Abstract
One of the most remarkable characteristics of stem cells is their ability to perpetuate themselves through self-renewal while concomitantly generating differentiated cells. In the hematopoietic system, stem cells balance these mechanisms to maintain steady-state hematopoiesis for the lifetime of the organism, and to effectively regenerate the system following injury. Defects in the proper control of self-renewal and differentiation can be potentially devastating and contribute to the development of malignancies. In this review, we trace the emerging role of Wnt signaling as a critical regulator of distinct aspects of self-renewal and differentiation, its contribution to the maintenance of homeostasis and regeneration, and how the pathway can be hijacked to promote leukemia development. A better understanding of these processes could pave the way to enhancing recovery after injury and to developing better therapeutic approaches for hematologic malignancies.
Wnt signaling is a critical regulator of hematopoietic stem cell self-renewal and differentiation. The pathway can be hijacked to promote the development of leukemia and lymphoma.
Hematopoietic stem cells (HSCs) give rise to all the cells of the blood. The ability of HSCs to successfully balance self-renewal to permit regeneration with differentiation to produce mature blood cells is critical to survival. Regulation of this balance recapitulates events that occur during embryonic development, and thus often uses many of the key signaling pathways found in ontogeny. In this context the Wnt pathway, which is a key player in pattern formation and other aspects of early development, has emerged as an important new regulator of many aspects of hematopoiesis, including stem cell establishment, homeostatic maintenance, and regeneration. Furthermore, Wnt signaling can also influence many differentiated cells of the blood. Given the close connections between the self-renewal that occurs during normal development and the uncontrolled self-renewal that can occur in cancers, Wnt signaling has also been studied in the context of cancers of the blood and has been shown to contribute to the aberrant renewal that drives leukemia formation. As with all pleiotropic factors, the Wnt pathway has a multifaceted influence on hematopoiesis. The diversity of effects comes in part from the complex nature of the signal itself: the family represents 19 different ligands and up to ten different receptors, and the nature of the ligand and receptor combination can activate either canonical or noncanonical cascades. Further, distinct cellular contexts provided by the multitude of developing and mature blood lineage cells can lead to differential outcomes following activation or inhibition of the pathway. Despite this complexity, experiments from many different angles are leading to consensus views of the nature of Wnt signaling in the hematopoietic system and how it can contribute to hematopoietic stem cell and progenitor cell function in specific contexts, raising the possibility that if correctly modulated it could be used to enhance hematopoietic regeneration. Perhaps even more exciting is the emerging role of Wnt signaling in driving the growth of numerous leukemias and lymphomas, suggesting that its effective blockade could have a profound impact on the treatment of hematologic malignancies in the clinic.
HSCs
Activation of Wnt Signaling during Hematopoiesis
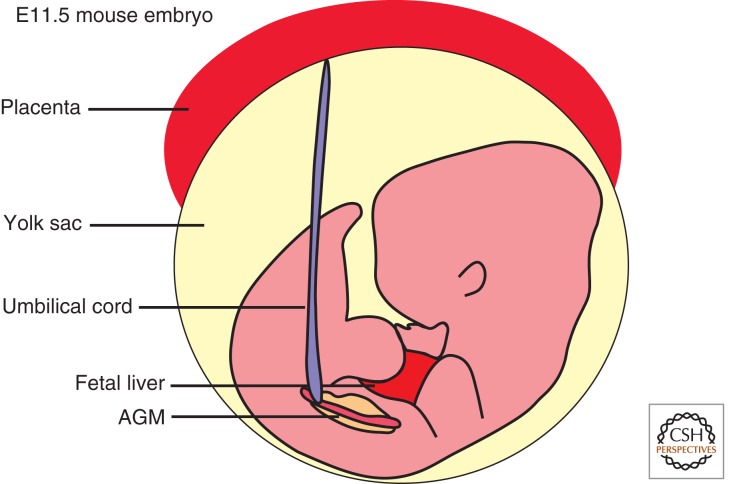
During development, hematopoiesis occurs at distinct anatomical sites. Murine hematopoietic development begins in the extraembryonic yolk sac at E7.5, continues in the chorioallantoic placenta, para-aortic splanchnopleura, and aorta–gonad–mesonephros (AGM) at E9 and the fetal liver at E10, and culminates in the colonization of the bone marrow at E15 (Fig. 1) (Medvinsky et al. 2011). In adult life all hematopoiesis stably occurs in the bone marrow.
Figure 1.
Murine embryonic hematopoiesis occurs simultaneously in several organs during development. The mouse embryo generates de novo hematopoietic stem cells (HSCs) in the placenta, yolk sac, and aorta–gonad–mesonephros (AGM) beginning at E7.5. These HSCs then migrate into and seed various organs at the start of definitive hematopoiesis around E11. Normal adult hematopoiesis only occurs in the bone marrow. (Figure created from data in Medvinsky et al. 2011.)
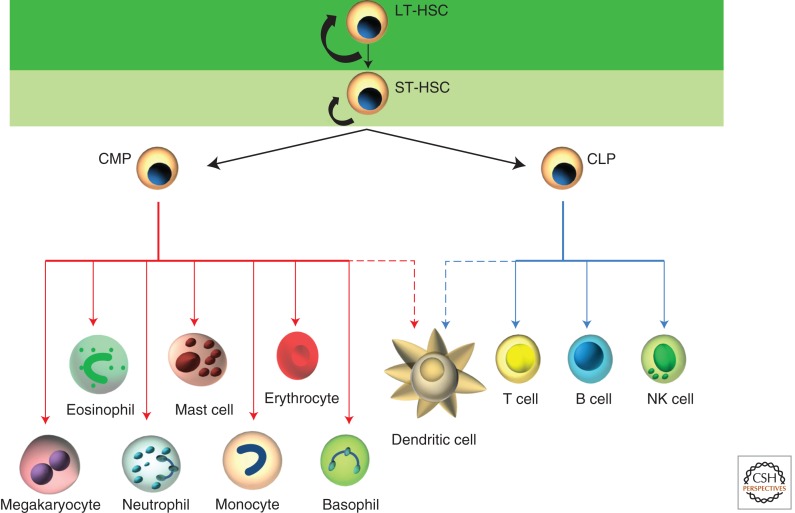
At each of these sites in fetal and adult life the maturation of blood cells from the hematopoietic stem cell follows a series of commitment steps (Fig. 2). Long-term HSCs lie at the apex of the hierarchy and display an extensive capacity for self-renewal, allowing them to reconstitute the hematopoietic system in a recipient for the long term. These cells give rise to the “short-term” HSCs, which have more limited self-renewal capacity and are only able to support reconstitution for a few weeks following transplantation. These short-term HSCs give rise to multipotent progenitor cells, which have no self-renewal capacity but which can differentiate to the full complement of hematopoietic cells, in part by stepping through semicommitted states such as the common myeloid progenitor, a precursor of cells like macrophages and granulocytes, and the common lymphoid progenitor, a precursor of B, T, and NK cells.
Figure 2.
The hematopoietic stem cell hierarchy in adult bone marrow. All differentiated blood cells are generated from a small pool of self-renewing long-term hematopoietic stem cells (LT-HSCs) (dark green area). LT-HSCs are capable of both self-renewal and differentiation. When LT-HSCs differentiate, they create short-term HSCs (ST-HSCs) with limited self-renewal potential (light green area). The ST-HSCs produce progenitors that give rise to multipotent non-self-renewing common myeloid progenitors (CMP) and common lymphoid progenitors (CLP). These progenitor populations generate mature hematopoietic cells such as macrophages, B and T cells, and red blood cells (erythrocytes). The dashed lines indicate partial lineage connections.
Early evidence that Wnt signaling may be involved in orchestrating hematopoietic ontogeny came from studies showing that Wnt ligands, including Wnt-5a and Wnt-10b, are expressed by the murine fetal liver (Austin et al. 1997). Subsequent studies, including observations in both mouse and Xenopus embryos that Wnt signaling is found in sites of primitive erythropoiesis (Cheng et al. 2008; Tran et al. 2010), as well as the demonstration that Wnt ligands and frizzled receptors are expressed in the murine yolk sac, aorta–gonad–mesonephros, and fetal liver, support a role for Wnt signaling in developmental hematopoiesis (Corrigan et al. 2009). More recently, studies using zebrafish have shown that early embryonic expression of the noncanonical ligand Wnt-16 in the somites is required to initiate definitive hematopoiesis in the prehematopoietic mesoderm and dorsal aorta (Clements et al. 2011).
In the adult bone marrow, Wnts are expressed in the microenvironment surrounding the HSCs (Table 1). For example, studies performed on human bone marrow indicate that Wnt-2b, Wnt-5a, and Wnt-10b are expressed in unfractionated bone marrow cells, and that Wnt-5a is expressed in populations enriched for HSCs and progenitors (Van den Berg et al. 1998). In the mouse bone marrow, Wnt-5a is expressed by mature B cells (Liang et al. 2003), whereas Wnt-10b is produced by myeloid cells, erythrocytes, and immature B cells (Congdon et al. 2008). Moreover, the precise expression of Wnt ligand seems to be regulated dynamically during hematopoietic regeneration; whereas Wnt-3a, Wnt-5a, and Wnt-10b are all expressed in the homeostatic murine bone marrow microenvironment (Reya et al. 2000), Wnt-10b is specifically up-regulated in the injured murine bone marrow microenvironment (Congdon et al. 2008), suggesting that specific Wnts may be particularly adept at or used during regeneration. The receptors for these ligands are also extensively expressed (Table 1), with long-term human HSCs expressing Frizzled 6 (Wagner et al. 2004). These studies collectively indicate that Wnt signaling components are found in both fetal and adult sites of hematopoiesis. Further, the possibility that ligand and receptor expression is indicative of physiologic utilization is supported by reporter expression in HSCs (Nemeth et al. 2007).
Table 1.
Expression of Wnt pathway elements in the bone marrow microenvironment
| Cell source | Wnt ligands | FZD/LRP | sFRP/Dkk/WIF | Transcription factors | References |
|---|---|---|---|---|---|
| Osteoblasts | Wnt2, Wnt2B2, Wnt3A, Wnt4, Wnt5AQ, Wnt10A, Wnt11 | FZD2, FZD3, FZD4, FZD5, FZD6 | sFRP1, sFRP2, sFRP3, sFRP4 | TCF1, TCF2, TCF3, TCF4, TLE1, CTBP1, β-catenin | Spencer et al. 2006 |
| Mesenchymal stem cell | Wnt2B, Wnt5A, Wnt2B, Wnt11 | FZD1, FZD2, FZD3, FZD4, FZD5, FZD6 | sFRP2, sFRP2, Dkk2 | β-catenin, TCF1 | Dufourcq et al. 2008; Li et al. 2010; Qui et al. 2011 |
| Endothelial cells | Wnt2, Wnt2b, Wnt3, Wnt5A, Wnt7A, Wnt 11, Wnt14 | FZD3, FZD4, FZD6, LRP5, LRP6 | sFRP1, sFRP3, Dkk1, Dkk2, Dkk3 | β-catenin, TCF1, Lef1 | Li et al. 2010; Planutiene et al. 2011 |
Gain-of-Function Studies
In an effort to understand whether the observed expression of Wnt/Fz is functionally important, numerous gain-of-function and loss-of-function experiments have been performed in model organisms as well as in human cells. Gain-of-function experiments to assess Wnt signaling in hematopoiesis have been approached in several ways, including exposure to soluble Wnt proteins, inhibition of GSK-3β, and activation of β-catenin.
Independent studies have shown that the addition of Wnt-3a can increase hematopoietic output in murine embryonic stem cell cultures and that canonical Wnt signaling can promote proliferation of committed hematopoietic progenitors in human embryonic stem cell cultures (Corrigan et al. 2009; Goessling et al. 2009). These studies are consistent with reports of Wnt-10b-induced proliferation of cultured murine fetal liver hematopoietic progenitor cells (Austin et al. 1997). Signaling via Wnt-11 can also increase the number of hematopoietic progenitors, possibly implicating noncanonical Wnts in hematopoietic lineage specification (Vijayaragavan et al. 2009).
Wnt signaling has also been triggered in murine and human HSCs using inhibitors of GSK-3β, a strategy that leads to activation of β-catenin. In vivo administration of GSK-3β inhibitors has been shown to promote the engraftment of HSCs in bone marrow transplantation models (Trowbridge et al. 2006), and short-term pretreatment of human HSC with a GSK-3β inhibitor can enhance the engraftment of human hematopoietic progenitor cells in xenograft models (Ko et al. 2011). In wild-type bone marrow, GSK-3β inhibition resulted in transient expansion of phenotypic hematopoietic stem cells, but concurrently resulted in progressive depletion of the long-term repopulation ability of these HSCs (Huang et al. 2009).
Studies based on the more direct activation of β-catenin have also provided important insight into the role of Wnt signaling in hematopoiesis. Transduction of the constitutively active form of β-catenin into HSCs of transgenic mice expressing the prosurvival protein Bcl-2 increased the number of hematopoietic stem cells during culture in vitro and enhanced reconstitution in vivo (Reya et al. 2003). Activated β-catenin can also reprogram CLPs to display more characteristics of undifferentiated multipotent cells (Baba et al. 2005) and its expression in murine bone marrow cells dramatically enhanced the ability of these cells to expand in culture. The cells maintained many primitive characteristics and responsiveness to normal signals, although they appeared arrested in an undifferentiated state (Baba et al. 2006). Interestingly, using a genetic approach, mice expressing stabilized β-catenin in the hematopoietic system were generated; these mice showed expansion of HSCs, arrested differentiation, and subsequent defects in hematopoietic reconstitution (Kirstetter et al. 2006; Scheller et al. 2006). By combining β-catenin activation with inhibition of apoptosis through PTEN deletion, recent studies have also shown that induction of prosurvival pathways in combination with Wnt activation drives the self-renewal and long-term reconstitution ability of HSCs (Perry et al. 2011). This study together with previous work (Reya et al. 2003) suggests that the cellular context of β-catenin activation and whether it is coexpressed with a survival factor may dictate its ultimate effect on expansion or exhaustion. Additionally, the levels at which β-catenin is overexpressed may also dictate the outcome. This was most effectively shown in recent studies in which a genetic series of adenomatous polyposis coli (APC) alleles was used to activate β-catenin at different levels, resulting in either expansion of HSCs at low doses or in their exhaustion at high doses (Luis et al. 2011).
Work from model organisms such as zebrafish is consistent with a role for Wnt signaling in the induction of long-term HSCs (Goessling et al. 2009). Prostaglandin E2 regulates hematopoietic stem cell specification in zebrafish as well (North et al. 2007) and promotes increased proliferation, survival, homing, and engraftment of murine HSC (Hoggatt et al. 2009; Durand and Zon 2010). Importantly, PGE2 can activate β-catenin-responsive Wnt reporter activity in the zebrafish AGM, the site of long-term hematopoietic stem cell potential. Studies in mice further showed that PGE2-dependent Wnt signaling enhanced hematopoietic progenitor cell generation from differentiating embryonic stem cells. Cumulatively, the work over the last decade indicates that the Wnt pathway is active in HSCs and that triggering it either at low levels or at high levels in the context of survival cues can lead to expansion of the stem cell pool.
Loss-of-Function Studies
To define whether Wnt signaling may be required for HSC specification, growth, and function, many studies targeting deletion of β-catenin and other components of the Wnt signaling pathway have been undertaken. Germline deletion of Wnt-3a leads to a dramatic reduction in the numbers of hematopoietic stem and progenitor cells in the fetal liver and results in embryonic lethality at E12.5 (Luis et al. 2009). Interestingly, the impaired self-renewal capacity of Wnt-3a null cells is not reversed by a normal microenvironment after transplantation, suggesting that the developmental absence of Wnt-3a may affect multiple aspects of stem cell function. Subsequent studies using a Vav-Cre transgene to conditionally delete β-catenin in the hematopoietic system of developing mice supported these findings. Although HSCs were established in these mice, they were deficient in their long-term maintenance and reconstitution capacity in transplantation studies (Zhao et al. 2007). Interestingly, mice in which an IFN-inducible Mx-Cre transgene was used to delete β-catenin in bone marrow progenitor cells did not display defects in the ability of HSC to self-renew or reconstitute hematopoietic lineages. The different consequences of β-catenin deletion in vivo are very likely attributable to the different contexts in which the deletions were initiated. The impact of deleting β-catenin in embryonic versus adult life could lead to the differences observed; alternatively, interferon (IFN) exposure, which can activate HSCs (Baldridge et al. 2010) in the Mx-Cre mice, could change the transcriptional context within the HSCs in which β-catenin is deleted and mask the effects of β-catenin loss.
Wnt Signaling in Lymphocyte Development
As stem cells differentiate to generate functional cells of the blood, they give rise to committed cells of both myeloid and lymphoid lineages. Components of the Wnt pathway, such as the downstream transcription factors Lef and Tcf, are expressed in developing lymphocytes (Oosterwegel et al. 1991; Travis et al. 1991; van Genderen et al. 1994). Gain- and loss-of-function approaches indicate that Wnt/β-catenin signaling is a key regulator of T-cell development during thymocyte differentiation (Molenaar et al. 1996; Schilham et al. 1998; Nusse 1999; Ioannidis et al. 2001; Staal et al. 2001; Mulroy et al. 2002, 2003; Weerkamp et al. 2006). Overexpression of Dkk1, a Wnt antagonist that prevents interactions between Wnt and Frizzled, blocks T-cell development at the double-negative (CD4−/CD8−) stage in fetal thymic organ cultures (Weerkamp et al. 2006). Furthermore, in vivo genetic deletion of β-catenin in T cells using Lck-Cre causes a maturation defect in double-positive T cells at the β-selection checkpoint and significantly reduces the peripheral splenic T-cell population (Xu et al. 2003). The Wnt/β-catenin signaling pathways also continue to influence T cells post differentiation (Gattinoni and Restifo 2010). In cytotoxic T cells, Wnt signaling promotes the formation of CD8+ memory T cells by inhibiting effector cell differentiation. In the helper T-cell lineage, Wnt signaling enhances the survival of regulatory CD4+ T cells and influences CD4+ Th-cell polarization, favoring the formation of Th2 over Th1 cells (Yu et al. 2009; Notani et al. 2010). In the B-cell lineage, genetic studies using Lef-1-deficient mice show that loss of Lef-1 leads to defects in pro-B-cell proliferation and survival in vitro and in vivo and that purified Wnt3a can induce proliferation of immature B cells (Reya et al. 2000). Consistent with this, B-cell defects were reported in the absence of Wnt receptors in Frizzled 9-deficient mice (Ranheim et al. 2005). The fact that activated β-catenin can lead to B-cell leukemia in transplant assays in the long term is consistent with the possibility that activation of this pathway promotes the growth and survival of B cells.
A few studies have also examined the role of the noncanonical Wnt signaling pathway in lymphocyte development. Wnt-5a-deficient mice display abnormal B-cell development; in this setting, Wnt-5a was found to signal at least in part through the noncanonical Wnt/Ca2+ pathway to interfere with pro-B-cell responses to IL-7 (Liang et al. 2003). The possibility that Wnt-5a may oppose canonical signaling was also suggested by the increased canonical signaling observed in thymocytes and distal limb buds from Wnt-5a−/− mice (Topol et al. 2003; Liang et al. 2007). In addition, whereas canonical Wnt signaling mediated by Wnt-3a overexpression in stromal cells from osteopetrotic mice inhibited B-cell and plasmacytoid dendritic cell development, Wnt-5a increased B-cell lymphopoiesis (Malhotra et al. 2008). Interestingly, in this setting the addition of Wnt-3a triggered increased expression of stem cell markers, suggesting the possibility that these culture conditions can induce an undifferentiated state.
These studies cumulatively indicate that Wnt signaling is important not only in HSCs but also in more committed lymphoid progenitor cells, including pro-B cells and pre-B cells in the bone marrow, as well as in the most immature subsets of thymocytes and in mature T cells (Staal and Clevers 2005). Emerging studies also indicate that Wnt signaling can influence nonlymphoid lineages. For example, GSK-3β negatively regulates megakaryocyte differentiation and platelet production from primary human bone marrow cells in vitro. In addition, numerous Wnt canonical effectors have been detected in human platelets, and Wnt-3a negatively regulates platelet function, including adhesion, activation, granule secretion, and aggregation (Steele et al. 2009).
LEUKEMIA
Wnt Signaling in Myeloid Leukemia
In addition to its role in normal hematopoietic function, activation of Wnt signaling is intricately involved in cancer formation in blood cells. Several studies in a variety of leukemic cell lines and patient samples support this possibility. For example, pre-B-cell leukemia lines carrying the E2A-PbX translocation overexpress Wnt proteins (McWhirter et al. 1999), and survival of these cells in vitro can be inhibited by blocking Wnt signaling (Mazieres et al. 2005). Similarly, studies of acute myelogenous leukemia show that associated fusion proteins enhance replating efficiency of HSCs and that this is abrogated on inhibition of the Wnt pathway (Muller-Tidow et al. 2004; Zheng et al. 2004). Most importantly, primary cells from patients with chronic myelogenous leukemia (CML) display activated Wnt signaling and a dependence on this pathway for their growth in vitro (Jamieson et al. 2004). An investigation into the molecular basis of Wnt activation in CML patient samples identified a novel missplicing of GSK-3β RNA that deletes exons 8 and 9 (Abrahamsson et al. 2009). The resulting truncated GSK-3β protein lacks a critical axin binding domain and is therefore unable to phosphorylate β-catenin to initiate its degradation. These experiments were complemented with genetic experiments showing that the Wnt pathway was required for CML initiation and maintenance in vivo. Specifically, whether β-catenin activity was essential for initiation and maintenance of leukemia stem cells was tested by transducing β-catenin null stem cells with BCR-ABL translocation and transferring them into recipient mice (Zhao et al. 2007). Overall, the loss of β-catenin led to a significant reduction in leukemia incidence and increase in leukemia latency in vivo (Zhao et al. 2007). Interestingly, BCR-ABL-induced B-ALL still occurred, suggesting that the dependence of leukemia on β-catenin may differ with cellular context and the cell of origin for the leukemia. The possibility that Wnt is an independent regulator of CML is particularly important because current evidence indicates that although Gleevec blocks BCR-ABL in CML cancer stem cells, these cells are no longer dependent on BCR-ABL and continue to propagate the tumor. Thus, Wnt signaling could represent an effective ABL-independent target for pharmacological intervention in CML.
Following the work performed in CML, the dependence of acute myelogenous leukemia on β-catenin in vivo was also tested. Importantly, the genetic deletion of β-catenin led to reduced leukemia incidence and a blockade of leukemia stem cell self-renewal (Wang et al. 2010) in mouse models of acute myelogenous leukemia driven either with a combination of the oncogenes Hoxa9 and Meis1 or by viral delivery of the translocation MLL-AF9. Interestingly, activation of β-catenin directly imparted self-renewal capacity to otherwise nonrenewing granulocyte-macrophage progenitors when expressed in conjunction with HoxA9/Meis1a (Wang et al. 2010).
The loss of β-catenin can also affect AML driven by other oncogenes. β-catenin deletion in preleukemia stem cells showed that β-catenin is required for initiation of mixed lineage leukemia-eleven nineteen leukemia (MLL-ENL)- driven AML (Yeung et al. 2010). Viral knockdown of β-catenin in patient samples decreases colony formation and reduces the frequency of long-term initiating cells in vitro. Additionally, β-catenin activation is required for drug resistance to GSK3 inhibitors in MLL-ENL leukemia stem cells (Yeung et al. 2010). These data collectively indicate that propagation of acute myeloid leukemia requires intact activity of β-catenin for leukemia stem cell self-renewal and resistance to GSK3 inhibitors and suggests that directly targeting β-catenin could impact multiple aspects of leukemia pathogenesis. It is important to note that in leukemia driven by MLL-AF4 and MLL-AF5 translocations, GSK3 inhibition paradoxically inhibits leukemia clonogenic potential through regulation of the cell-cycle inhibitor p27kip1 (Wang et al. 2008). These data suggest that specific MLL translocations may differentially use Wnt signaling, and that the context set up by the different oncogenic alleles governs leukemia stem cell behavior in distinct ways.
Wnt Signaling in T-Cell Leukemia and Lymphoma
To study whether activation of β-catenin can lead to T-cell leukemia, transgenic mice in which activated β-catenin is driven by Lck (LckCre-CtnnbDex3) or CD4 (CD4Cre-CtnnbDex3) have been generated (Guo et al. 2007). These mice display CD4+CD8+ thymic lymphoma within 60 days. In other studies in which β-catenin was driven with the Lck promoter, tumors only arose in the absence of p53 (Xu et al. 2008). The fact that PTEN mutant fetal liver stem cells drive a combination of acute myeloid leukemia and CD4+CD8+ T-acute lymphoblastic leukemia (T-ALL) also suggests an oncogenic role for β-catenin in c-KitmidCD3+lineage− T-ALL (Guo et al. 2008). Consistent with a role for β-catenin in mediating self-renewal of the leukemia stem cell population, β-catenin heterozygous T-ALLs show premature exhaustion and lose tumor-initiating ability. Although most studies have focused on aspects of β-catenin and its role in leukemia, the noncanonical β-catenin-independent Wnt pathway may also modulate leukemia propagation and survival. A synthetic lethal screen for short hairpin RNAs (shRNAs) that cooperate with imatinib mesylate to induce apoptosis identified the Wnt/Ca+/NFAT (nuclear factor associated with T cells) signaling pathway as required for CML survival in vitro (Gregory et al. 2010) Interestingly, the requirement for NFAT signaling was also shown in B-cell acute lymphoblastic leukemia survival in vivo (Gregory et al. 2010).
SUMMARY
The activation of Wnt signaling from development to oncogenesis in the hematopoietic system is indicative of a crucial role for the pathway. In many ways, this is an exciting time, with opportunities to pursue a deeper understanding of the mechanisms Wnt signaling controls and its interactions with other key developmental signals. Importantly, this understanding should provide a strong foundation for initiating concerted efforts to identify therapeutics in the Wnt cascade that could prove broadly effective in enhancing stem-cell based therapies and targeting leukemia.
Footnotes
Editors: Roel Nusse, Xi He, and Renee van Amerongen
Additional Perspectives on Wnt Signaling available at www.cshperspectives.org
REFERENCES
- Abrahamsson AE, Geron I, Gotlib J, Dao K-HT, Barroga CF, Newton IG, Giles FJ, Durocher J, Creusot RS, Karimi M, et al. 2009. Glycogen synthase kinase 3β missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci 106: 3925–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W 1997. A role for the Wnt gene family in hematopoiesis: Expansion of multilineage progenitor cells. Blood 89: 3624–3635 [PubMed] [Google Scholar]
- Baba Y, Garrett KP, Kincade PW 2005. Constitutively active β-catenin confers multilineage differentiation potential on lymphoid and myeloid progenitors. Immunity 23: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, PW K 2006. Constitutively active β-catenin promotes expansiion for multipotent hematopoietic progenitors in culture. J Immunol 4: 2294–2303 [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA 2010. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 465: 793–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Huber TL, Chen VC, Gadue P, Keller GM 2008. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development 135: 3447–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D 2011. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474: 220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, Reya T 2008. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells 26: 1202–1210 [DOI] [PubMed] [Google Scholar]
- Corrigan PM, Dobbin E, Freeburn RW, Wheadon H 2009. Patterns of Wnt/Fzd/LRP gene expression during embryonic hematopoiesis. Stem Cells Dev 18: 759–772 [DOI] [PubMed] [Google Scholar]
- Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamazière JM, Couffinhal T, Duplàa C 2008. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 26: 2991–3001 [DOI] [PubMed] [Google Scholar]
- Durand EM, Zon LI 2010. Newly emerging roles for prostaglandin E2 regulation of hematopoiesis and hematopoietic stem cell engraftment. Curr Opin Hematol 17: 308–312 [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Ji Y, Restifo NP 2010. Wnt/β-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res 16: 4695–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moot RT, et al. 2009. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136: 1136–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M, Phang T, Neviani P, Alvarez-Calderon F, Eide C, O’Hare T, Zaberezhnyv V, Williams RT, Druker BJ, Perrotti D, et al. 2010. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell 18: 74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Dose M, Kovalovsky D, Chang R, O’Neil J, Look AT, von Boehmer H, Khazaie K, Gounari F 2007. β-Catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood 109: 5463–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Lasky JL, Chang C-J, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, et al. 2008. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 453: 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM 2009. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113: 5444–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang Y, Bersenev A, O’Brien WT, Tong W, Emerson SG, Klein PS 2009. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin Invest 12: 3519–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis V, Beermann F, Clevers H, Held W 2001. The β-catenin-TCF-1 pathway ensures CD4+CD8+ thymocyte survival. Nat Immunol 2: 691–697 [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens MS, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. 2004. Granulocyte–macrophage progenitors in chronic myelogenous leukemia are candidate leukemia stem cells in blast-crisis CML. New Engl J Med 351: 657–667 [DOI] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SEW, Nerlov C 2006. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol 7: 1048–1056 [DOI] [PubMed] [Google Scholar]
- Ko KH, Holmes T, Palladinetti P, Song E, Nordon R, O’Brien TA, Dolnikov A 2011. GSK-3β inhibition promotes engraftment of ex vivo-expanded hematopoietic stem cells and modulates gene expression. Stem Cells 1: 101–118 [DOI] [PubMed] [Google Scholar]
- Li H, Daculsi R, Grellier M, Bareille R, Bourget C, Amedee J 2010. Role of neural-cadherin in early osteoblastic differentiation of human bone marrow stromal cells cocultured with human umbilical vein endothelial cells. Am J Physiol Cell Physiol 299: C422–C430 [DOI] [PubMed] [Google Scholar]
- Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN 2003. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4: 349–360 [DOI] [PubMed] [Google Scholar]
- Liang H, Coles AH, Zhu Z, Zayas J, Jurecic R, Kang J, Jones SN 2007. Noncanonical Wnt signaling promotes apoptosis in thymocyte development. J Exp Med 204: 3077–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis TC, Weerkamp F, Naber BAE, Baert MRM, de Haas EFE, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJ, Staal FJ 2009. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 113: 546–554 [DOI] [PubMed] [Google Scholar]
- Luis TC, Naber BA, Fibbe WE, van Dongen JJ, Staal FJ . 2011. Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9: 345–356 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R 2008. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol 181: 3955–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazieres J, You L, He B, Xu Z, Lee AY, Makami I, McCormick F, Jablons DM 2005. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene 24: 5396–5400 [DOI] [PubMed] [Google Scholar]
- McWhirter JR, Neuteboom ST, Wancewicz EV, Monia BP, Downing JR, Murre C 1999. Oncogenic homeodomain transcription factor E2A-Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc Natl Acad Sci 96: 11464–11469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S, Taoudi S 2011. Embryonic origin of the adult hematopoietic system: Advances and questions. Development 138: 1017–1031 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Muller-Tidow C, Steffen B, Cauvet T, Tickenbrock L, Ji P, Diederichs S, Sargin B, Köhler G, Stelljes M, Puccetti E, et al. 2004. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol Cell Biol 24: 2890–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J 2002. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol 32: 967–971 [DOI] [PubMed] [Google Scholar]
- Mulroy T, Xu Y, Sen JM 2003. β-catenin expression enhances generation of mature thymocytes. Int Immunol 15: 1485–1494 [DOI] [PubMed] [Google Scholar]
- Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM 2007. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci 104: 15436–15441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. 2007. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447: 1007–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S 2010. Global regulator SATB1 recruits β-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R 1999. WNT targets: Repression and activation. Trends Genet 15: 1–3 [DOI] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Dooijes D, Klomp L, Winoto A, Georgopoulos K, Meijlink F, Clevers H 1991. Cloning of murine TCF-1, a T cell-specific transcription factor interacting with functional motifs in the CD3-e and TCR-a enhancers. J Exp Med 173: 1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S, Li L 2011. Cooperation between both Wnt/β-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev 25: 1928–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planutiene M, Planutis K, Holcombe RF 2011. Lymphoid enhancer-binding factor 1, a representative of vertebrate-specific Lef1/Tcf1 sub-family, is a Wnt-β-catenin pathway target gene in human endothelial cells which regulates matrix metalloproteinase-2 expression and promotes endothelial cell invasion. Vasc Cell 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Chen L, Kassem M 2011. Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem sells. Biochem Biophys Res Commun 413: 98–104 [DOI] [PubMed] [Google Scholar]
- Ranheim EA, Kwan HC, Reya T, Wang YK, Weissman IL, Francke U 2005. Frizzled 9 knock-out mice have abnormal B-cell development. Blood 105: 2487–2494 [DOI] [PubMed] [Google Scholar]
- Reya T, O’Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R 2000. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 13: 15–24 [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409–414 [DOI] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A 2006. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat Immunol 7: 1037–1047 [DOI] [PubMed] [Google Scholar]
- Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC 1998. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol 161: 3984–3991 [PubMed] [Google Scholar]
- Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG 2006. Wnt signaling in osteoblasts regulates expression of the receptor activator of NFκB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 119: 1283–1296 [DOI] [PubMed] [Google Scholar]
- Staal FJ, Clevers H 2005. WNT signalling and haematopoiesis: A WNT-WNT situation. Nat Rev Immunol 5: 21–30 [DOI] [PubMed] [Google Scholar]
- Staal FJ, Meeldijk J, Moerer P, Jay P, van de Weerdt BC, Vainio S, Nolan GP, Clevers H 2001. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol 31: 285–293 [DOI] [PubMed] [Google Scholar]
- Steele BM, Harper MT, Macaulay IC, Morrell CN, Perez-Tamayo A, Foy M, Habas R, Poole AW, Fitzgerald DJ, Maguire PB 2009. Canonical Wnt signaling negatively regulates platelet function. Proc Natl Acad Sci 106: 1936–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ 2003. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol 162: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Sekkali B, Van Imschoot G, Janssens S, Vleminckx K 2010. Wnt/β-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc Natl Acad Sci 107: 16160–16165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A, Amsterdam A, Belanger C, Grosschedl R 1991. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain regulates T-cell receptor a enhancer function. Genes Dev 5: 880–894 [DOI] [PubMed] [Google Scholar]
- Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M 2006. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med 1: 89–98 [DOI] [PubMed] [Google Scholar]
- Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R 1998. Role of members of the Wnt gene family in human hematopoiesis. Blood 92: 3189–3202 [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Fariñas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R 1994. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1 deficient mice. Genes Dev 8: 2691–2703 [DOI] [PubMed] [Google Scholar]
- Vijayaragavan K, Szabo E, Bosse M, Menendez P, Weisel K, Bhatia M . 2009. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell 4: 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Ansorge A, Wirkner U, Eckstein V, Schwager C, Blake J, Miesala K, Selig J, Saffrich R, Ansorge W, et al. 2004. Molecular evidence for stem cell function of the slow dividing fraction among human hematopoietic progenitor cells by genome wide analysis. Blood 104: 675–686 [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TCP, Cleary ML 2008. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature 455: 1205–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA 2010. The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science 327: 1650–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR, van Dongen JJ, Herzenberg LA, Staal FJ 2006. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci 103: 3322–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen J 2003. Deletion of β-catenin impairs T cell development. Nat Immunol 4: 1177–1182 [DOI] [PubMed] [Google Scholar]
- Xu M, Yu Q, Subrahmanyam R, Difilippantonio MJ, Ried T, Sen JM 2008. β-Catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo. Mol Cell Biol 28: 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, So CW 2010. β-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 18: 606–618 [DOI] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, et al. 2009. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-γ. Nat Immunol 9: 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T 2007. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 12: 528–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Beissert T, Kukoc-Zivojnov N, Puccetti E, Altschmied J, Strolz C, Boehrer S, Gul H, Schneider O, Ottmann OG, et al. 2004. γ-Catenin contributes to leukemogenesis induced by AML-associated translocation products by increasing the self-renewal of very primitive progenitor cells. Blood 103: 3535–3543 [DOI] [PubMed] [Google Scholar]