Abstract
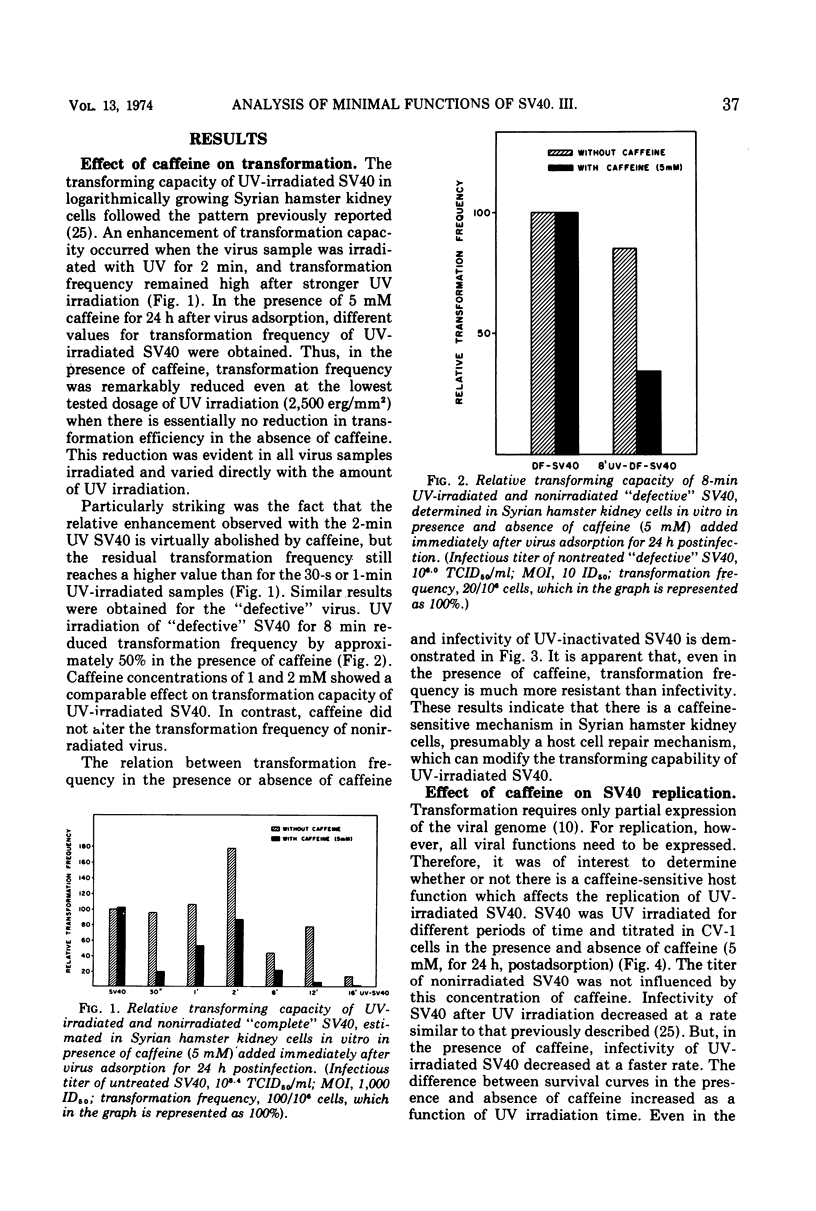
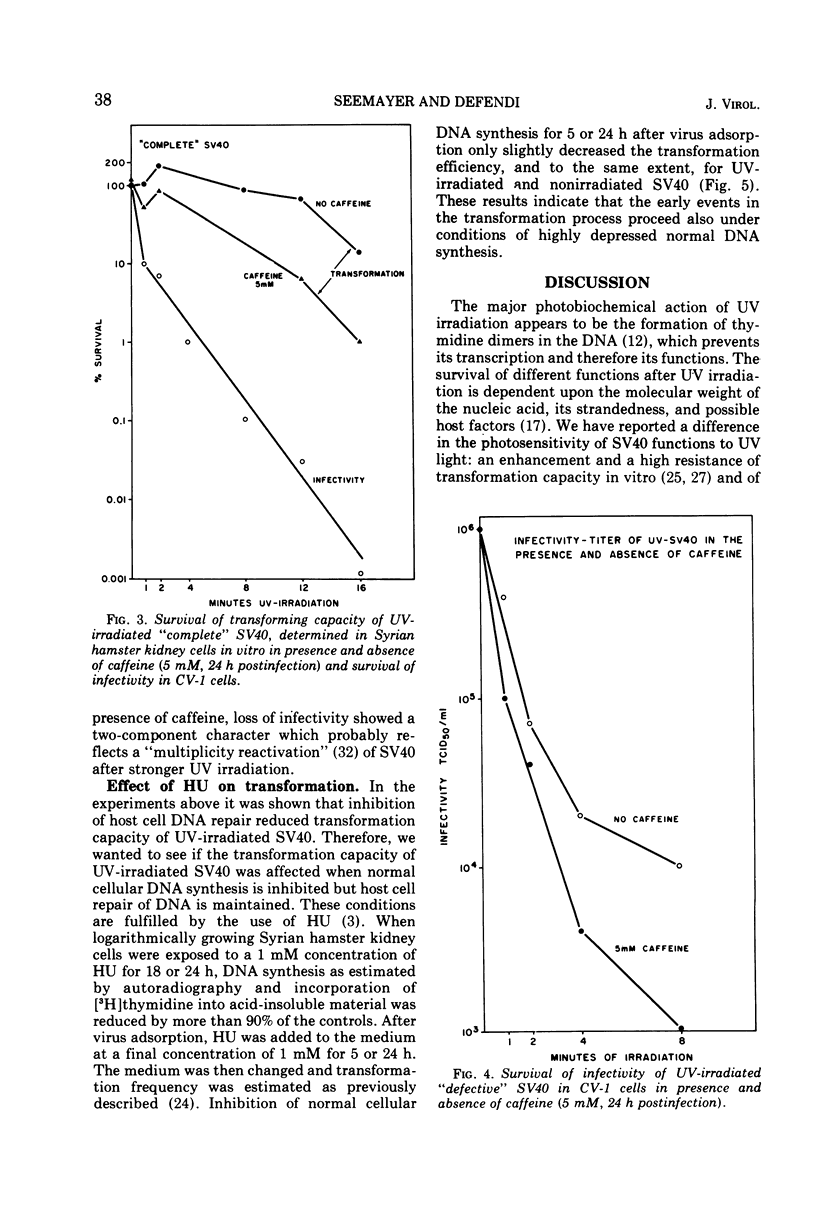
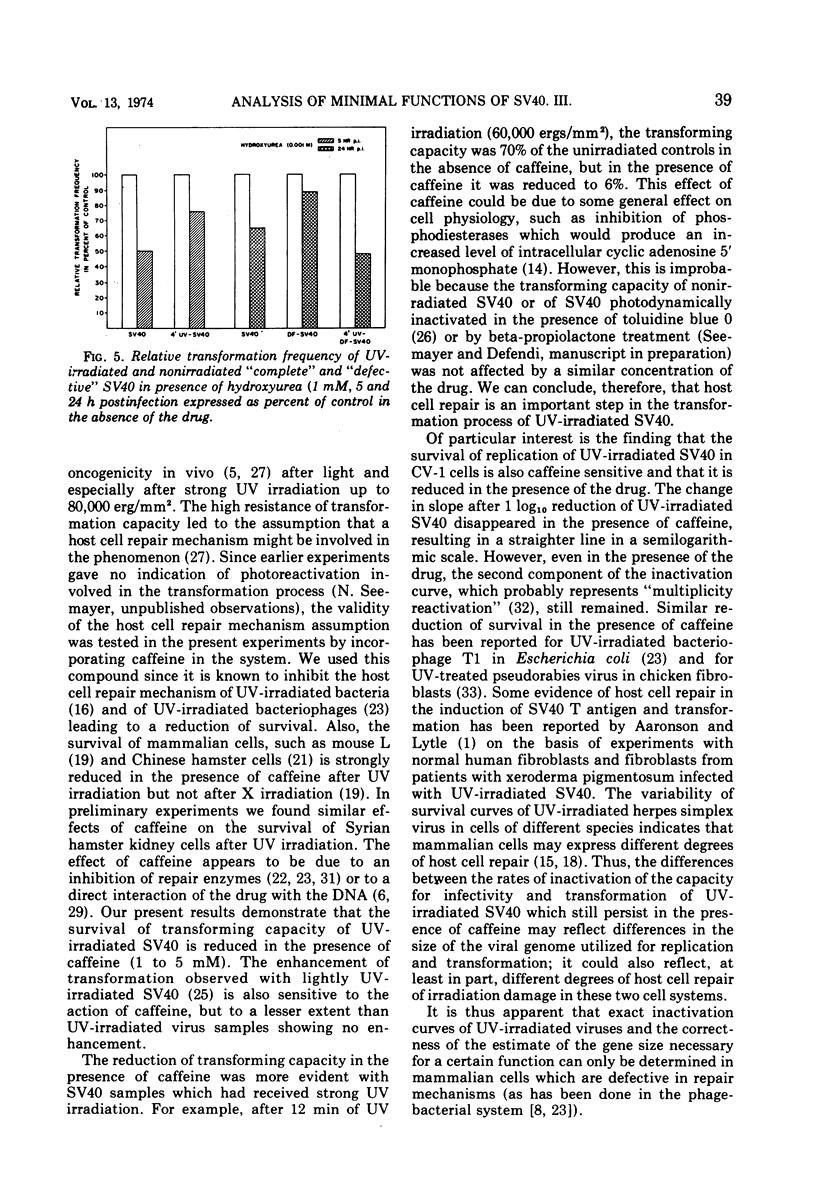
The in vitro transforming capacity of simian virus 40 (SV40) for Syrian hamster cells is highly resistant to inactivation by UV light in comparison to infectivity. In the same cell system, we demonstrated a “host cell repair mechanism” sensitive to caffeine which is, to a large extent, responsible for the high resistance to UV inactivation of the transforming capacity of SV40. The survival of infectivity of UV-irradiated SV40 in CV-1 cells was also sensitive to caffeine, again indicating host cell repair. On the other hand, depression of normal cell DNA synthesis by hydroxyurea during the first 24 h postinfection only modestly reduced, and to a similar extent, the transforming capacity of UV-irradiated and nonirradiated SV40.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Lytle C. D. Decreased host cell reactivation of irradiated SV40 virus in xeroderma pigmentosum. Nature. 1970 Oct 24;228(5269):359–361. doi: 10.1038/228359a0. [DOI] [PubMed] [Google Scholar]
- Brandt W. N., Flamm W. G., Bernheim N. J. The value of hydroxyurea in assessing repair synthesis of DNA in HeLa cells. Chem Biol Interact. 1972 Oct;5(5):327–339. doi: 10.1016/0009-2797(72)90072-5. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication of mammalian cell DNA: effects of compounds that inhibit DNA synthesis or dark repair. Radiat Res. 1969 Feb;37(2):334–348. [PubMed] [Google Scholar]
- Collins C. J., Sauer G. Fate of infecting simian virus 40-deoxyribonucleic acid in nonpermissive cells: integration into host deoxyribonucleic acid. J Virol. 1972 Sep;10(3):425–432. doi: 10.1128/jvi.10.3.425-432.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon M., Barton B., Porte A., Rauth A. M. The interaction of caffeine with ultra-violet-light-irradiated DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(4):395–399. doi: 10.1080/09553007014550481. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kondo T. Caffeine-sensitive repair of ultraviolet light-damaged DNA of mouse L cells. Biochem Biophys Res Commun. 1972 May 12;47(3):557–564. doi: 10.1016/0006-291x(72)90915-1. [DOI] [PubMed] [Google Scholar]
- GAREN A., ZINDER N. D. Radiological evidence for partial genetic homology between bacteriophage and host bacteria. Virology. 1955 Nov;1(4):347–376. doi: 10.1016/0042-6822(55)90030-1. [DOI] [PubMed] [Google Scholar]
- Girardi A. J. Prevention of SV40 virus oncogenesis in hamsters. I. Tumor resistance induced by human cells transformed by SV40. Proc Natl Acad Sci U S A. 1965 Aug;54(2):445–451. doi: 10.1073/pnas.54.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hirai K., Lehman J., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of primary infected Chinese hamster cells. J Virol. 1971 Nov;8(5):708–715. doi: 10.1128/jvi.8.5.708-715.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. D. Host-cell reactivation in mammalian cells. I. Survival of ultra-violet-irradiated herpes virus in different cell-lines. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(4):329–337. doi: 10.1080/09553007114550451. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Cook J. S., Tennant R. W. Ultraviolet photobiology of Kilham rat virus and the absolute ultraviolet photosensitivities of other animal viruses: influence of DNA strandedness, molecular weight, and host-cell repair. Virology. 1972 Aug;49(2):368–378. doi: 10.1016/0042-6822(72)90489-8. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., Tyrrell S. A., Legallais F. Y. Growth of ultraviolet-damaged herpesvirus in xeroderma pigemntosum. Proc Soc Exp Biol Med. 1969 Nov;132(2):802–806. [PubMed] [Google Scholar]
- Rauth A. M. Evidence for dark-reactivation of ultraviolet light damage in mouse L cells. Radiat Res. 1967 May;31(1):121–138. [PubMed] [Google Scholar]
- Rommelaere J., Errera M. The effect of caffeine on the survival of U.V.-irradiated diploid and tetraploid Chinese-hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Sep;22(3):285–291. doi: 10.1080/09553007214551061. [DOI] [PubMed] [Google Scholar]
- Roulland-Dussoix D. Dégradation par la cellule hôte du DNA du bacteriophage lambda irradié par le rayonnement ultraviolet. Mutat Res. 1967 May-Jun;4(3):241–252. doi: 10.1016/0027-5107(67)90019-x. [DOI] [PubMed] [Google Scholar]
- Sauerbier W. Inhibition of host cell reactivation in phage T1 by caffeine. Biochem Biophys Res Commun. 1964;14:340–346. doi: 10.1016/s0006-291x(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Seemayer N. H., Hirai K., Defendi V. Analysis of minimal functions of simian virus 40. I. Oncogenic transformation of Syrian hamster kidney cells in vitro by photodynamically inactivated SV40. Int J Cancer. 1973 Sep 15;12(2):524–531. doi: 10.1002/ijc.2910120224. [DOI] [PubMed] [Google Scholar]
- Seemayer N., Seemayer G., Hass R. Untersuchungen über die onkogene Transformation, Induktion der DNS-Synthese und T-Antigenbildung durch UV-bestrahltes Simian Virus (SV-) 40. Z Med Mikrobiol Immunol. 1969;155(2):123–132. doi: 10.1007/BF02123856. [DOI] [PubMed] [Google Scholar]
- Sinclair W. K. Hydroxyurea: differential lethal effects on cultured mammalian cells during the cell cycle. Science. 1965 Dec 24;150(3704):1729–1731. doi: 10.1126/science.150.3704.1729. [DOI] [PubMed] [Google Scholar]
- TSO P. O., LU P. INTERACTION OF NUCLEIC ACIDS, I. PHYSICAL BINDING OF THYMINE, ADENINE, STEROIDS, AND AROMATIC HYDROCARBONS TO NUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1964 Jan;51:17–24. doi: 10.1073/pnas.51.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]


