Abstract
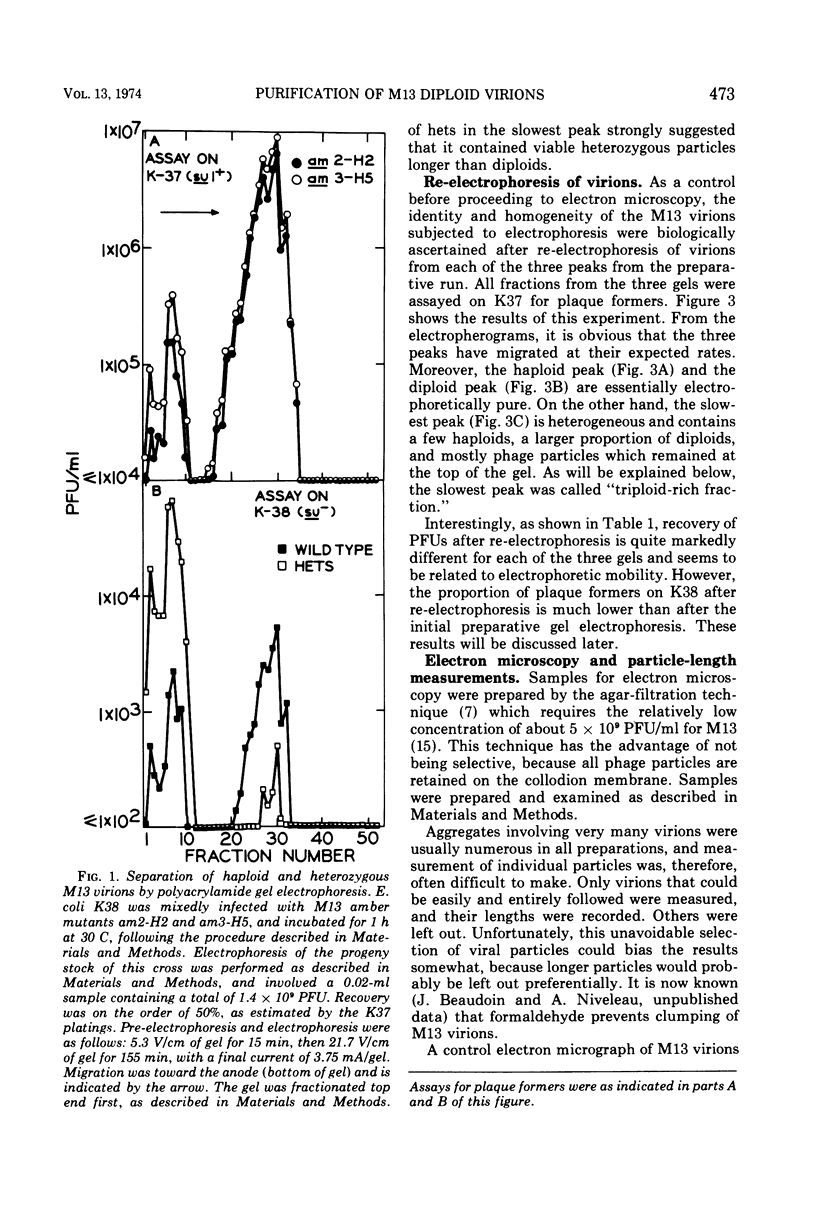
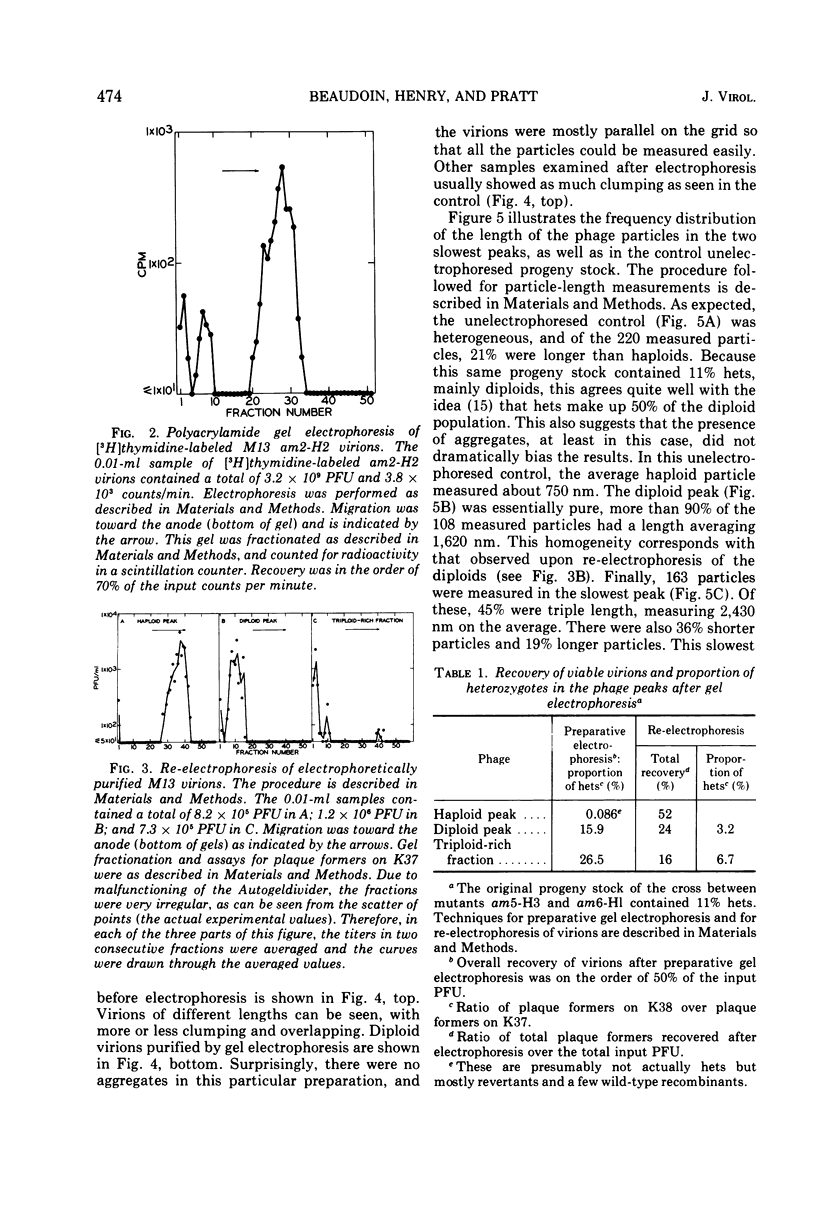
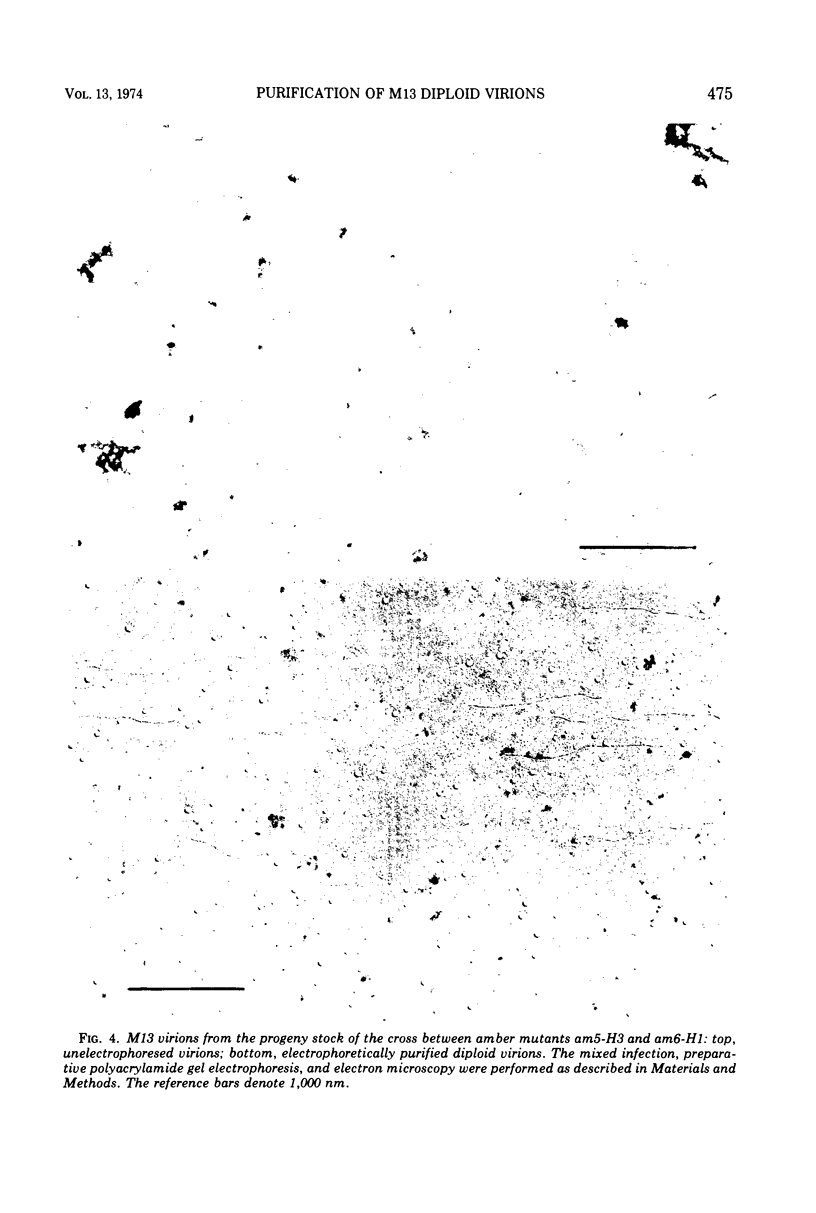
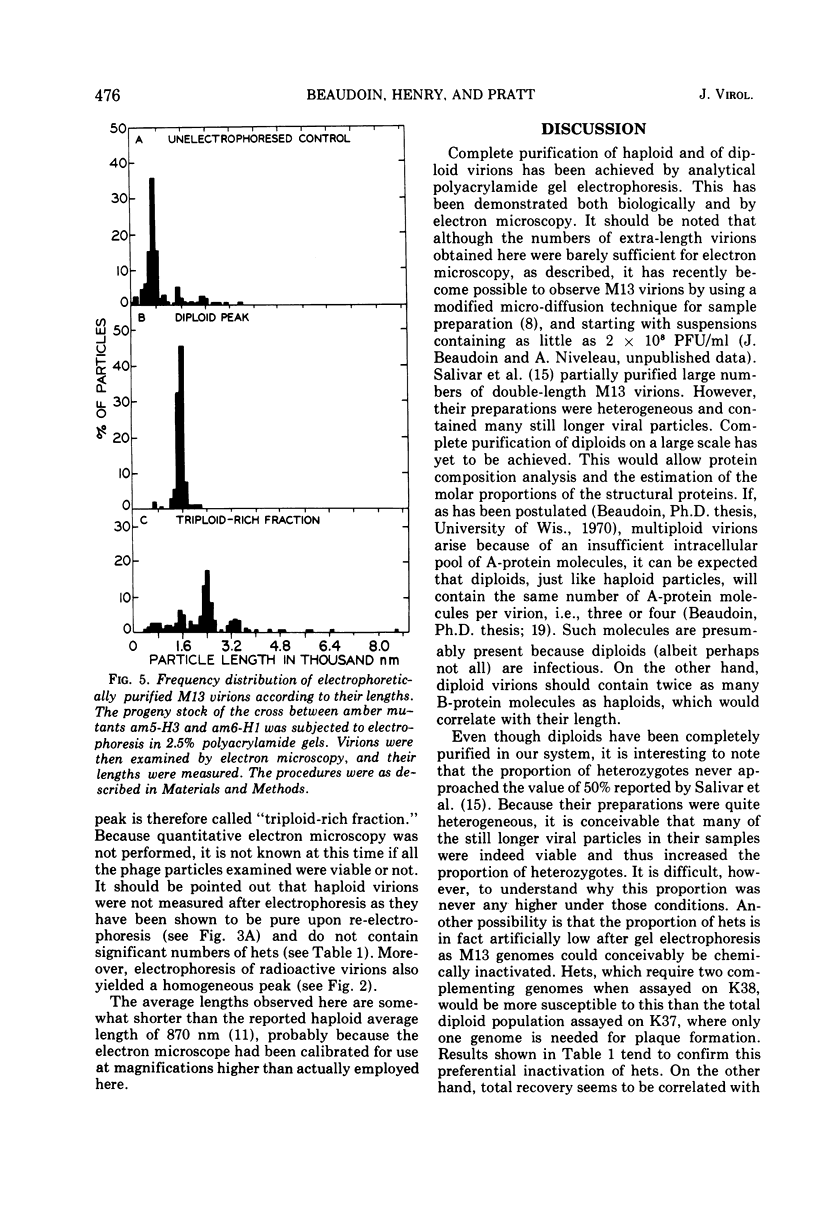
Complete separation of single- and double-length M13 virions from each other and from still-longer phage particles was achieved by electrophoresis of phage stocks through 2.5% polyacrylamide gels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., MORSE M. L. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. VI. EFFECTS ON BACTERIAL CONJUGATION. Genetics. 1965 Jan;51:137–148. doi: 10.1093/genetics/51.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY D. E. THE STRUCTURE OF SOME BACTERIOPHAGES ASSOCIATED WITH MALE STRAINS OF ESCHERICHIA COLI. J Gen Microbiol. 1964 Jun;35:471–482. doi: 10.1099/00221287-35-3-471. [DOI] [PubMed] [Google Scholar]
- Beaudoin J., Pratt D. Antiserum inactivation of electrophoretically purified M13 diploid virions: model for the F-specific filamentous bacteriophages. J Virol. 1974 Feb;13(2):466–469. doi: 10.1128/jvi.13.2.466-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R. Restriction of infectious bacteriophage fd DNA's and an assay for in vitro host-controlled restriction and modification. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1294–1299. doi: 10.1073/pnas.59.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjertén S., Jerstedt S., Tiselius A. Some aspects of the use of "continuous" and "discontinuous" buffer systems in polyacrylamide gel electrophoresis. Anal Biochem. 1965 May;11(2):219–223. doi: 10.1016/0003-2697(65)90008-4. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A. Use of tetrazolium for improved resolution of bacteriophage plaques. J Bacteriol. 1966 Sep;92(3):787–788. doi: 10.1128/jb.92.3.787-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Henry T. J., Pratt D. Purification and properties of diploid particles of coliphage M13. Virology. 1967 May;32(1):41–51. doi: 10.1016/0042-6822(67)90250-4. [DOI] [PubMed] [Google Scholar]
- Simon E. H. The distribution and significance of multiploid virus particles. Prog Med Virol. 1972;14:36–67. [PubMed] [Google Scholar]
- Wiseman R. L., Dunker A. K., Marvin D. A. Filamentous bacterial viruses. 3. Physical and chemical characterization of the If1 virion. Virology. 1972 Apr;48(1):230–244. doi: 10.1016/0042-6822(72)90130-4. [DOI] [PubMed] [Google Scholar]



