Abstract
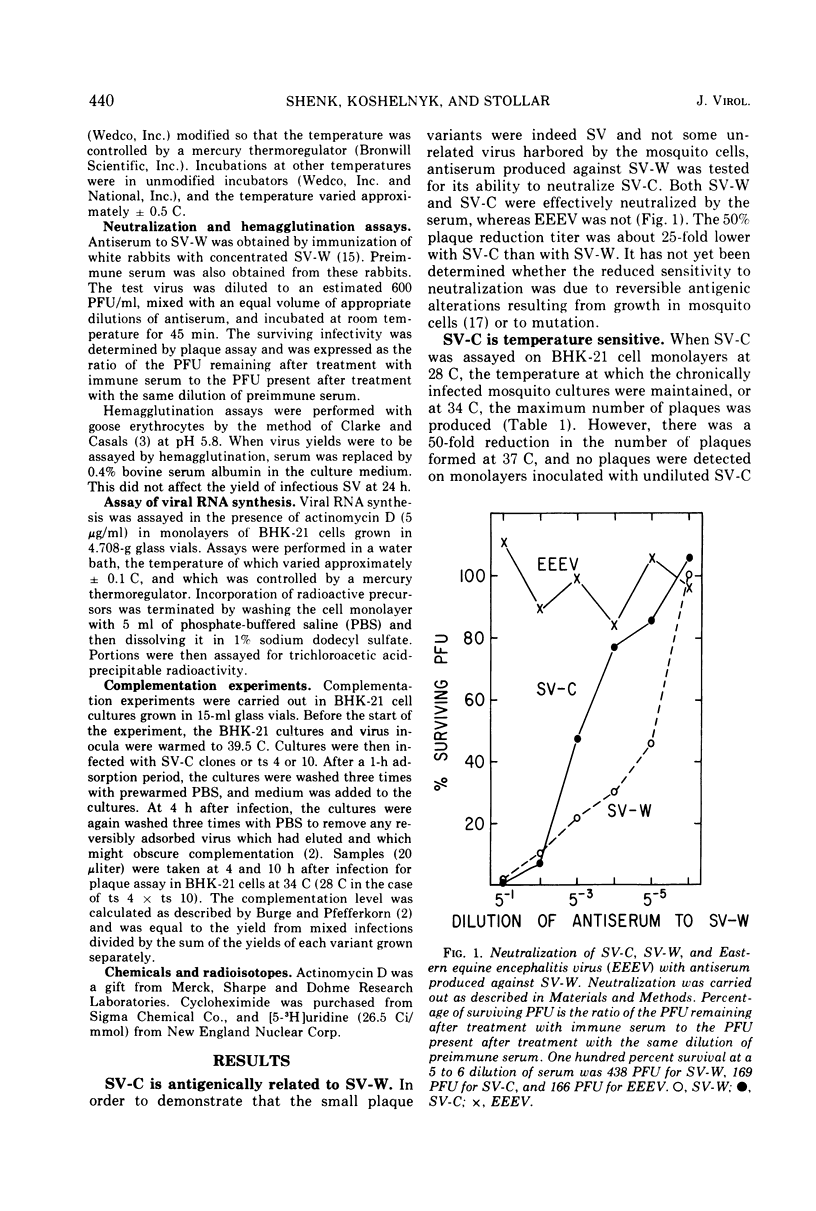
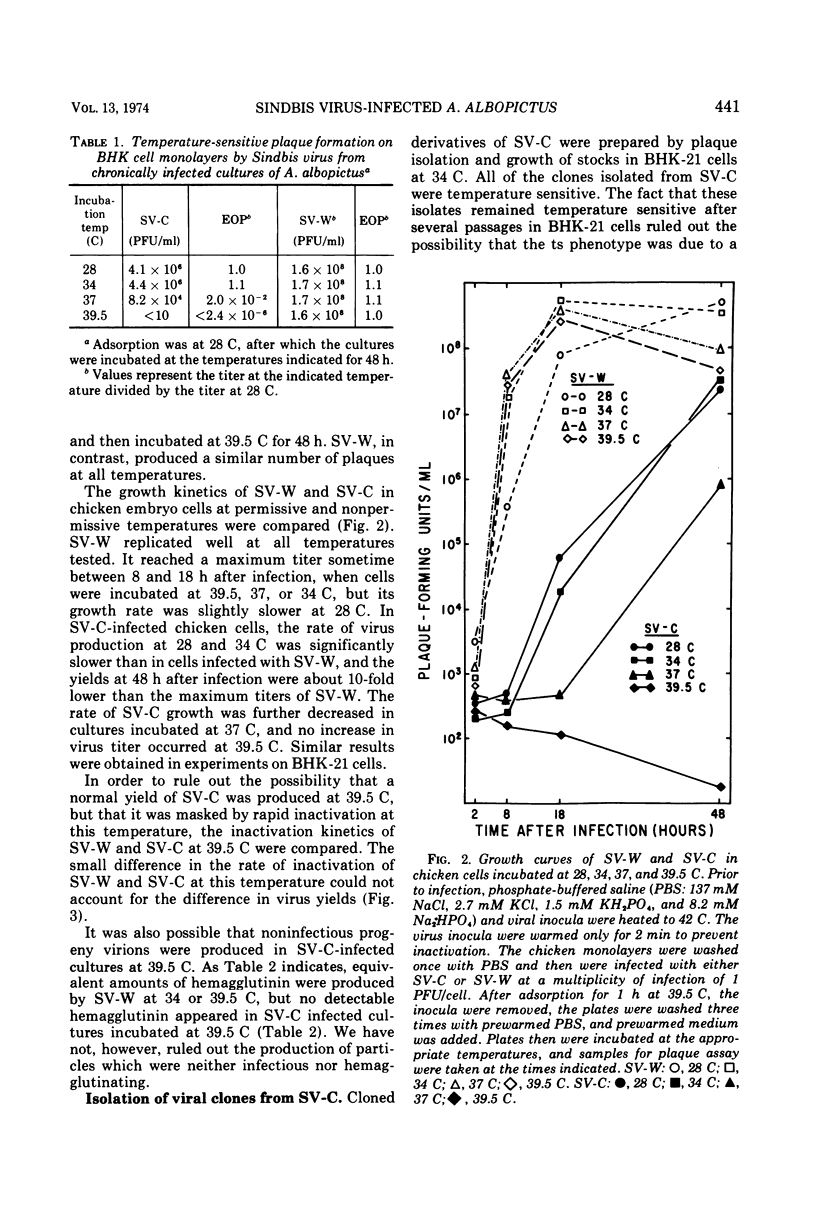
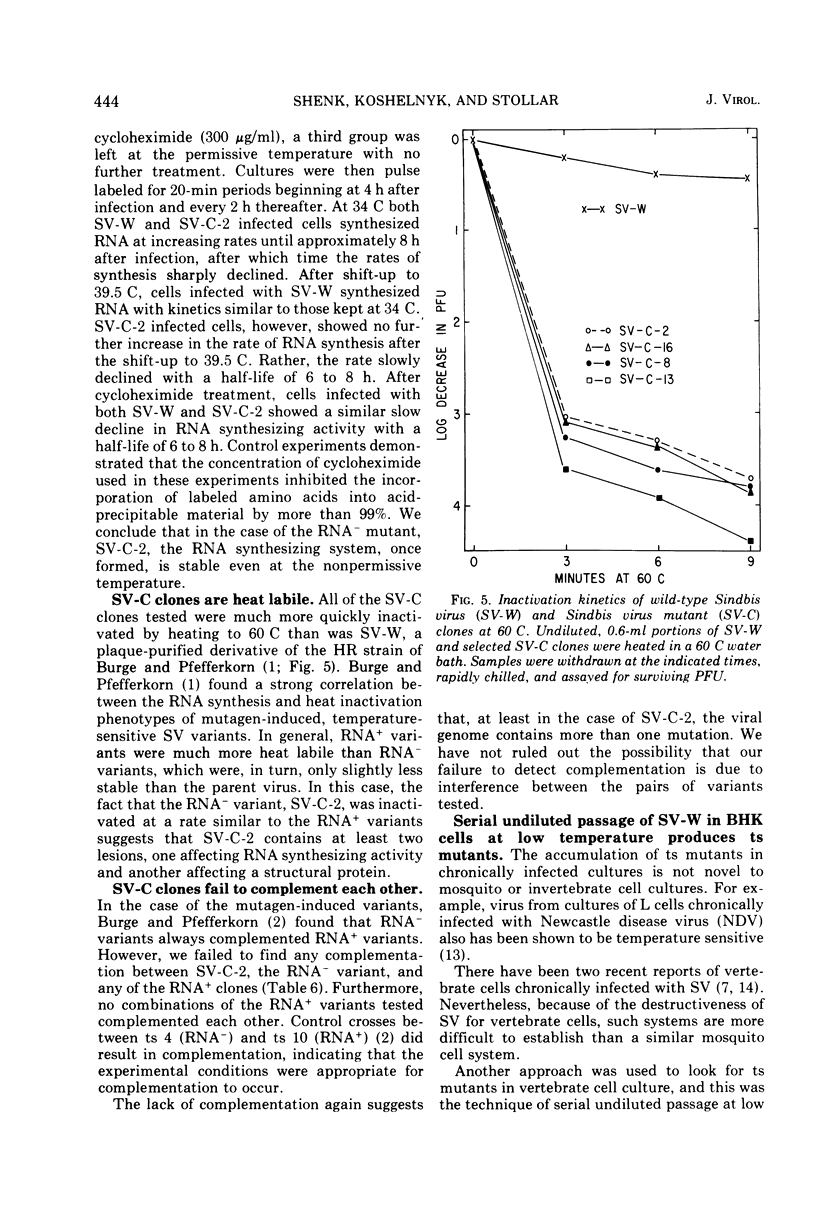
Cultures of Aedes albopictus cells persistently infected with wild-type Sindbis virus (SV-W) give rise to small plaque-forming mutants which are also temperature sensitive. These mutants, designated SV-C, are neutralized by antiserum produced against SV-W. Mutant ts clones were isolated from SV-C by plaque purification. After serial undiluted passage in BHK or mosquito cells, each of the clones gave rise to ts+ revertants which, however, remained mutant with respect to plaque morphology. Nineteen of 20 clones derived from SV-C were RNA+, and one was RNA− (SV-C-2). The RNA synthesizing activity, once induced in infected cells by SV-C-2, was stable at the nonpermissive temperature (39.5 C). All clones derived from SV-C were inactivated at 60 C much more quickly than was SV-W. It was not possible to demonstrate complementation between any of the SV-C clones.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- CHAPIN M., DUBES G. R. Cold-adapted genetic variants of polio viruses. Science. 1956 Sep 28;124(3222):586–587. doi: 10.1126/science.124.3222.586-a. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Buckler C. E., Baron S. Kinetics of the development of factors responsible for inerferon-induced resistance. Proc Soc Exp Biol Med. 1968 Nov;129(2):535–538. doi: 10.3181/00379727-129-33363. [DOI] [PubMed] [Google Scholar]
- Gaidamovich S. Y., Tsilinsky Y. Y., Lvova A. I., Khutoretskaya N. V. Aëdes aegypti mosquitoes as an experimental model for studies on the ecology and genetics of Venezuelan equine encephalomyelitis virus. Acta Virol. 1971 Jul;15(4):301–308. [PubMed] [Google Scholar]
- Inglot A. D., Albin M., Chudzio T. Persistent infection of mouse cells with Sindbis virus: role of virulence of strains, auto-interfering particles and interferon. J Gen Virol. 1973 Jul;20(1):105–110. doi: 10.1099/0022-1317-20-1-105. [DOI] [PubMed] [Google Scholar]
- LWOFF A. The thermosensitive critical event of the viral cycle. Cold Spring Harb Symp Quant Biol. 1962;27:159–174. doi: 10.1101/sqb.1962.027.001.018. [DOI] [PubMed] [Google Scholar]
- Martin E. M. Studies on the RNA polymrase of some temperature-sensitive mutants of Semliki Forest virus. Virology. 1969 Sep;39(1):107–117. doi: 10.1016/0042-6822(69)90352-3. [DOI] [PubMed] [Google Scholar]
- Nagata I., Kimura Y., Ito Y., Tanaka T. Temperature-sensitive phenomenon of viral maturation observed in BHK cells persistently infected with HVJ. Virology. 1972 Aug;49(2):453–461. doi: 10.1016/0042-6822(72)90497-7. [DOI] [PubMed] [Google Scholar]
- Pattyn S. R., De Vleesschauwer L. The multiplication of Middleburg s and 1 plaque viruses in Aëdes aegypti mosquitoes. Acta Virol. 1968 Jul;12(4):347–354. [PubMed] [Google Scholar]
- Peleg J. Growth of viruses in arthropod cell cultures: applications. I. Attenuation of Semliki Forest (SF) virus in continuously cultured Aedes aegypti mosquito cells (Peleg) as a step in production of vaccines. Curr Top Microbiol Immunol. 1971;55:155–161. doi: 10.1007/978-3-642-65224-0_26. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwöbel W., Ahl R. Peristence of sindbis virus in BHK-21 cell cultures. Arch Gesamte Virusforsch. 1972;38(1):1–10. doi: 10.1007/BF01241350. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Stollar V. Defective-interfering particles of Sindbis virus. I. Isolation and some chemical and biological properties. Virology. 1973 May;53(1):162–173. doi: 10.1016/0042-6822(73)90475-3. [DOI] [PubMed] [Google Scholar]
- Simizu B., Takayama N. Relationship between neurovirulence and temperature sensitivity of an attenuated western equine encephalitis virus. Arch Gesamte Virusforsch. 1971;35(2):242–250. doi: 10.1007/BF01249716. [DOI] [PubMed] [Google Scholar]
- Sinarachatanant P., Olson L. C. Replication of dengue virus type 2 in Aedes albopictus cell culture. J Virol. 1973 Aug;12(2):275–283. doi: 10.1128/jvi.12.2.275-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E. Homologous viral interference in Aedes albopictus cultures chronically infected with Sindbis virus. J Virol. 1973 Apr;11(4):592–595. doi: 10.1128/jvi.11.4.592-595.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E., Stollar B. D. Double-stranded RNA in hamster, chick, and mosquito cells infected with Sindbis virus. Virology. 1972 Jan;47(1):122–132. doi: 10.1016/0042-6822(72)90245-0. [DOI] [PubMed] [Google Scholar]
- Waite M. R. Protein synthesis directed by an RNA temperature-sensitive mutant of Sindbis virus. J Virol. 1973 Feb;11(2):198–206. doi: 10.1128/jvi.11.2.198-206.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


