Abstract
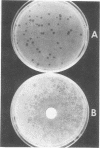
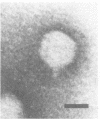
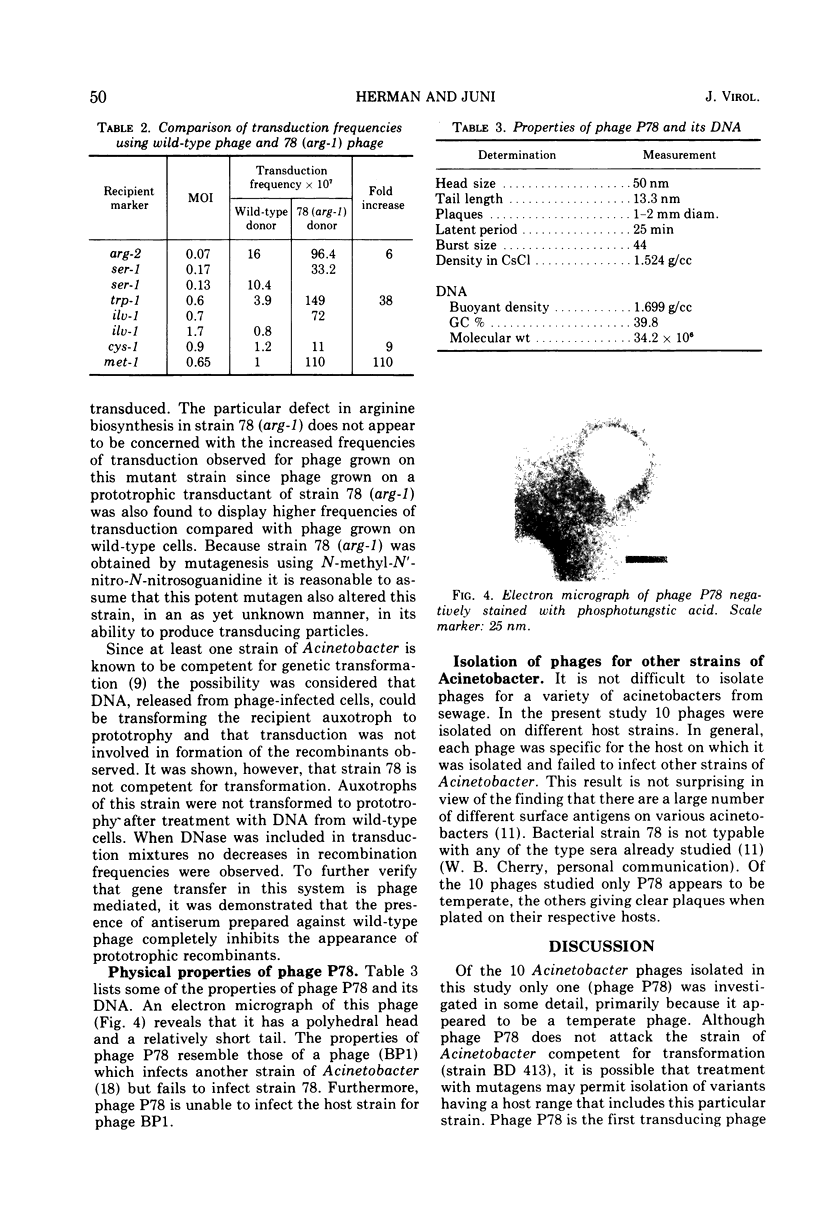
A series of bacteriophages which grow in various strains of Acinetobacter have been isolated. One of these, phage P78, which forms turbid plaques on Acinetobacter strain 78 is specific for this particular host and fails to attack 389 other independently isolated strains of Acinetobacter. Phage P78 appears to be a temperate phage which lysogenizes its host. Various agents such as N-methyl-N′-nitro-N-nitrosoguanidine, diethyl sulfate, mitomycin C, and ultraviolet light are effective inducers of the lysogen. Phage lysates of wild-type cells are capable of transducing auxotrophs of strain 78 to prototrophy at frequencies ranging from 0.3 × 10−7 to 34 × 10−7 per plaque-forming unit adsorbed. To date, no linkage has been detected between any of the markers studied in two-factor crosses. Donor phage grown in one particular mutant, strain 78 (arg-1), has been shown to give rise to significantly higher transduction frequencies than when phage is grown on wild-type or other auxotrophic strains. Phage P78 is rapidly adsorbed to its bacterial host and has a latent period of 25 min, and infection results in a burst size of approximately 50. Some of the physical properties of phage P78 and its DNA are described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus B. B., Samuels S. B., Pittman B., Cherry W. B. A serologic study of Herellea vaginicola and its identification by immunofluorescent staining. Am J Clin Pathol. 1969 Sep;52(3):309–319. doi: 10.1093/ajcp/52.3.309. [DOI] [PubMed] [Google Scholar]
- Mayer V. W., Gabridge M. G., Oswald E. J. Rapid plate test for evaluating phage induction capacity. Appl Microbiol. 1969 Oct;18(4):697–698. doi: 10.1128/am.18.4.697-698.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Mapping of the tryptophan genes of Acinetobacter calcoaceticus by transformation. J Bacteriol. 1972 Nov;112(2):797–805. doi: 10.1128/jb.112.2.797-805.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961 May;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog R., Blouse L. E. Isolation and characterization of transducing bacteriophage BP1 for Bacterium anitratum (Achromobacter sp.). J Virol. 1968 Jul;2(7):716–722. doi: 10.1128/jvi.2.7.716-722.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Thorne C. B. Comparison of Bacillus cereus bacteriophages CP-51 and CP-53. J Virol. 1971 Aug;8(2):242–253. doi: 10.1128/jvi.8.2.242-253.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]