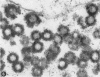Abstract
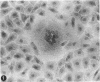
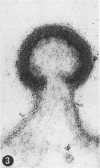
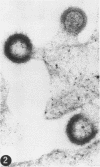
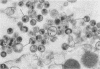
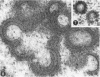
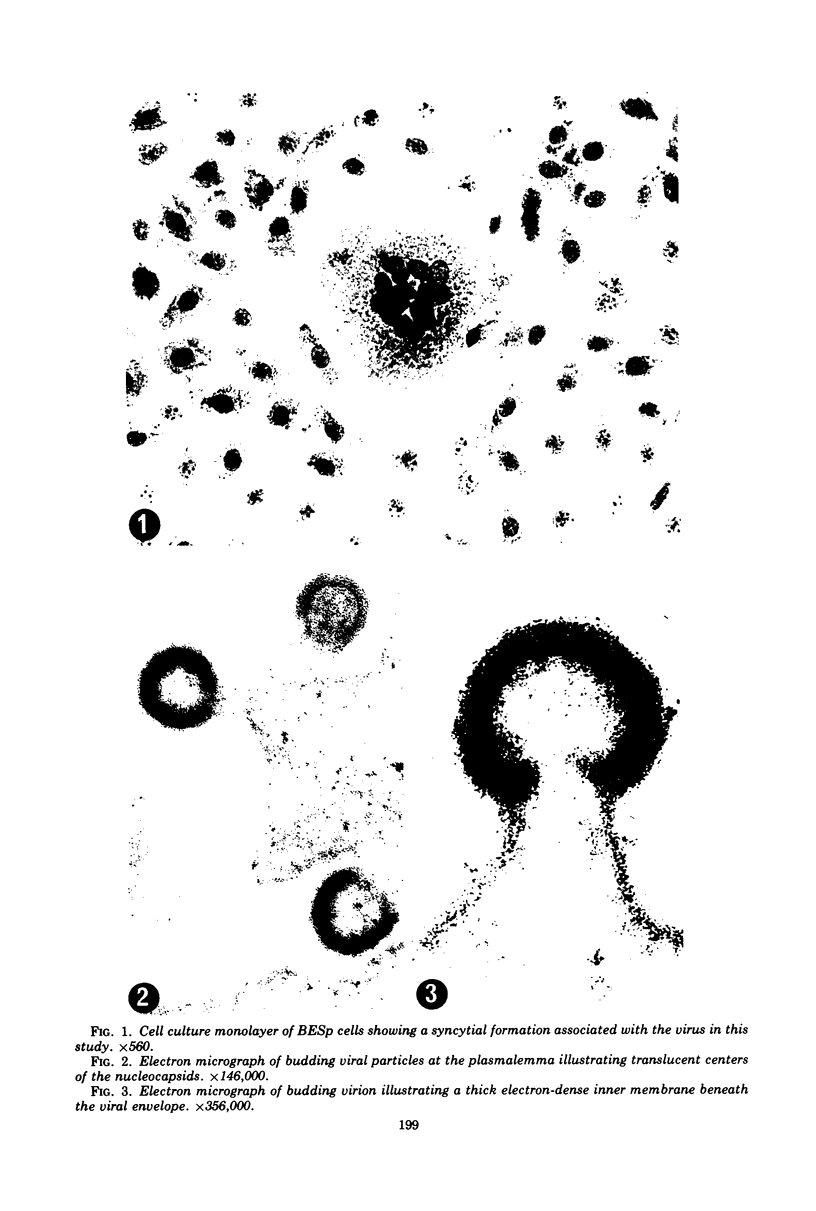
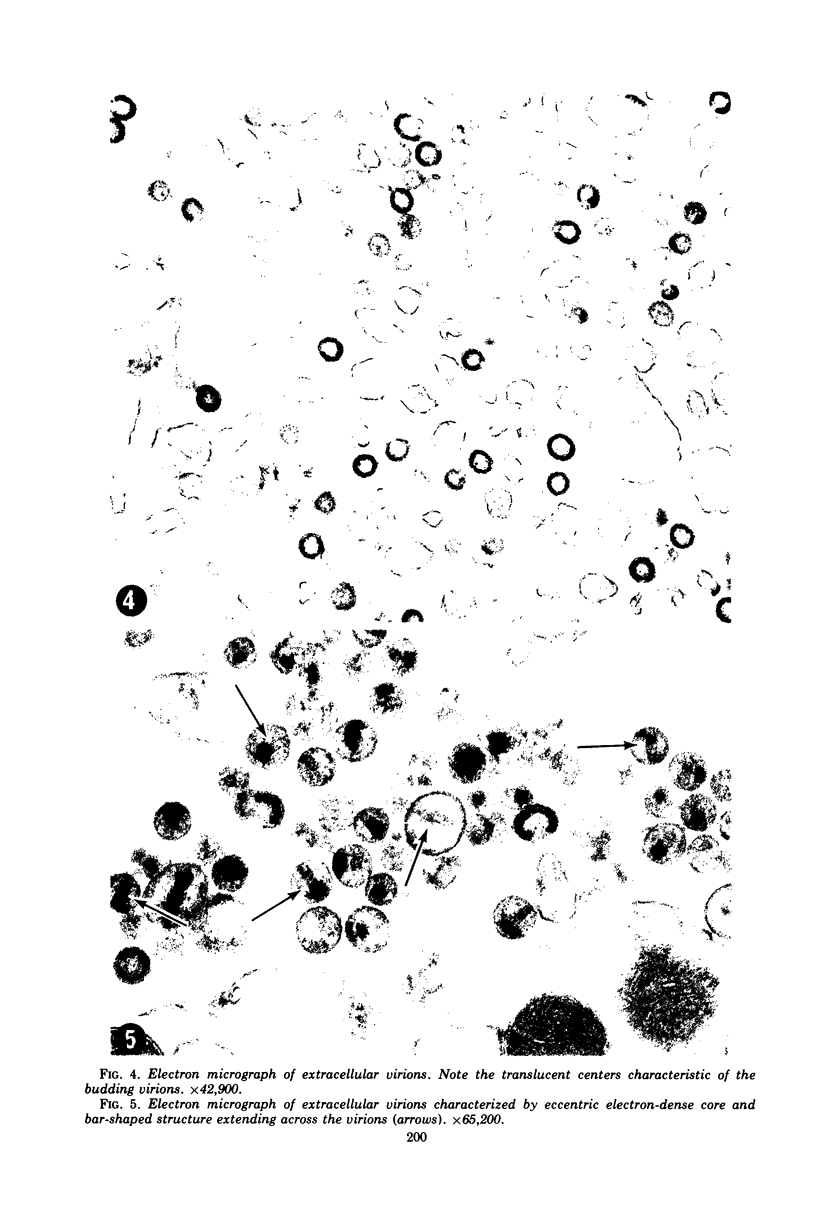
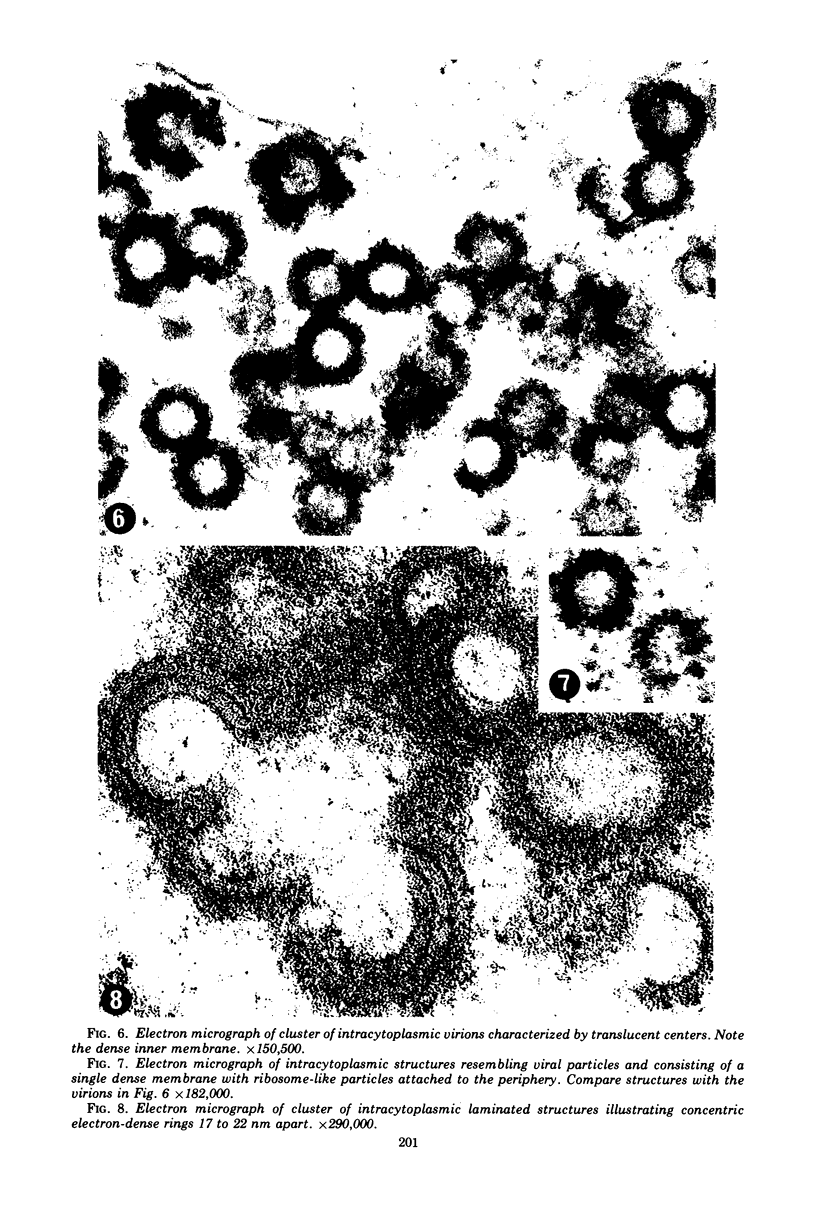
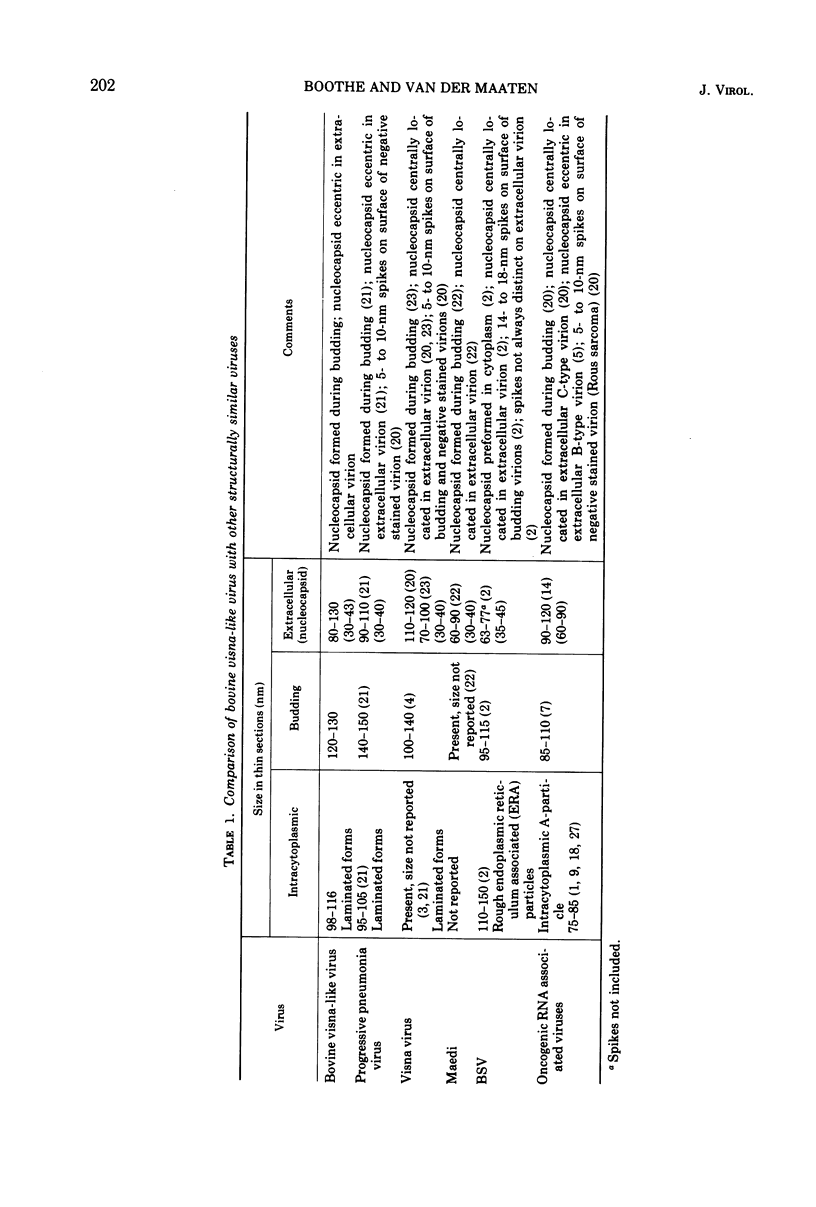
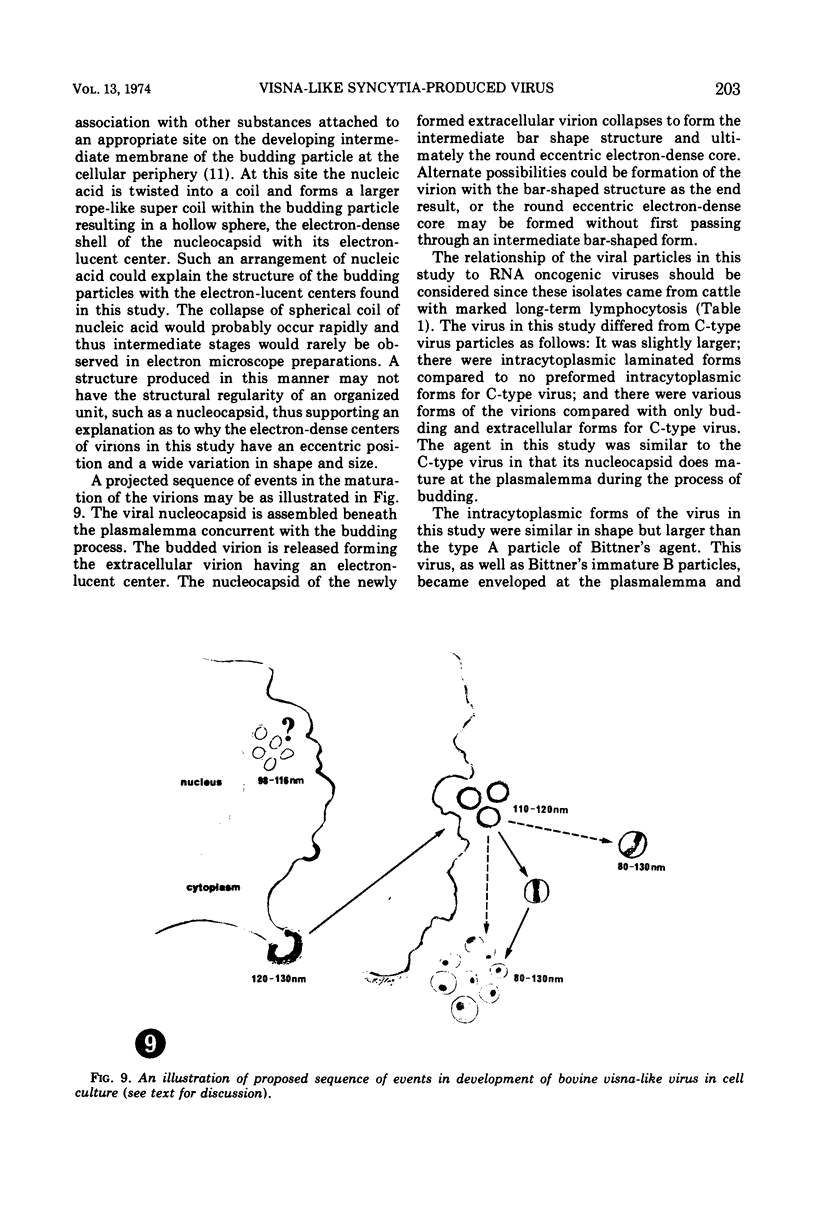
A virus structurally similar to viruses associated with maedi, progressive pneumonia, and visna of sheep has been isolated from buffy coat cells of cattle with chronic lymphocytosis. Electron microscope studies revealed three variants of the virion: (i) an intracytoplasmic form 98 to 116 nm in diameter when occurring in a nonlaminated form, (ii) a budding form 120 to 130 nm in diameter, and (iii) an extracellular form 80 to 130 nm in diameter and containing a 30 to 43 nm eccentrically located electron-dense core.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- Boothe A. D., Van der Maaten M. J., Malmquist W. A. Morphological variation of a syncytial virus from lymphosarcomatous and apparently normal cattle. Arch Gesamte Virusforsch. 1970;31(3):373–384. doi: 10.1007/BF01253771. [DOI] [PubMed] [Google Scholar]
- Coward J. E., Harter D. H., Hsu K. C., Morgan C. Ferritin-conjugated antibody labeling of visna virus. Virology. 1972 Dec;50(3):925–930. doi: 10.1016/0042-6822(72)90449-7. [DOI] [PubMed] [Google Scholar]
- Coward J. E., Harter D. H., Morgan C. Electron microscopic observations of Visna virus-infected cell cultures. Virology. 1970 Apr;40(4):1030–1038. doi: 10.1016/0042-6822(70)90149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J. Further analysis of the detailed structure of type B and C particles. J Natl Cancer Inst. 1972 Apr;48(4):1095–1099. [PubMed] [Google Scholar]
- Dermott E., Clarke J. K., Samuels J. The morphogenesis and classification of bovine syncytial virus. J Gen Virol. 1971 Aug;12(2):105–119. doi: 10.1099/0022-1317-12-2-105. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Stock N. D., Lin P. Detection of replicating C-type viruses in continuous cell cultures established from cows with leukemia: effect of the culture medium. J Natl Cancer Inst. 1971 Sep;47(3):613–621. [PubMed] [Google Scholar]
- Frank G. H. Serial passage of two plaque types of parainfluenza-3 virus: changes in hemagglutinating properties and cytopathic effect. Am J Vet Res. 1970 Jun;31(6):1085–1091. [PubMed] [Google Scholar]
- Gay F. W., Clarke J. K., Dermott E. Morphogenesis of Bittner virus. J Virol. 1970 Jun;5(6):801–806. doi: 10.1128/jvi.5.6.801-816.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasty V., Chang P. W. Infectious bovine rhinoracheitis virus in bovine kidney cells: sequence of viral production, cellular changes, and localization of viral nucleic acid and protein. Am J Vet Res. 1969 Aug;30(8):1325–1332. [PubMed] [Google Scholar]
- Kakefuda T., Bader J. P. Electron microscopic observations on the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):460–474. doi: 10.1128/jvi.4.4.460-474.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmquist W. A., Van der Maaten M. J., Boothe A. D. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969 Jan;29(1):188–200. [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Parks W. P., Todaro G. J. Biological properties of syncytium-forming ("foamy") viruses. Virology. 1972 Mar;47(3):673–683. doi: 10.1016/0042-6822(72)90557-0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J., Aaronson S. A. Immunological characterization of primate C-type virus reverse transcriptases. Nat New Biol. 1972 Jan 12;235(54):35–40. doi: 10.1038/newbio235035a0. [DOI] [PubMed] [Google Scholar]
- Smith G. H. Cytochemical studies on the mouse mammary tumor virus. Cancer Res. 1967 Nov;27(11):2179–2196. [PubMed] [Google Scholar]
- Stone L. B., Scolnick E., Takemoto K. K., Aaronson S. A. Visna virus: a slow virus with an RNA dependent DNA polymerase. Nature. 1971 Jan 22;229(5282):257–258. doi: 10.1038/229257a0. [DOI] [PubMed] [Google Scholar]
- THORMAR H. A COMPARISON OF VISNA AND MAEDI VIRUSES. I. PHYSICAL, CHEMICAL AND BIOLOGICAL PROPERTIES. Res Vet Sci. 1965 Jan;6:117–129. [PubMed] [Google Scholar]
- THORMAR H. An electron microscope study of tissue cultures infected with visna virus. Virology. 1961 Aug;14:463–475. doi: 10.1016/0042-6822(61)90339-7. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Aoki T., Garon C., Sturm M. M. Comparative studies on Visna, progressive pneumonia, and Rous sarcoma viruses by electron microscopy. J Natl Cancer Inst. 1973 Feb;50(2):543–547. doi: 10.1093/jnci/50.2.543. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Mattern C. F., Stone L. B., Coe J. E., Lavelle G. Antigenic and morphological similarities of progressive pneumonia virus, a recently isolated "slow virus" of sheep, to visna and maedi viruses. J Virol. 1971 Mar;7(3):301–308. doi: 10.1128/jvi.7.3.301-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D. Isolation of a herpes-like virus from lymphosarcomatous cattle. Arch Gesamte Virusforsch. 1972;37(1):85–96. doi: 10.1007/BF01241154. [DOI] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Wivel N. A., Smith G. H. Distribution of intracisternal A-particles in a variety of normal and neoplastic mouse tissues. Int J Cancer. 1971 Jan 15;7(1):167–175. doi: 10.1002/ijc.2910070119. [DOI] [PubMed] [Google Scholar]