valley Test Co; 1985. The River-mead Behavioral Memory Test. [Google Scholar]
- Yu DS, Lee DT, Man NW. Fatigue among older people: a review of the research literature. International Journal of Nursing Studies. 2009;47:216–228. doi: 10.1016/j.ijnurstu.2009.05.009. [DOI] [PubMed] [Google Scholar]