Abstract
We have previously demonstrated that the reaction of a physiological dicarbonyl, methylglyoxal (MGO) enhances the chaperone function of human αA-crystallin. MGO can react with cysteine, arginine, and lysine residues in proteins. Although the role of arginine and lysine residues in the enhancement of chaperone function has been investigated, the role of cysteine residues is yet to be determined. In this study, we have investigated the effect of MGO modification on the structure and chaperone function of αA-crystallin mutant proteins in which C131 and C142 were replaced either individually or simultaneously with isoleucine. MGO-modification resulted in improved chaperone function in all three αA-crystallin mutants, including the cysteine-free double mutant. The enhanced chaperone function was due to increased surface hydrophobicity and increased binding of client proteins. These results suggest that the two cysteine residues, even though they could be modified, do not take part in the MGO-induced improvement in the chaperone function of human αA-crystallin.
Keywords: Crystallin, Chaperone, Methylglyoxal, Hemithioacetal, Cysteine, Site-directed mutagenesis
Introduction
Alpha-crystallin is a major protein in the ocular lens. It consists of two proteins, αA- and αB-crystallin, that share > 50% of sequence homology. It is now well established that both αA- and αB-crystllins function as molecular chaperones [1]. They prevent the aggregation of denatured proteins. This function appears to be important for the lens, as αA- and αB-crystallin double knockout animals exhibit developmental defects in the lens, and αA-crystallin knockout animals develop dense nuclear cataracts [2, 3]. In addition, overexpression of mutant αA-crystallin proteins that have lost the chaperone function leads to cataracts in mice [4, 5]. These observations imply that α-crystallin’s chaperone function is important for the transparency of the lens.
Lens proteins, including α-crystallin, are possibly the most long-lived proteins in the human body. Therefore, they accumulate post-translational modifications over many decades during aging. Among many types of post-translational modifications, chemical modification by methylglyoxal (MGO) has attracted considerable attention recently. MGO is produced mainly by non-enzymatic reactions from the triose phosphate intermediates of glycolysis. Its concentration in the lens is estimated to be ~ 20-times higher than that in plasma [6].
Methylglyoxal is a highly reactive molecule, and it reacts with cysteine, arginine, and lysine residues in proteins. The reaction with arginine and lysine produces stable adducts. Nε-carboxyethyllysine (CEL) and methylglyoxal lysine dimer (MOLD) are lysine-derived products, hydroimidazolones (MGH1, MGH2, and MGH3) and argpyrimidine are arginine-derived molecules, and methylglyoxal-derived crosslink structure (MODIC) is a lysine-arginine crosslinking structure, all of which are present in the human lens [7–10]. Unlike the reactions with arginine and lysine, the reaction with cysteine produces reversible hemithioacetal product [11].
Recently, we observed that the reaction of MGO with αA-crystallin enhances its chaperone activity [12]. We found that several arginine residues are converted to argpyrimidine in the MGO-modified protein. Replacement of argpyrimidine modifiable arginine residues (R21 and R103) with alanine by site-directed mutagenesis replicated the effects of MGO-modification, suggesting that neutralization of positive charge on those specific arginine residues was the cause for improvement in the chaperone function [13]. Further studies showed that conversion of lysine to ‘arginine-like product’ followed by reaction with MGO also makes αA-crystallin a better chaperone [14]. Although we found evidence for the participation of arginine residues in MGO-mediated enhancement in the chaperone function, we found no evidence for participation of lysine residues in such enhancement [12].
Human αA-crystallin contains two cysteine residues, one at position 131 and the other at position 142. Both could potentially react with MGO to form hemithioacetal adducts. Thus, the enhancement of chaperone function by MGO could be due to the modification of cysteine residues, in addition to arginine residues, in αA-crystallin. To explore this possibility, we have generated recombinant mutant proteins in which the cysteine residues are replaced with isoleucine and studied the effect of MGO modification on their structure and chaperone function.
Materials and methods
Materials
Dithiothreitol (DTT), bovine insulin, carbonic anhydrase (CA), citrate synthase (CS), and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) were obtained from Sigma Chemical Co., St. Louis, MO, USA. CS was dialyzed against 40 mM HEPES buffer, pH 7.4, for 24 h before use. 2-(p-toluidinyl) naphthalene-6-sulfonate (TNS) was obtained from Molecular Probes (Invitrogen, Carlsbad, CA, USA). All other chemicals were of analytical grade. MGO (Sigma) was purified as previously described [12].
Cloning and purification of wild type and cysteine mutants of human αA-crystallin
The wild type αA-crystallin inserted into pET-23d (+) vector (cloning as described in [13]) was used as template for PCR. Primers used for the substitution of cysteines at positions 131 and 142 with isoleucine and site-directed mutagenesis were as described by Chen et al [15]. Plasmids were sequenced to confirm the presence of mutations. The proteins were overexpressed in E. coli strain BL21(DE3) as described previously [12]. The recombinant proteins were purified from the supernatant by the following successive steps: fractionation with 30–60% saturated ammonium sulfate, gel filtration on Sephacryl S-300, and ion exchange chromatography on DEAE-Sepharose [15]. The purity of the recombinant αA-crystallins was confirmed by SDS-PAGE and Western blotting using polyclonal rabbit anti-αA-crystallin antibody (Assay Designs, Ann Arbor, MI, USA) (Fig. 1). The purified proteins were stored at −20°C in 0.05 M phosphate buffer until use.
Fig. 1.

Western blotting of purified recombinant human αA-crystallin. Proteins were electrophoresed on a 12% SDS-polyacrylamide gel and stained with Bio-Rad Bio-Safe Coomassie Stain (A) or electrophoretically transferred to a nitrocellulose membrane for Western blotting (B). The membrane was blocked with 5% non-fat dry milk/phosphate buffered saline-Tween (NFDM/PBST) overnight at 4°C and then incubated for 1 h at 4°C with 10 ml of diluted αA-crystallin-pAb in 5% NFDM/PBS. After washing five times (5 min each) with PBS-T, the membrane was incubated for 1 h at room temperature with diluted goat anti-rabbit IgG conjugated to horseradish peroxidase. Following five washes (5 min each) with PBS-T, the membrane was treated with SuperSignal West Pico chemiluminescence substrate (Pierce) for 5 min and exposed to X-ray film (Pierce). Lane 1 molecular weight markers; lane 2 Wt; lane3 C131I; lane 4 C142I, and lane 5 C131I/C142I
MGO modification
AlphaA-crystallin (5 mg/ml in 0.1M sodium phosphate buffer, pH 7.4) was incubated with or without 500 μM MGO for 2 days at 37°C under sterile conditions. Incubated proteins (without dialysis) were used in all experiments.
CD-spectroscopy
Secondary and tertiary structure of αA-crystallin were examined by recording far- and near-UV CD and at 25°C in a Jasco 810 spectropolarimeter (Jasco, Inc.) flushed with dry nitrogen as described previously [13]. Briefly, far-UV CD spectra of 0.2 mg/ml protein in 25 mM phosphate buffer pH 7.4 were collected from 250 to 195 nm in a CD quartz cell of 1 mm path length using a slit width of 1 nm and a scan speed of 30 nm/min. Spectra obtained from the average of three scans were analyzed for secondary structure by the curve-fitting program K2D software. Near-UV CD spectra of 1 mg/ml protein in 25 mM phosphate buffer pH 7.4 were collected from 300 to 250 nm using a CD cell of 10 mm path length.
Chaperone assays
The chaperone function was determined using two client proteins, insulin and citrate synthase (CS), as previously described [13].
Chemical modification of cysteine residues
The cysteine residues were modified by incubating 5 mg/ml αA-crystallin with N-ethylmalemide or iodoacetamide (each 2.0 mM) at room temperature for 2 h in 50 mM sodium phosphate buffer pH 7.4 followed by dialysis against 50 mM phosphate buffer pH 7.4 for 24 h.
Determination of sulphydryl content
The protein thiol content was assessed by the method previously described using DTNB [16]. Samples (500 μg protein in 100 μl 25 mM phosphate buffer) were mixed with 360 μl of 1 mM DTNB. The absorbance readings were made at 412 nm. The sulfhydryl content was determined using a molar extinction of 13,600 M−1 cm−1.
Surface hydrophobicity of proteins
The surface hydrophobicity of αA-crystallin was determined using TNS, a specific hydrophobic probe, as previously described [13].
Carbonic anhydrase binding assay
Wild-type or mutant αA-crystallin (12 μM) was incubated at 60°C for 1 h with 5–18 μM CA in 50 mM phosphate buffer containing 100 mM NaCl (pH 7.2). No protein aggregation was observed under these conditions. The solutions were cooled to 25°C for 1 h. Unbound CA was then separated by centrifugation at 4,000g through a 100 kDa cutoff membrane filter. The amount of remaining CA associated with αA-crystallin was calculated by subtracting free CA from the total CA concentration. Protein concentrations were determined by the Bradford assay using BSA as the standard. The number of binding sites (n) and dissociation constant (Kd) were determined as described previously [13].
Results
The Wt and mutant proteins were judged to be pure on the basis of SDS-PAGE and Western blotting, where proteins showed single band of ~20 kDa and strong reaction with αA-crystallin antibody (Fig. 1). As previously suggested [15], the choice of isoleucine to replace cysteine was based on the similar hydrophobicity of cysteine deficient mutant (C131I/C142I) with the Wt protein. Data on secondary structure of protein are shown in Table 1, which showed no significant change in the secondary structure in any of the mutant proteins compared to the Wt protein. MGO modification did not appear to have any significant effect on the secondary structure, either.
Table 1.
Secondary structural elements in Wt and mutant αA-crystallin
| α-Helix (%) | β-Sheet (%) | Random (%) | |
|---|---|---|---|
| Wt | 6 | 46 | 48 |
| Wt+MGO | 5 | 47 | 48 |
| C131I | 5 | 47 | 48 |
| C131I+MGO | 7 | 43 | 50 |
| C142I | 7 | 43 | 50 |
| C142I+MGO | 8 | 42 | 50 |
| C131I/C142I | 8 | 42 | 50 |
| C131I/C142I+MGO | 7 | 43 | 50 |
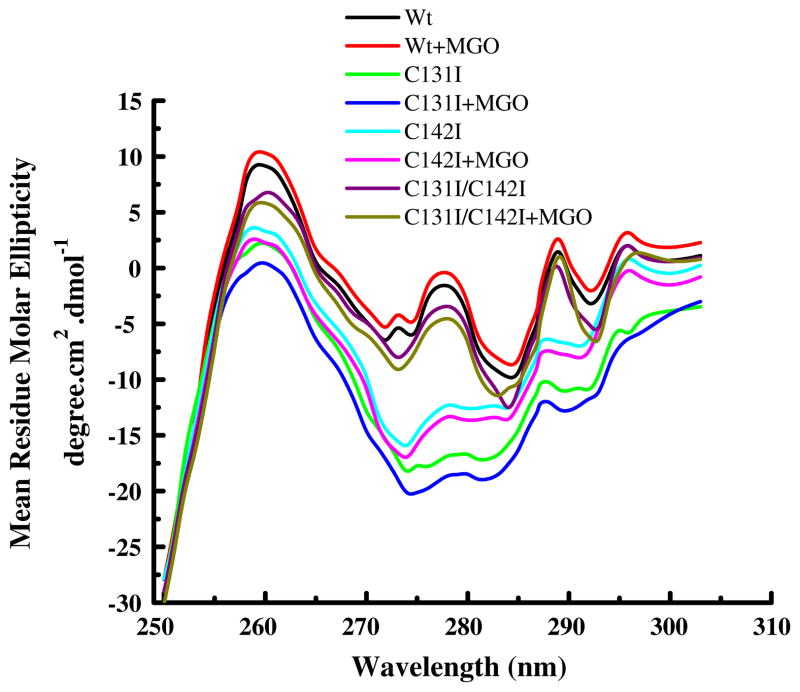
The near-UV CD spectra of the mutant and Wt protein are shown in Fig. 2. The data suggest that the mutant proteins have altered tertiary structure. The spectra between 270–280 nm and 290–320 nm, which report predominantly on the tyrosine and tryptophan microenvironment, respectively, were altered in the mutant proteins. The lower intensity could be due to tyrosine residues being more buried in mutant proteins than in the Wt protein. MGO modification appeared to have further changed, although marginally, the microenvironment of tryptophan residues.
Fig. 2.
Near-UV circular dichroic spectra of human αA-crystallin. Proteins (1.0 mg/ml) were in 25 mM phosphate buffer (pH 7.4) at 25°C. Spectra were measured in a 10 mm path length CD quartz cell
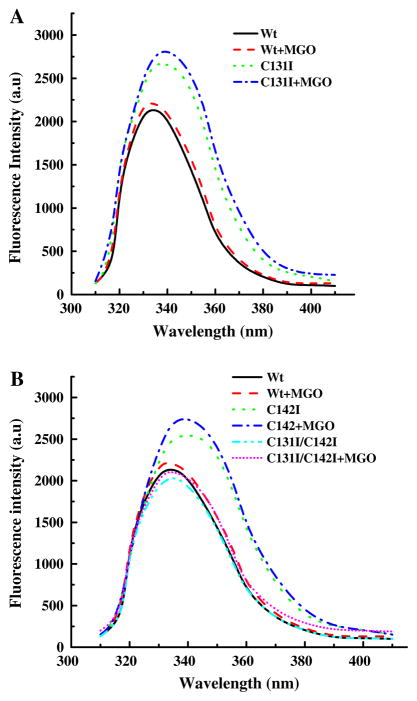
Intrinsic tryptophan fluorescence spectra of the Wt and mutant proteins (Fig. 3) support the near-UV CD data. An increase in fluorescence intensity with ~4 nm red shift in the emission maxima was observed in mutant proteins. The fluorescence intensities of the C131I and C142I proteins increased by 22% and 18%, respectively, when compared to the Wt protein. In the double mutant, however, the fluorescence intensity was decreased relative to the Wt protein. Upon MGO modification, the fluorescence intensity increased by ~5% in all proteins. These data suggest that the microenvironment of tryptophan was altered in mutant proteins and was further altered by MGO modification.
Fig. 3.
Intrinsic fluorescence of human αA-crystallin. The fluorescence spectrum of unmodified and MGO-modified samples (0.1 mg/ml protein) was recorded from 310 to 450 nm at 27°C. The excitation wavelength was 295 nm. Data were collected at 0.5 nm wavelength resolution. A Wt and C131I, B Wt, C142I and C131I/C142I
To examine the role of cysteine residues in the chaperone function of αA-crystallin, we first chemically modified the cysteine residues in the Wt protein and assessed the chaperone function using CS and insulin as client proteins. Upon chemical modification with either N-ethylmalemide or iodoacetamide, the chaperone function was almost completely abolished (data not shown), suggesting that the thiol groups on the two cysteine residues are required for the chaperone function. We then determined whether the reaction with MGO modified cysteine residues, by estimating the thiol content in the unmodified and the MGO-modified αA-crystallin. The thiol content in unmodified protein was 2.0 moles/mole, which is in agreement with two cysteine residues/molecule. However, it was reduced to zero upon modification with MGO, indicating that MGO reacted with cysteine residues and possibly formed hemithioacetal adducts.
We then assessed the effect of mutation and modification by MGO on the chaperone function using two client proteins, insulin and CS. In the DTT-induced insulin aggregation assay, we observed nearly 30% protection by Wt protein. The mutant proteins were less effective, displaying 18% in C131I and 25% in C142I. Interestingly, the double mutant protein showed better protection (~27%) than the two single mutant proteins. The relative chaperone activity of the constructs followed the order: Wt >C131I/C142I >C131I >C142I. Upon MGO modification, protection against insulin aggregation increased between 60% and 135% in all mutant and Wt proteins (Fig. 4A). The highest increase was seen with C131I protein. With CS as the client protein, we observed a similar trend; the mutant proteins were weaker chaperones than the Wt protein. The protection against CS aggregation was 22%, 38%, and 40% in the C131I, C142I, and C131I/C142I, respectively, when compared to 45% protection seen with the Wt protein (Fig. 4B). MGO modification enhanced the chaperone function in all proteins, although not as dramatically as in the insulin aggregation assay. The chaperone function increased by 21% (in C131I/C142I) and 133% (in C131I), and ranked as follows: C131I >Wt >C142I >C142I/C131I. These data demonstrate that replacement of cysteine residues with isoleucine in αA-crystallin results in a loss of chaperone function, but subsequent modification by MGO results in a gain of chaperone function.
Fig. 4.
Chaperone function of human αA-crystallin. Panels A and B show the percent protection ability of αA-crystallin against insulin and CS aggregation, respectively. The chaperone:client protein ratio (w/w) was 1:5 and 1:15 for insulin and CS aggregation assays, respectively. n = 3 in each bar
Figure 5 shows fluorescence spectra of protein-bound TNS in the Wt and mutant proteins. Upon binding to hydrophobic regions in αA-crystallin, TNS displays intense fluorescence with an emission maximum at 430 nm. This emission maximum did not change in mutant proteins when compared to the Wt protein. We found that C131I and C142I have 38% and 12% more surface hydrophobicity, respectively, when compared to the Wt protein (Fig. 5A), the C131I/C142I mutant had only 10% lower surface hydrophobicity relative to the Wt protein (Fig. 5B). Modification by MGO increased the surface hydrophobicity in all proteins; the increase was 6–10%. The highest increase was seen with C131I mutant protein.
Fig. 5.
Surface hydrophobicity of αA-crystallin. The protein concentration was 0.1 mg/ml and the TNS concentration was 100 μM. The fluorescence spectrum of samples at 27°C was recorded from 350 to 520 nm. The excitation wavelength was 320 nm. A Wt and C131I, B Wt, C142I and C131I/C142I
We measured binding parameters using CA as the client protein. This was done to determine whether client protein binding parameters were altered in MGO-modified αA-crystallin. First, we determined the chaperone function of Wt and mutant proteins against CA aggregation. The chaperone function followed the same pattern as observed for insulin and CS (Fig. 3). The activities of the constructs followed the order Wt >C131I/C142I >C142I >C131I (data not shown). To determine binding constants, we incubated 12.0 μM αA-crystallin at 60°C for 1 h with varying concentrations (5–18 μM) of CA. The unbound (S) and bound CA were measured by filtration of the samples. The dissociation constant (Kd) was analyzed by the Scat-chard equation: ν̃/S = n/Kd − 1/Kdν̃ where ν̃ is the number of moles of substrate bound per mole of chaperone, n is the number of binding sites, and Kd is the dissociation constant. The stoichiometry n and dissociation constant Kd were obtained from the plot of ν̃/S against ν̃ (Table 2). All of the mutants appeared to lose binding sites for CA compared to the Wt protein, with the number of binding sites (n) per subunit decreased by 16%, 10% and 9%, in C131I, C142I, and in the double mutant, respectively. The number of binding sites in all proteins increased by 13–20% and the Kd values slightly reduced after MGO modification. From these data, we conclude that substitution of cysteine residues by isoleucine residues in αA-crystallin reduced the binding affinity for CA, but MGO-modification enhanced the affinity.
Table 2.
Determination of n and dissociation constant values for interaction of Wt and mutant αA-crystallin with CA
| Protein | n | Kd (μM) |
|---|---|---|
| Wt | 2.42 ± 0.02 | 3.92 ± 0.07 |
| Wt+MGO | 2.78 ± 0.04 | 3.25 ± 0.22 |
| C131I | 2.02 ± 0.07 | 2.98 ± 0.17 |
| C131I+MGO | 2.52 ± 0.02 | 2.02 ± 0.23 |
| C142I | 2.16 ± 0.08 | 3.12 ± 0.19 |
| C142I+MGO | 2.60 ± 0.12 | 2.84 ± 0.20 |
| C131I/C142I | 2.20 ± 0.14 | 3.42 ± 0.18 |
| C131I/C142I+MGO | 2.67 ± 0.09 | 2.92 ± 0.16 |
Discussion
The purpose of this study was to determine if the two cysteine residues in human αA-crystallin participated in MGO-induced improvement of chaperone function. The results indicated that MGO-induced enhancement of chaperone function occurred in the absence of cysteines.
The C131I mutation inhibited chaperone function more than either C142I or double mutation in both the CS and insulin aggregation assays. This does not appear to be due to a higher reduction of surface hydrophobicity, as both C131I and in C142I had higher surface hydrophobicities than the Wt protein, while the double mutant protein displayed decreased surface hydrophobicity. Although the chaperone function appears to be due to interaction of the client proteins with hydrophobic patches on αA-crystallin, several previous studies have shown that the relationship between surface hydrophobicity and chaperone function is not strong (reviewed in [17]). One reason could be that the hydrophobic probe used in many studies, including the TNS used in this study, may bind to hydrophobic sites other than the chaperone sites. Competition experiments with TNS and client proteins for binding to α-crystallin show some degree of competition [18], which underscores the idea that the two may bind at different sites in α-crystallin. The observation that the double mutant was similar to C142I, but a better chaperone than C131I mutant, was somewhat unexpected. It indicates that the C142I mutation overrides the negative effect of C131I. This property was retained even after MGO modification. The results also suggest that C131 is more important than C142 for chaperone function.
The reaction of αA-crystallin with MGO slightly enhanced the surface hydrophobicity in all proteins, regardless of the type of mutation. This subtle change appears to be sufficient to enhance the chaperone function. However, the extent of enhancement of the chaperone function varied among the mutants. This was more evident in the CS aggregation assay, where the highest increase in the chaperone function was seen with C131I mutant. The C142I and the double mutant proteins showed smaller increases relative to C131I and Wt protein. These results suggest that the MGO-induced enhancement in chaperone function may depend, to some extent, on C142. Whether it is due to the modification of hemithioacetal requires further investigation. However, our observation that C142I and the double mutant protein are better chaperones than C131I suggests that C142 may not be as important as C131 for the chaperone function of αA-crystallin. These data imply that C131 is important for chaperone function, but its absence does not hinder the enhancement of chaperone function by MGO.
In addition to TNS binding experiments, far-UV CD spectroscopy revealed no major changes in the secondary structure either in unmodified or MGO-modified mutant proteins. However, significant changes were noticeable in the near-UV CD spectra of the mutant proteins, suggesting that the microenvironments of tyrosine and tryptophan were altered. MGO modification appeared to have further changed the tertiary structure, although only slightly. These data imply that the loss in chaperone function in the mutant proteins could be due to the changes in tertiary structure as revealed by near-UV CD spectroscopy.
The binding constants using CA as the client protein showed that replacement of cysteine residues with isoleucine slightly reduced the binding of CA to αA-crystallin. Modification with MGO increased the binding of CA to all of the mutants. The increased binding was accompanied by stronger binding of CA to αA-crystallin, as evident from the lower Kd values. Such changes in client protein binding to modified αA-crystallin were also seen in our previous study where MGO-modifiable arginine residues were replaced with alanine [13].
In summary, this study demonstrates that the enhancement of the chaperone function of human αA-crystallin by MGO-modification occurs in the absence of cysteine residues. In the lens, where MGO is constantly present, formation of reversible hemithioacetal adducts with αA-crystallin is likely at all times, but it may neither change the structure of αA-crystallin nor contribute to enhancement in the chaperone function. However, the two cysteine residues appear to be required for proper folding of the protein and chaperone function, as their replacement changed the tertiary structure and reduced the chaperone function. In the human lens, a significant portion of αA-crystallin is known to exist as intra- and inter-molecular disulfide crosslinked species [19]. In addition, αA-crystallin may exist as a mixed disulfide from reaction with glutathione [20]. These reactions might limit reaction of cysteine residues with MGO. Nevertheless, this study further supports the view that modification of arginine residues, as previously suggested [12, 13], is the main cause for enhancement in the chaperone function in MGO-modified human αA-crystallin.
Acknowledgments
This study was supported by NIH grants R01EY-016219 and R01EY-09912, Carl F. Asseff, M.D. Professorship (RHN), P30EY-11373 (Visual Sciences Research Center of CWRU), Research to Prevent Blindness, NY, and the Ohio Lions Eye Research Foundation. We thank Dr. Ashis Biswas for helpful discussions.
References
- 1.Augusteyn RC. Alpha-crystallin: a review of its structure and function. Clin Exp Optom. 2004;87:356–366. doi: 10.1111/j.1444-0938.2004.tb03095.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyle DL, Takemoto L, Brady JP, Wawrousek EF. Morphological characterization of the alpha A- and alpha B-crystallin double knockout mouse lens. BMC Ophthalmol. 2003;3:3. doi: 10.1186/1471-2415-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CD, Kymes S, Petrash JM. A transgenic mouse model for human autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2006;47:2036–2044. doi: 10.1167/iovs.05-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 6.Haik GM, Jr, Lo TW, Thornalley PJ. Methylglyoxal concentration and glyoxalase activities in the human lens. Exp Eye Res. 1994;59:497–500. doi: 10.1006/exer.1994.1135. [DOI] [PubMed] [Google Scholar]
- 7.Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999;368:98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- 8.Padayatti PS, Ng AS, Uchida K, Glomb MA, Nagaraj RH. Argpyrimidine, a blue fluorophore in human lens proteins: high levels in brunescent cataractous lenses. Invest Ophthalmol Vis Sci. 2001;42:1299–1304. [PubMed] [Google Scholar]
- 9.Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 10.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of Major Maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002;277:24907–24915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 11.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- 12.Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 13.Biswas A, Miller A, Oya-Ito T, Santhoshkumar P, Bhat M, Nagaraj RH. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry. 2006;45:4569–4577. doi: 10.1021/bi052574s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas A, Lewis S, Wang B, Miyagi M, Santoshkumar P, Gangadhariah MH, Nagaraj RH. Chemical modulation of the chaperone function of human αA-crystallin. J Biochem. 2008;144:21–32. doi: 10.1093/jb/mvn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SJ, Sun TX, Akhtar NJ, Liang JJ. Oxidation of human lens recombinant alphaA-crystallin and cysteine-deficient mutants. J Mol Biol. 2001;305:969–976. doi: 10.1006/jmbi.2000.4348. [DOI] [PubMed] [Google Scholar]
- 16.Liang JN, Pelletier MR. Spectroscopic studies on the mixed disulfide formation of lens crystallin with glutathione. Exp Eye Res. 1987;45:197–206. doi: 10.1016/s0014-4835(87)80143-4. [DOI] [PubMed] [Google Scholar]
- 17.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 18.Biswas A, Wang B, Miyagi M, Nagaraj RH. Effect of methylglyoxal modification on stress-induced aggregation of client proteins and their chaperoning by human alphaA-crystallin. Biochem J. 2008;409:771–777. doi: 10.1042/BJ20071006. [DOI] [PubMed] [Google Scholar]
- 19.Takemoto L. Increase in the intramolecular disulfide bonding of alpha-A crystallin during aging of the human lens. Exp Eye Res. 1996;63:585–590. doi: 10.1006/exer.1996.0149. [DOI] [PubMed] [Google Scholar]
- 20.Lou MF. Thiol regulation in the lens. J Ocul Pharmacol Ther. 2000;16:137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]