Abstract
Millions of molecules of lipopolysaccharide (LPS) must be assembled on the E. coli cell surface every division. Biogenesis of LPS requires seven essential lipopolysaccharide transport (Lpt) proteins to move LPS from the inner membrane (IM) through the periplasm to the cell surface. However, no intermediate transport states have been observed. We developed methods to observe intermediate LPS molecules bound to Lpt proteins in the process of being transported in vivo. Movement of individual LPS molecules along these binding sites required multiple rounds of ATP hydrolysis in vitro, which suggests that ATP is used to push a continuous stream of LPS through a transenvelope bridge in discrete steps against a concentration gradient.
The outer membrane (OM) of Gram-negative bacteria is an asymmetric bilayer with the inner leaflet composed of phospholipids and the outer leaflet composed of lipopolysaccharide (LPS). The LPS layer allows Gram-negative bacteria to survive in harsh environments and in the presence of many antibiotics. However, phospholipids and LPS do not spontaneously assemble into an asymmetric bilayer, and the cell devotes significant resources to synthesizing LPS at the inner membrane (IM) and assembling it in the OM (1). The process of how hydrophobic LPS molecules are transported across two membranes and the intervening, aqueous periplasmic space is not understood but is known to be mediated by seven essential Lpt (lipopolysaccharide transport) proteins. The heteromeric ABC transporter, LptBFG, forms a complex in the inner membrane with a membrane-bound protein, LptC (Fig. 1A) (2–5). Using homologous domains, the C-terminus of LptC interacts with the N-terminus of LptA, and the C-terminus of LptA interacts with the N-terminal periplasmic domain of LptD (6–9). The resulting head-to-tail oligomer produced creates a transenvelope protein bridge that connects the IM Lpt components with the OM LPS translocon (LptD and E) (6, 10–15). Defects in any of the Lpt proteins cause LPS to accumulate in the outer leaflet of the IM (2, 16). However, the mechanism of LPS transport has been difficult to study because it involves two membranes and seemingly cannot be broken into different steps.
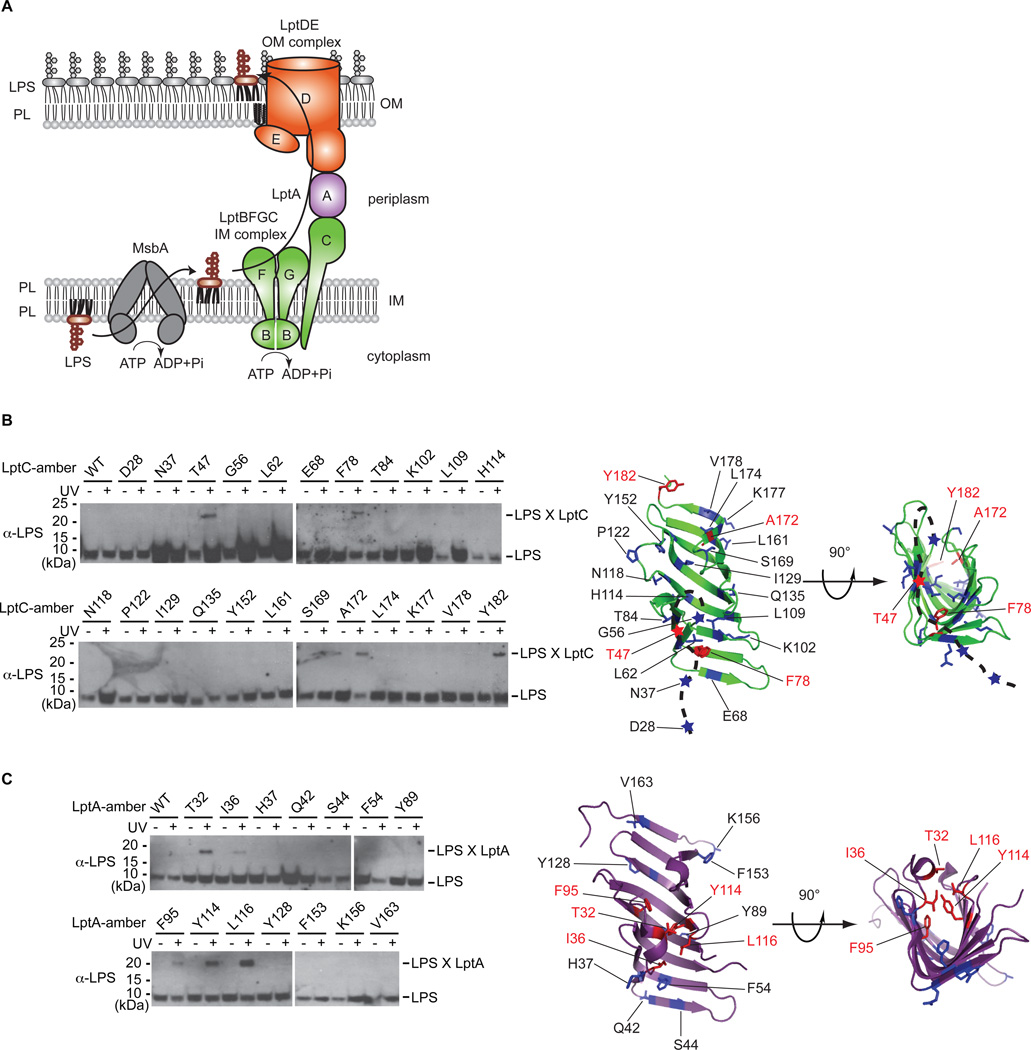
Fig. 1. LPS crosslinks to the inner surface of LptC and LptA in vivo.
(A) Cartoon of LPS transport in E. coli. MsbA flips LPS across the IM, and seven Lpt proteins transport it to the cell surface. (B) Specific amino acid positions in LptC crosslinked to LPS. In His-tagged LptC, eight residues on the inside surface, 11 residues on the outside surface, and four residues in the disordered region (shown as a dashed line) were mutated to incorporate pBPA. Four positions (red), three on the inside surface and one in the disordered region, crosslinked to LPS upon UV-irradiation. Crosslinking adducts (LPS X LptC) were detected by nickel affinity chromatography followed by immunoblotting with anti-LPS antibodies. Non-crosslinked LPS was also detected. (C) Specific amino acid positions in LptA crosslinked to LPS. As in (B), six and eight positions on the inside and outside surfaces, respectively, of the β-jellyroll were mutated to pBPA and evaluated following UV-irradiation by immunoblotting. Five specific positions (red) on the inside surface of LptA crosslinked to LPS.
To elucidate the mechanism of LPS transport, we first sought to identify sites where LPS interacts with the Lpt proteins in vivo. We incorporated an unnatural amino acid containing a photocrosslinker (p-benzoylphenylalanine, pBPA) at 23 positions in LptC and 14 positions in LptA (17–20). Four of the LptC mutants and five of the LptA mutants formed crosslinks to LPS in a UV-dependent manner (Fig. 1B and 1C). LptC adducts contained the crosslinker at positions 47, 78, 172, and 182 (Fig. 1B), while LptA adducts contained the crosslinker at positions 32, 36, 95, 114, and 116 (Fig. 1C). Except for residue 47 in LptC, all of these LPS adducts formed on the inside of the β-jellyroll structures. Residue 47 in LptC is in a disordered region of the protein (9), which may become ordered when it interacts with the LptBFG complex or LPS. No crosslinks from side chains on the outside surface of the β-jellyroll of LptC or LptA were observed. Thus, we propose that LPS specifically binds inside these proteins and transits the periplasm on the inside of the β-jellyroll of these proteins.
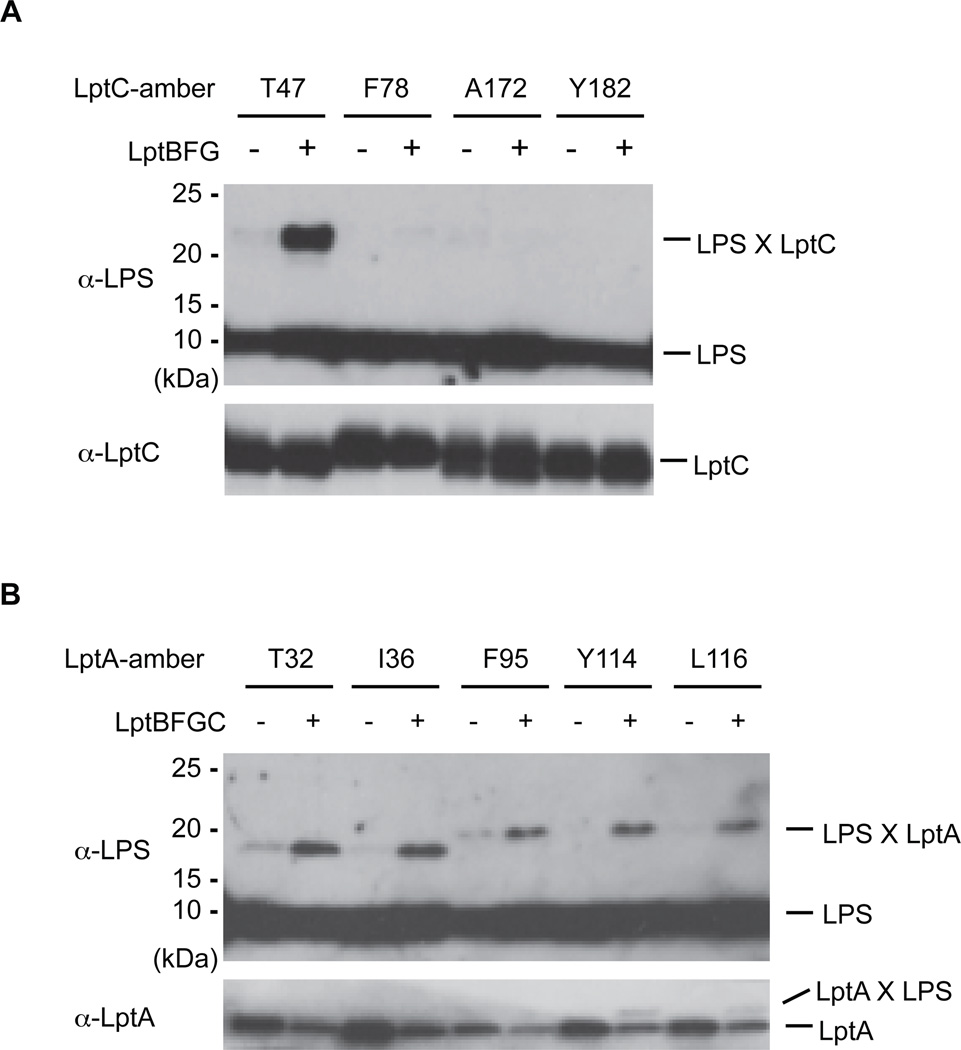
We tested whether LptC and LptA alone can extract LPS from the IM or whether cross-linking to LPS is dependent upon the entire transenvelope complex by manipulating the expression levels of the Lpt components. We overexpressed LptC or LptA alone or in combination with the other IM Lpt proteins (i.e. LptBFG or LptBFGC), reasoning that LptC and LptA would mostly not reside in Lpt complexes if they were overexpressed individually. Crosslinking of LPS to LptC at position 47, but not to position 78, 172, or 182, increased substantially when LptBFG were co-overexpressed (Fig. 2A). Thus, when LptA, D and E are limiting, LPS accumulates near residue 47 of LptC. Position 47 may be located near a strong binding site for LPS, whereas the other crosslinking positions may reflect more transient interactions that occur during the movement of LPS through fully assembled bridges. Because LPS accumulation at the binding site around residue 47 depends on LptBFG, it is also clear that LptC cannot extract LPS directly from the IM unaided.
Fig. 2. LPS cross-linking in LptA and LptC depends on the IM components of the Lpt machine.
(A) LPS accumulates near residue T47 of LptC in LptBFGC complexes. LptC mutants that crosslink to LPS were overexpressed with or without co-overexpression of LptBFG. Crosslinking was detected similarly to Fig. 1B. Crosslinking at position 47 significantly increases when LptBFG are co-overexpressed. (B) LPS accumulates at several positions of LptA in LptBFGC-dependent manner. In analogous method to that in (A), LptA mutants that crosslink to LPS were overexpressed with or without co-overexpression of LptBFGC. Crosslinking at all five positions increases when LptBFGC are co-overexpressed.
All five LptA mutants showed increased crosslinking with LPS when they were co-overexpressed with LptBFGC (Fig. 2B). Thus, LptA cannot receive LPS directly from the IM, but requires LptBFGC complex. LPS must migrate out of the IM in an ordered process that requires LptBFG to get to LptC and LptBFGC to get to LptA. Furthermore, because LPS transport between these proteins can occur in incomplete Lpt bridges, it was possible to develop a biochemical system to separate the individual step of LPS release from the membrane from that of the transport step along the Lpt bridge.
In order to study the ATP-dependence of LPS extraction from the IM, we first prepared spheroplasts containing overexpressed LptC(T47pBPA) and LptBFG by disrupting the OM and cell wall with ethylenediaminetetraacetic acid (EDTA) and lysozyme. The IM was then permeabilized by osmotic shock to introduce buffer with or without ATP into the cytoplasm to generate right side-out (RSO) membrane vesicles (21–25). These vesicles were then irradiated with UV light. Substantial LPS crosslinking to residue 47 of LptC was only observed when LptBFG was co-overexpressed and ATP was present (Fig. 3A). A small amount of LPS was crosslinked to LptC in the absence of ATP, but this amount did not increase over time (Fig. 3A). Presumably this small amount of LPS was present prior to generating the RSO fraction. Because crosslinking increased as a function of time in the presence of ATP, transfer of LPS from the IM to the membrane-bound LptC appears to require energy.
Fig. 3. LPS transport can be reconstituted in vitro.
(A) LPS accumulates in LptC in membrane vesicles over time in an LptBFG and ATP-dependent manner. RSO membrane vesicles containing overexpressed LptC(T47pBPA) with or without co-overexpression of LptBFG were prepared in the presence or absence of ATP. After incubation at 30°C for time indicated, the vesicles were UV-irradiated. Crosslinking was detected as in Fig. 1B. (B) LPS is released to LptA in an LptBFGC, time, and ATP-dependent manner. RSO membrane vesicles were prepared from wild-type cells or cells overexpressing LptC, LptBFG, or LptBFGC with or without added ATP. Purified LptA(I36pBPA or H37pBPA) was added to these vesicles, incubated at 30°C for time indicated, and then UV-irradiated. (C) Model of the stacked crystal structures of LptC (green) and LptA (purple). Residues I36 and H37 in LptA, which interact with LPS and LptC, respectively, are depicted as stick structures. (D) Periplasmic bridge components properly assemble in vitro. Purified LptA(I36pBPA or H37pBPA) was added to LptC- or LptBFGC-enriched RSO membrane vesicles. Samples were incubated at 30°C, and UV-irradiated. LptA(H37pBPA) crosslinked to LptC in an ATP, time, and LptBFG-independent manner.
We next addressed whether LPS could be transferred to the periplasmic component, LptA, in this in vitro system. This reconstitution requires release of LPS from a membrane fraction to a soluble periplasmic protein (Fig. S1). Purified LptA(I36pBPA), which crosslinked LPS in vivo, was added to RSO vesicles and analyzed for crosslinking to LPS. LPS crosslinked to LptA over time only in the presence of ATP and when all four IM components were overexpressed (Fig. 3B). Notably, LPS did not crosslink to LptA in the absence of LptC overexpression, indicating that LPS must first be transferred to LptC before being transferred to LptA, which is consistent with previous work (9). To confirm that the soluble periplasmic bridge component assembled in this in vitro system reflects the in vivo assembly process, we tested the specificity of this crosslinking. An LptA mutant, H37pBPA interacts directly with LptC (6) but not with LPS in vivo (Fig. 1C). Both residues I36 and H37 are located on the edge of the β-jellyroll that interacts with LptC, but their side chains are oriented in opposite directions (Fig. 3C). In this RSO vesicle reconstitution the H37 mutant did crosslink to LptC (Fig. 3D). However, although H37 is directly adjacent to I36, H37 did not crosslink to LPS in the presence of ATP (Fig. 3B). Thus our in vitro reconstitution cleanly recapitulates the specific protein-protein and the protein-substrate interactions observed in vivo. Because the binding of LptA to LptC was rapid and did not depend on time or ATP (Fig. 3D), energy appears not to be required for assembly of the bridge, but is required to transport LPS from the IM to LptA.
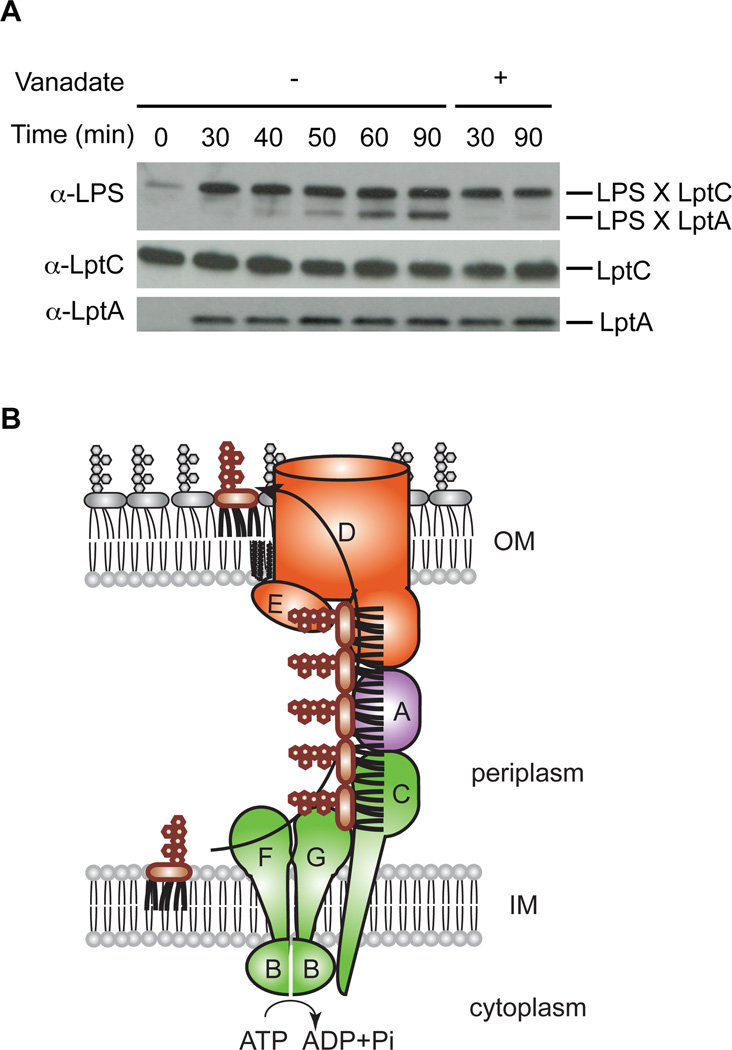
A small amount of LPS accumulated in LptC in the absence of ATP (Fig. 3A), but did not spontaneously move to LptA (Fig. 3B). We thus asked whether LPS transfer from LptC to LptA requires a separate energetic step. We incubated RSO vesicles overexpressing LptBFG-LptC(T47pBPA) with ATP to accumulate LPS in LptC, and then added purified LptA(I36pBPA) with or without an ATPase inhibitor, vanadate, prior to irradiating the samples. LPS did not crosslink to LptA in the inhibitor-treated samples, showing that LPS transfer from LptC to LptA required energy (Fig. 4A). In the absence of vanadate, LptC crosslinked adducts with LPS were not decreased even when LPS transferred to LptA, which suggests that the binding sites for LPS in the periplasmic bridge are always filled with LPS during its transport. Thus at least two energy-dependent steps are involved in LPS transport: one during extraction from the membrane to the periplasmic domain of the membrane-bound LptC, and another during transfer of LPS from LptC to LptA. These steps may occur synchronously. We propose a model for LPS transport in which ATP hydrolysis in the cytoplasm provides energy to push LPS molecules in discrete steps across the bridge (Fig. 4B).
Fig. 4. LPS is pushed in a continuous stream through the Lpt bridge.
(A) Inhibiting ATP hydrolysis inhibits transfer of LPS from LptC to LptA. RSO membrane vesicles overexpressing LptBFG-LptC(T47pBPA) were incubated at 30°C for 30 min in the presence of ATP to accumulate LPS in LptC. Purified LptA(I36pBPA) was then added with or without vanadate, and the samples were incubated for an additional 60 min. Crosslinking was detected as in Fig. 1B. (B) Model of LPS biogenesis. LptBFG extracts LPS from the IM and transports it to LptC using ATP hydrolysis energy. ATP hydrolysis is used again to push LPS from LptC to LptA.
The mechanism of LPS transport bears similarities with efflux pumps, which also form transenvelope bridges and use energy to drive a small molecule through a continuous channel to the exterior environment (26–28). The model for efflux pumps suggests that only one energy dependent step drives one toxic molecule out of the cell. This requirement reflects the fact the noxious molecules are released from the internal three-dimensional reservoir into the channel of the pump, which is continuous with the infinite exterior environment; diffusion can efficiently carry the molecule away. Movement of LPS from the IM to the OM across the periplasm proceeds through a series of energy dependent steps as sequential molecules are pushed in a continuous stream through the bridge. The problem of LPS transport is different from drug efflux in that LPS must move from a two-dimensional reservoir at the IM to another two-dimensional reservoir at the cell surface in opposition to the concentration gradient. In order to increase the efficiency of unidirectional transport, multiple rounds of ATP hydrolysis are required. If transport across the periplasmic bridge requires that multiple binding sites on Lpt proteins be filled with LPS during transport, it may be possible to design inhibitors that destroy the pumping mechanism by introducing air in the line.
Supplementary Material
Acknowledgements
The plasmid pSup-BpaRS-6TRN was a generous gift from Professor Peter G. Schultz (The Scripps Research Institute). We are grateful to Dr. C. Hagan for valuable discussion. This work was supported by National Institutes of Health Grants AI081059 and GM066174 (D.K.). E.F. was a graduate fellow of the Fannie and John Hertz Foundation. Materials and methods are available as supplementary materials on Science Online.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz N, Gronenberg LS, Kahne D, Silhavy TJ. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 2008;105:5537. doi: 10.1073/pnas.0801196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperandeo P, et al. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J Bacteriol. 2007;189:244. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz N, Kahne D, Silhavy TJ. Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat Rev Microbiol. 2009;7:677. doi: 10.1038/nrmicro2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narita S, Tokuda H. Biochemical characterization of an ABC transporter LptBFGC complex required for the outer membrane sorting of lipopolysaccharides. FEBS Lett. 2009;583:2160. doi: 10.1016/j.febslet.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 6.Freinkman E, Okuda S, Ruiz N, Kahne D. Regulated Assembly of the Transenvelope Protein Complex Required for Lipopolysaccharide Export. Biochemistry. 2012;51:4800. doi: 10.1021/bi300592c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperandeo P, et al. New insights into the Lpt machinery for lipopolysaccharide transport to the cell surface: LptA-LptC interaction and LptA stability as sensors of a properly assembled transenvelope complex. J Bacteriol. 2011;193:1042. doi: 10.1128/JB.01037-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suits MD, Sperandeo P, Deho G, Polissi A, Jia Z. Novel structure of the conserved gram-negative lipopolysaccharide transport protein A and mutagenesis analysis. J Mol Biol. 2008;380:476. doi: 10.1016/j.jmb.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 9.Tran AX, Dong C, Whitfield C. Structure and functional analysis of LptC, a conserved membrane protein involved in the lipopolysaccharide export pathway in Escherichia coli. J Biol Chem. 2010;285:33529. doi: 10.1074/jbc.M110.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun M, Silhavy TJ. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol. 2002;45:1289. doi: 10.1046/j.1365-2958.2002.03091.x. [DOI] [PubMed] [Google Scholar]
- 11.Chng SS, Gronenberg LS, Kahne D. Proteins required for lipopolysaccharide assembly in Escherichia coli form a transenvelope complex. Biochemistry. 2010;49:4565. doi: 10.1021/bi100493e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chng SS, Ruiz N, Chimalakonda G, Silhavy TJ, Kahne D. Characterization of the two-protein complex in Escherichia coli responsible for lipopolysaccharide assembly at the outer membrane. Proc Natl Acad Sci U S A. 2010;107:5363. doi: 10.1073/pnas.0912872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freinkman E, Chng SS, Kahne D. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc Natl Acad Sci U S A. 2011;108:2486. doi: 10.1073/pnas.1015617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu T, et al. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 2006;103:11754. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos MP, Tefsen B, Geurtsen J, Tommassen J. Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci U S A. 2004;101:9417. doi: 10.1073/pnas.0402340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperandeo P, et al. Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli. J Bacteriol. 2008;190:4460. doi: 10.1128/JB.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science. 2001;292:498. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 18.Chin JW, Martin AB, King DS, Wang L, Schultz PG. Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proc Natl Acad Sci U S A. 2002;99:11020. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu Y, Schultz PG. Efficient incorporation of unnatural amino acids into proteins in Escherichia coli. Nat Methods. 2006;3:263. doi: 10.1038/nmeth864. [DOI] [PubMed] [Google Scholar]
- 20.Liu CC, Schultz PG. Adding new chemistries to the genetic code. Annu Rev Biochem. 2010;79:413. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 21.Kaback HR. Bacterial Membranes. Methods Enzymol. 1971;XXII:99. [Google Scholar]
- 22.Kaback HR, Stadtman ER. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966;55:920. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YJ, Rajapandi T, Oliver D. SecA protein is exposed to the periplasmic surface of the E. coli inner membrane in its active state. Cell. 1994;78:845. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 24.Yakushi T, Yokota N, Matsuyama S, Tokuda H. LolA-dependent release of a lipid-modified protein from the inner membrane of Escherichia coli requires nucleoside triphosphate. J Biol Chem. 1998;273:32576. doi: 10.1074/jbc.273.49.32576. [DOI] [PubMed] [Google Scholar]
- 25.Materials and methods are available as supplementary materials on Science Online.
- 26.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 2006;443:173. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 27.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeger MA, et al. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006;313:1295. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.