Abstract
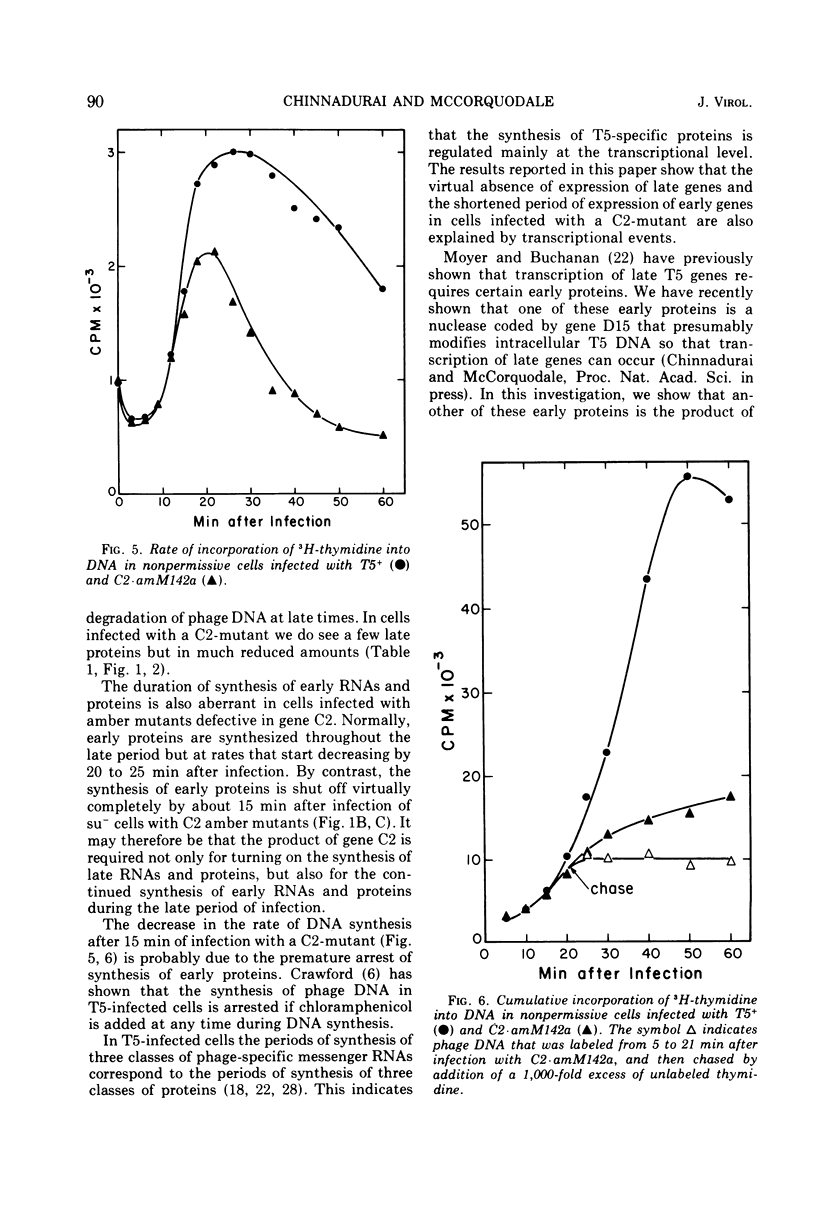
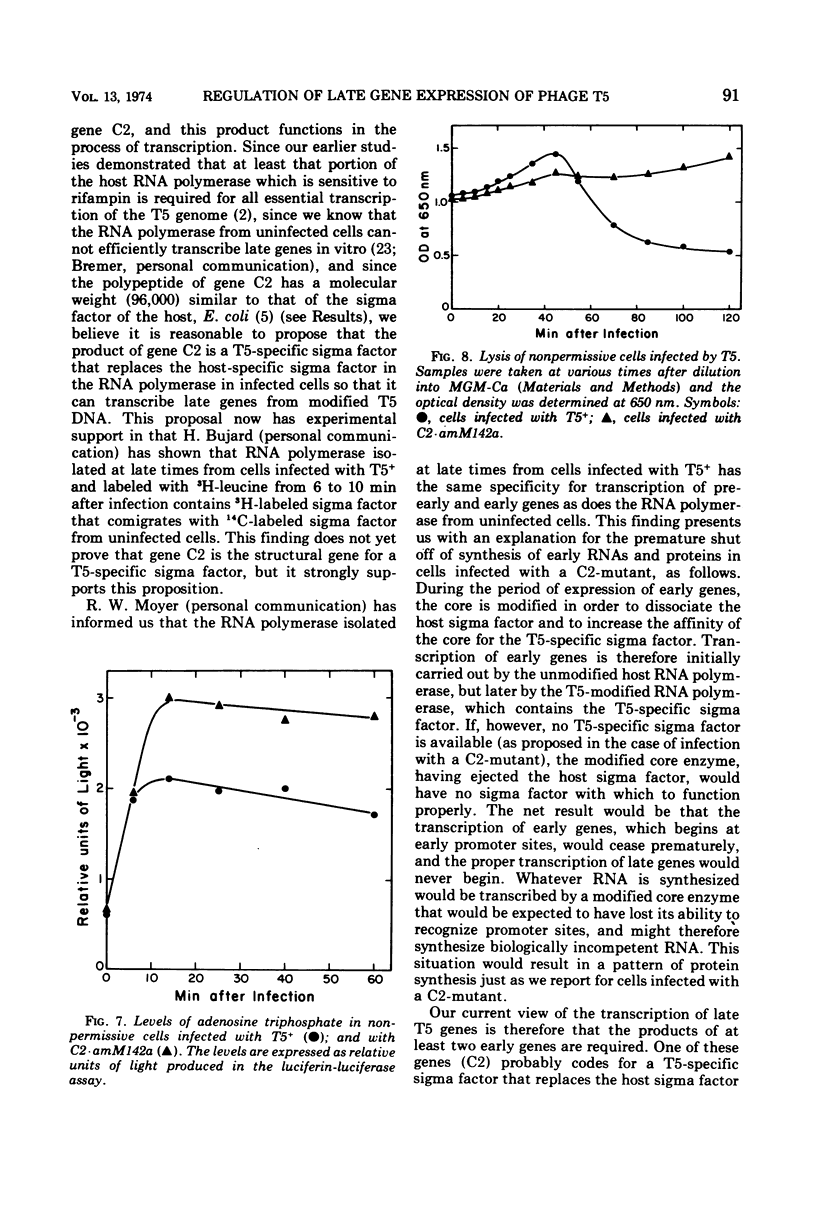
Amber mutants of bacteriophage T5 defective in gene C2 have been characterized. The product of this gene is required for the normal turn-on of synthesis of late RNA's and proteins, and, apparently, for the normal continued synthesis of early RNA's and proteins during late stages of infection. The inability of nonpermissive cells to synthesize any proteins, either late or early, during the late period after infection with a C2-mutant is not due to premature lysis of the infected cells, to a depletion of the cellular energy supply, or to degradation of phage DNA at late times. A possible role for the product of gene C2 in early and late transcription of the viral genome is suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman L. D., Hoffman M. S., McCorquodale D. J. Pre-early proteins of bacteriophage T5: structure and function. J Mol Biol. 1971 Dec 28;62(3):551–564. doi: 10.1016/0022-2836(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Beckman L. D., Witonsky P., McCorguodale D. J. Effect of rifampin on the growth of bacteriophage T5. J Virol. 1972 Aug;10(2):179–186. doi: 10.1128/jvi.10.2.179-186.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Bruner R., Cape R. E. The expression of two classes of late genes of bacteriophage T4. J Mol Biol. 1970 Oct 14;53(1):69–89. doi: 10.1016/0022-2836(70)90046-x. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. Nucleic acid metabolism in Escherichia coli infected with phage T5. Virology. 1959 Apr;7(4):359–374. doi: 10.1016/0042-6822(59)90065-0. [DOI] [PubMed] [Google Scholar]
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- FESSLER L. I., KELEMEN M. V., BURTON K. Synthesis of protein in a purine-requiring Escherichia coli infected with bacteriophage T2. Biochem J. 1960 Dec;77:558–563. doi: 10.1042/bj0770558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. E., McCorquodale D. J. Genetic and physiological studies of bacteriophage T5. 2. The relationship between phage DNA synthesis and protein synthesis in T5-infected cells. Biochem Biophys Res Commun. 1971 May 21;43(4):735–740. doi: 10.1016/0006-291x(71)90677-2. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. E., McCorquodale D. J. Genetic and physiological studies of bacteriophage t5 I. An expanded genetic map of t5. J Virol. 1971 May;7(5):612–618. doi: 10.1128/jvi.7.5.612-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. II. Dissociation of calcium-independent and calcium-dependent processes. Virology. 1960 Apr;10:514–529. doi: 10.1016/0042-6822(60)90133-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landy A., Spiegelman S. Exhaustive hybridization and its application to an analysis of the ribonucleic acid synthesized in T4-infected cells. Biochemistry. 1968 Feb;7(2):585–591. doi: 10.1021/bi00842a011. [DOI] [PubMed] [Google Scholar]
- MCCORQUODALE D. J., LANNI Y. T. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. I. CHARACTERIZATION OF FIRST-STEP-TRANSFER MATERIAL. J Mol Biol. 1964 Oct;10:10–18. doi: 10.1016/s0022-2836(64)80023-1. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- McCorquodale D. J., Lanni Y. T. Patterns of protein synthesis in Escherichia coli infected by amber mutants in the first-step-transfer DNA of T5. J Mol Biol. 1970 Feb 28;48(1):133–143. doi: 10.1016/0022-2836(70)90224-x. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Buchanan J. M. Patterns of RNA synthesis in T5-infected cells. I. As studied by the technique of DNA-RNA hybridization-competition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1249–1256. doi: 10.1073/pnas.64.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J. P., Buchanan J. M. Synthesis of bacteriophage T5 specific RNA in vitro. Biochim Biophys Acta. 1971 Sep 30;247(1):181–184. doi: 10.1016/0005-2787(71)90823-9. [DOI] [PubMed] [Google Scholar]
- Ramírez J., Smith L. Synthesis of adenosine triphosphate in intact cells of Rhodospirillum rubrum and Rhodopseudomonas spheroides on oxygenation or illumination. Biochim Biophys Acta. 1968 Feb 12;153(2):466–475. doi: 10.1016/0005-2728(68)90088-1. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A., Buchanan J. M. Patterns of ribonucleic acid synthesis in T5-infected Escherichia coli. 3. Separation of low molecular weight ribonucleic acid species by disc electrophoresis on acrylamide gel columns. J Biol Chem. 1970 May 25;245(10):2693–2703. [PubMed] [Google Scholar]
- Sirbasku D. A., Buchanan J. M. Patterns of ribonucleic acid synthesis in T5-infected Escherichia coli. II. Separation of high molecular weight ribonucleic acid species by disc electrophoresis on acrylamide gel columns. J Biol Chem. 1970 May 25;245(10):2679–2692. [PubMed] [Google Scholar]
- Steuart C. D., Anand S. R., Bessman M. J. Studies on the synthesis of deoxyribonucleic acid. I. Further purification and properties of the deoxyribonucleic acid polymerase induced by infection of Escherichia coli with bacteriophage T5. J Biol Chem. 1968 Oct 25;243(20):5308–5318. [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Structural proteins of bacteriophage T5. Virology. 1973 Feb;51(2):443–453. doi: 10.1016/0042-6822(73)90443-1. [DOI] [PubMed] [Google Scholar]