Abstract
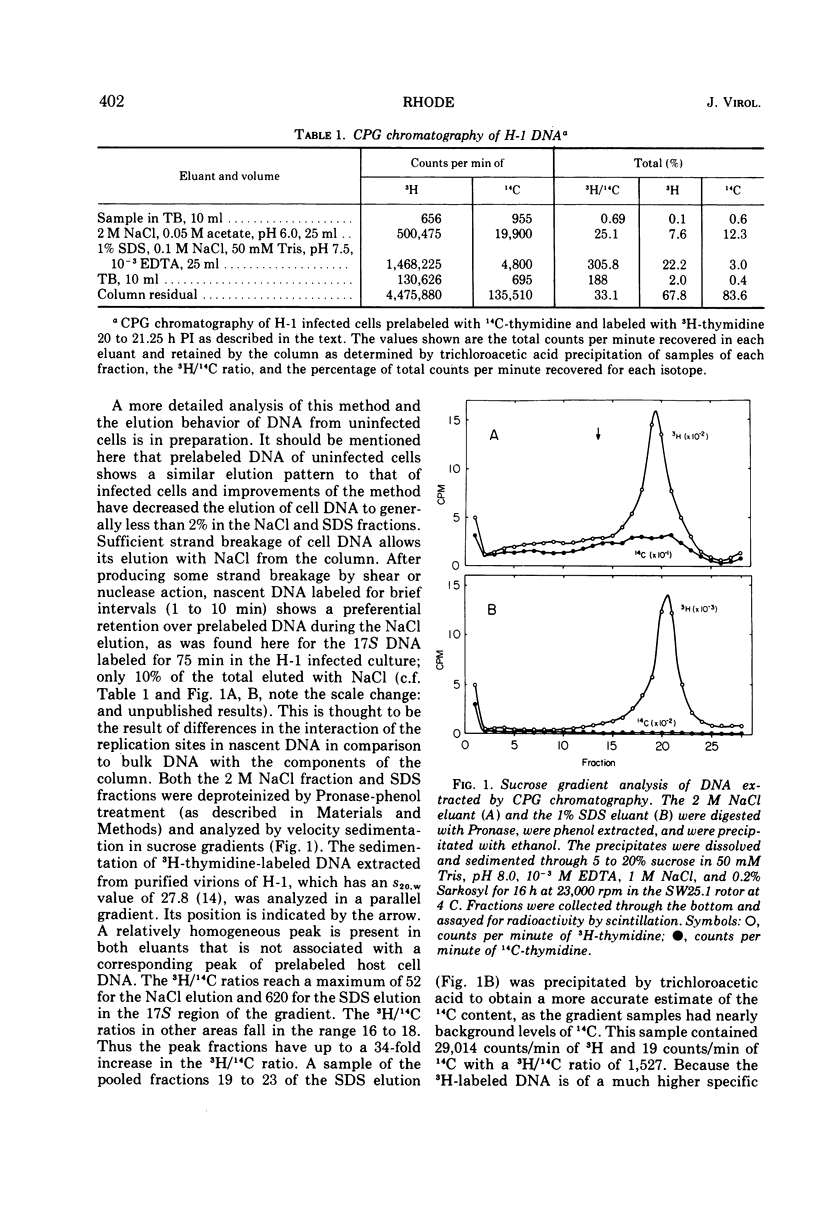
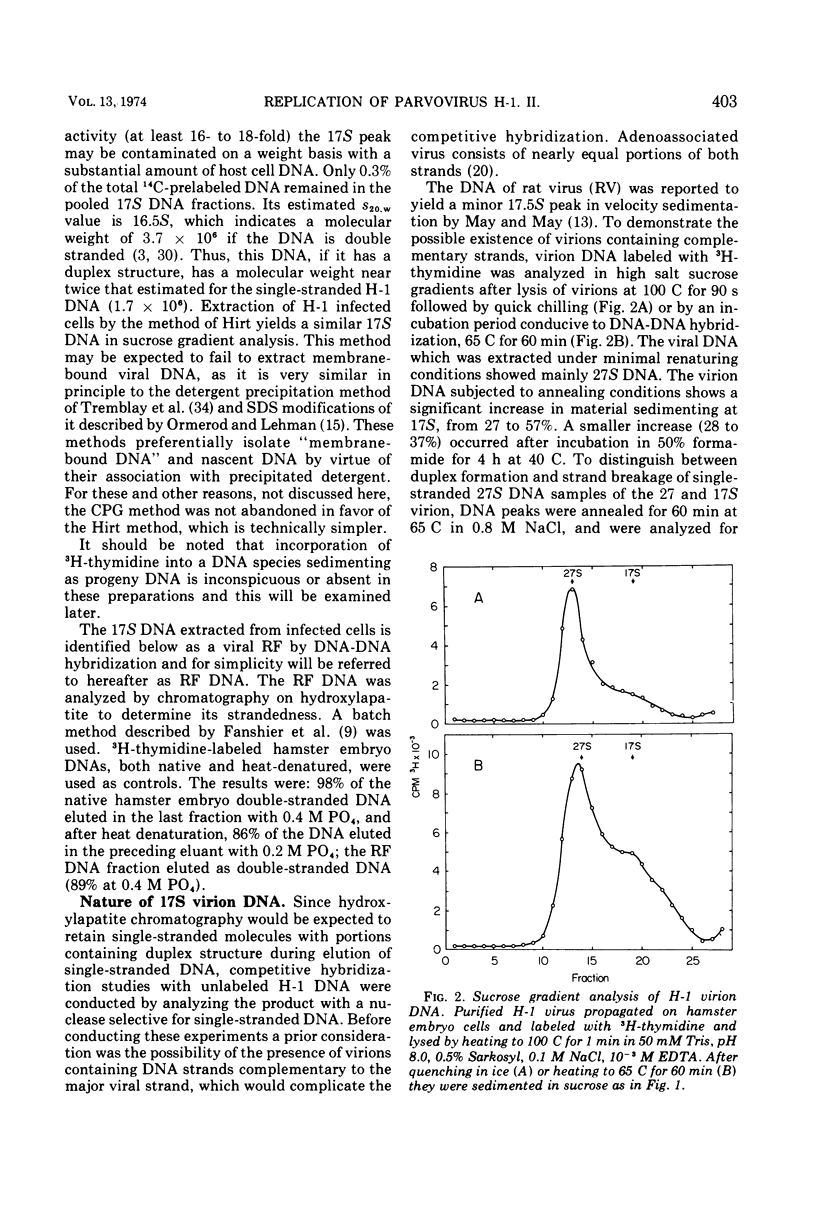
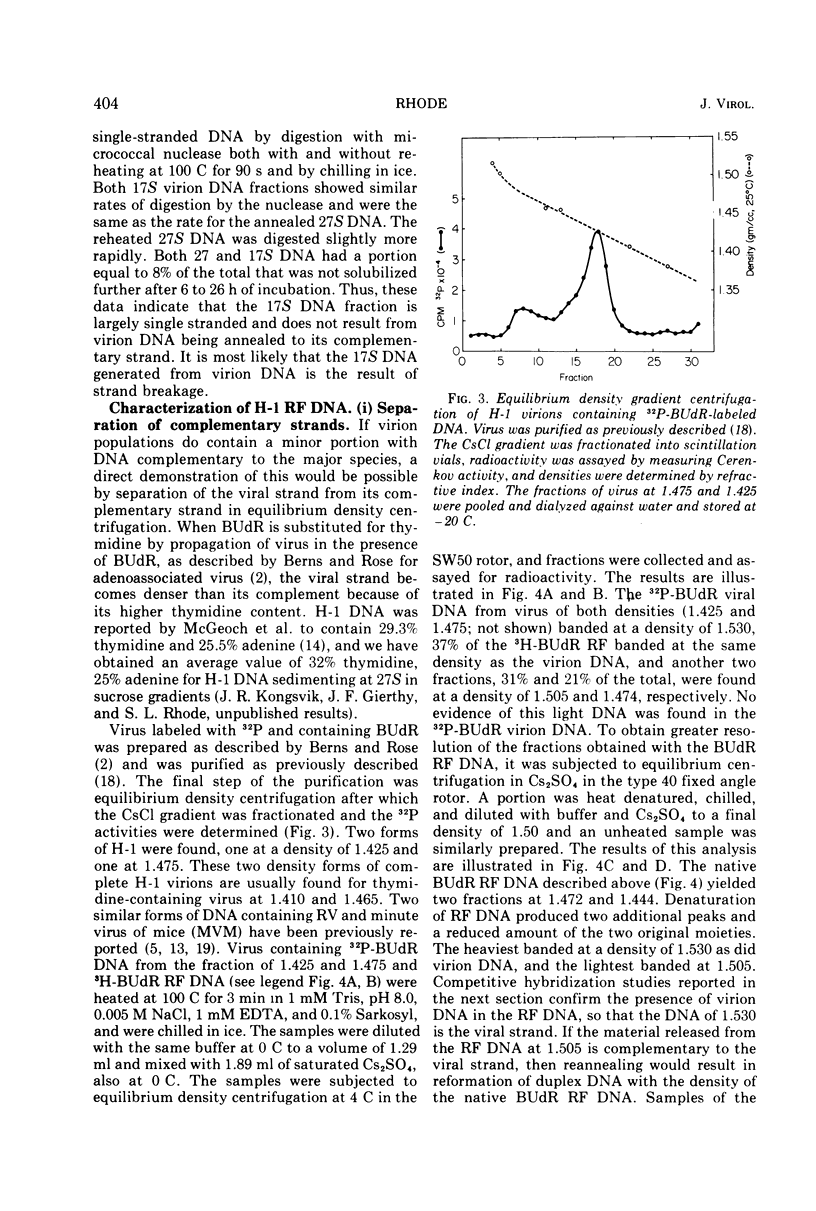
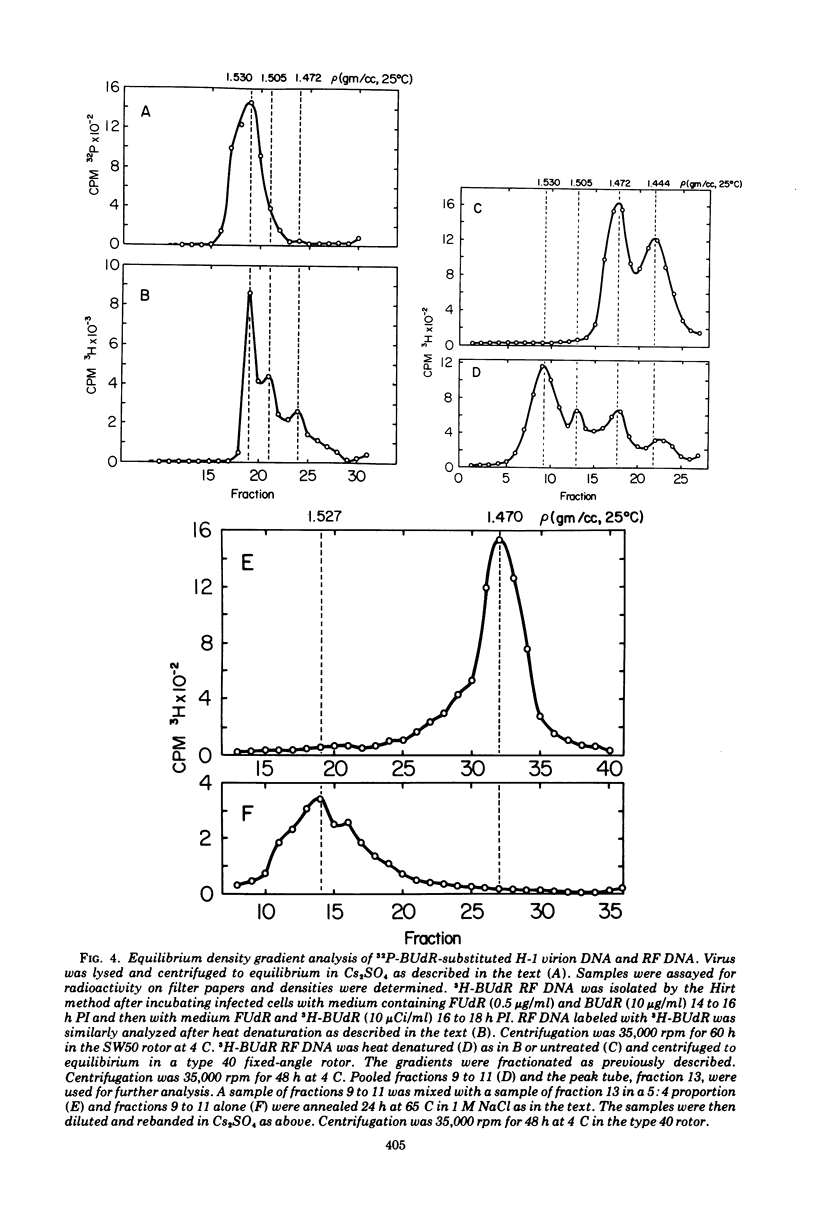
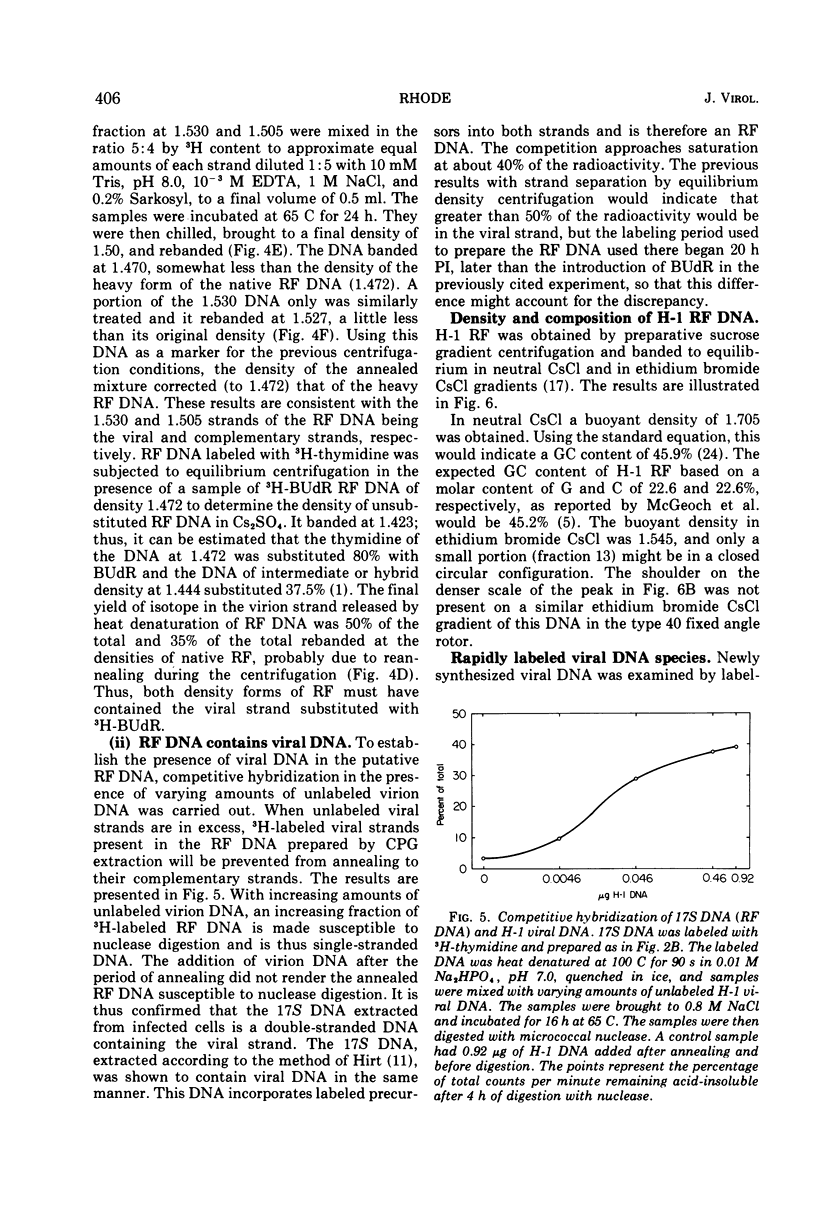
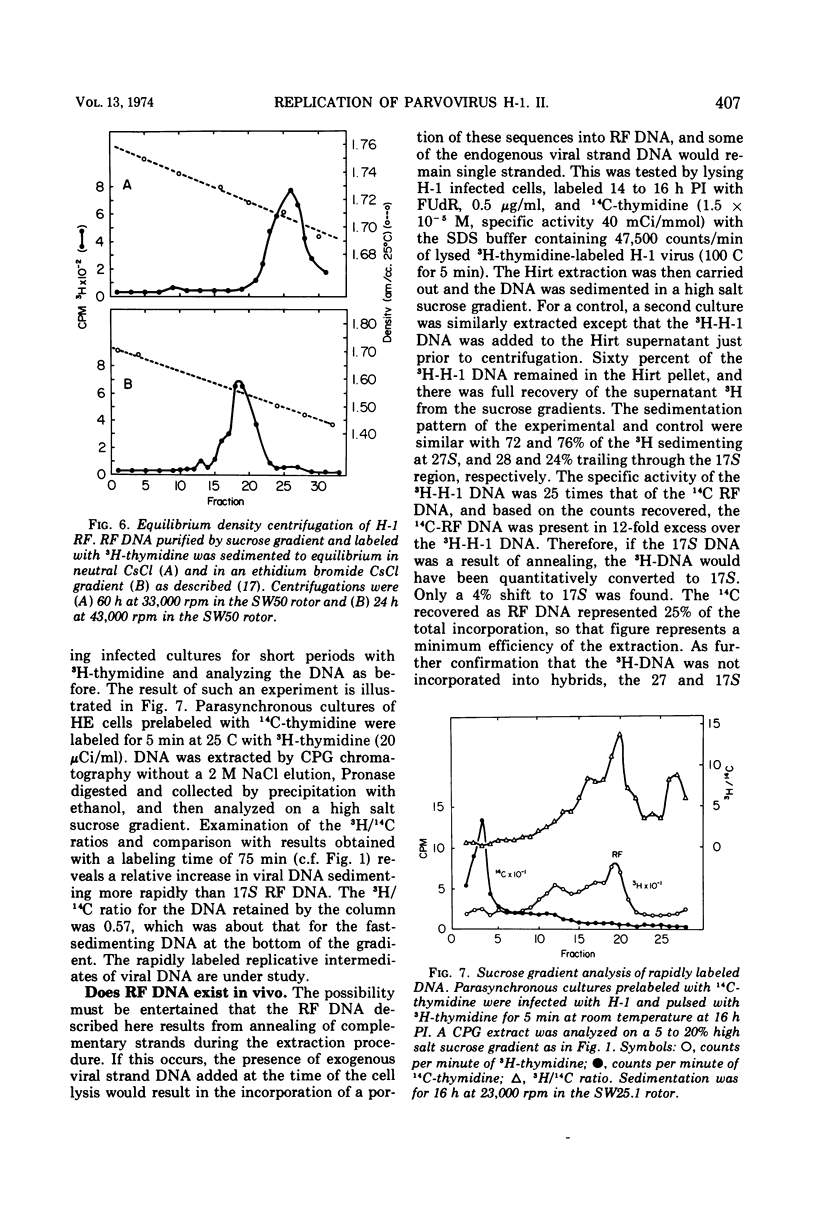
The Parvovirus H-1 replicates autonomously in hamster embryo cells. A DNA synthetic event, called HA-DNA synthesis, upon which subsequent viral RNA and viral hemagglutinin synthesis is dependent, is initiated in late S phase of the infected cell (18). It was postulated that HA-DNA represents parental viral replicative form DNA (RF DNA). This study describes the isolation and characterization of H-1 RF DNA as part of the continuing study of the mechanisms and control of DNA replication in the eukaryotic cell. The H-1 RF DNA is a linear duplex molecule containing the viral strand and its complement. The complementary strands of the RF DNA have been separated by equilibrium density gradient centrifugation. The RF DNA has a buoyant density of 1.705 in neutral CsCl and an estimated guanine plus cytosine (GC) content of 45.9%. It has a sedimentation coefficient of 17S. The calculated molecular weight of 3.7 × 106 is twice that of the single-stranded virion DNA. H-1 virions contain DNA that is homogeneous and free of complementary strands.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., SHOOTER E. M. THE ALKALINE TRANSITION OF BU-CONTAINING DNA AND ITS BEARING ON THE REPLICATION OF DNA. J Mol Biol. 1963 Nov;7:511–526. doi: 10.1016/s0022-2836(63)80098-4. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V. A minute virus of mice. Virology. 1966 Aug;29(4):605–612. doi: 10.1016/0042-6822(66)90284-4. [DOI] [PubMed] [Google Scholar]
- Dobson P. R., Helleiner C. W. A replicative form of the DNA of minute virus of mice. Can J Microbiol. 1973 Jan;19(1):35–41. doi: 10.1139/m73-005. [DOI] [PubMed] [Google Scholar]
- Ellem K. A. A dual-label technique for comparing the rates of synthesis of nucleic acid fractions separated by methylated albumin-kieselguhr chromatography from cells in different states of activity. Biochim Biophys Acta. 1967 Nov 21;149(1):74–87. doi: 10.1016/0005-2787(67)90692-2. [DOI] [PubMed] [Google Scholar]
- Ellem K. A., Rhode S. L., 3rd The use of guanidine thiocyanate to fractionate DNA-like RNA adsorbed on methylated bovine albumin-kieselguhr columns. Biochim Biophys Acta. 1969 Jan 21;174(1):117–123. doi: 10.1016/0005-2787(69)90234-2. [DOI] [PubMed] [Google Scholar]
- Fanshier L., Garapin A. C., McDonnell J., Faras A., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase associated with avian tumor viruses: secondary structure of the deoxyribonucleic acid product. J Virol. 1971 Jan;7(1):77–86. doi: 10.1128/jvi.7.1.77-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Bynum B. S., Shack J. Unique secondary structure of newly replicated HeLa DNA. Biochim Biophys Acta. 1969 Aug 20;186(2):412–414. doi: 10.1016/0005-2787(69)90025-2. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., May E. The DNA of kilham rat virus. J Gen Virol. 1970 Mar;6(3):437–439. doi: 10.1099/0022-1317-6-3-437. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Crawford L. V., Follett E. A. The DNAs of three parvoviruses. J Gen Virol. 1970 Jan;6(1):33–40. doi: 10.1099/0022-1317-6-1-33. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., Lehmann A. R. The release of high molecular weight DNA from a mammalian cell (L-5178Y). Attachment of the DNA to the nuclear membrane. Biochim Biophys Acta. 1971 Jan 28;228(2):331–343. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. M., Hetrick F. M. Single-stranded DNA from the Kilham rat virus. J Gen Virol. 1969 Mar;4(2):269–281. doi: 10.1099/0022-1317-4-2-269. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., Kakefuda T. Linear, single-stranded deoxyribonucleic acid isolated from Kilham rat virus. J Virol. 1971 Jun;7(6):830–835. doi: 10.1128/jvi.7.6.830-835.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., McKerlie L. Growth characteristics of Kilham rat virus and its effect on cellular cellular macromolecular synthesis. J Virol. 1972 Oct;10(4):573–577. doi: 10.1128/jvi.10.4.573-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. In vivo conversion of the single-stranded DNA of the kilham rat virus to a double-stranded form. J Virol. 1973 Feb;11(2):299–305. doi: 10.1128/jvi.11.2.299-305.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido K., Ikeda Y. Isolation of double-helical regions rich in adenine-thymine base pairing from bacteriophage f1 DNA. J Mol Biol. 1971 Jan 28;55(2):287–291. doi: 10.1016/0022-2836(71)90200-2. [DOI] [PubMed] [Google Scholar]
- Shishido K., Ikeda Y. Isolation of double-helical regions rich in guanine-cytosine base pairing from bacteriophage fl DNA. Biochem Biophys Res Commun. 1971 Feb 5;42(3):482–489. doi: 10.1016/0006-291x(71)90396-2. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. II. Biochemical characteristics of virus replication. Arch Gesamte Virusforsch. 1973;40(1):119–127. doi: 10.1007/BF01242643. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Hand R. E., Jr Requirement of cellular synthesis for Kilham rat virus replication. Virology. 1970 Dec;42(4):1054–1063. doi: 10.1016/0042-6822(70)90353-3. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W. The picodna viruses. H, RV, and AAV. Int Rev Exp Pathol. 1968;6:135–180. [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Usategui-Gomez M., Toolan H. W., Ledinko N., al-Lami F., Hopkins M. S. Single-stranded DNA from the Parvovirus, H-1. Virology. 1969 Nov;39(3):617–621. doi: 10.1016/0042-6822(69)90117-2. [DOI] [PubMed] [Google Scholar]


