Abstract
Melanoma is the most serious type of skin cancer with a high potential for metastasis and very low survival rates. The discovery of constitutive activation of the BRAF kinase caused by activating BRAF(V600E) kinase mutation in most melanoma patients led to the discovery of the first potent BRAF(V600E) signaling inhibitor, vemurafenib. Vemurafenib was effective in treating advanced melanoma patients and was proposed for the treatment of other BRAF(V600E) mutant cancers as well. Unfortunately, the success of vemurafenib was hampered by the rapid development of acquired resistance in different types of BRAF(V600E) mutant cancer cells. It becomes important to identify and evaluate all of the potential mechanisms of cellular resistance to vemurafenib. In this study, we characterized the interactions of vemurafenib with three major ATP-binding cassette (ABC) transporters, ABCB1, ABCC1 and ABCG2. We found that vemurafenib stimulated the ATPase activity and potently inhibited drug efflux mediated by ABCB1 and ABCG2. Vemurafenib also restored drug sensitivity in ABCG2-overexpressing cells. Moreover, we revealed that in the presence of functional ABCG2, BRAF kinase inhibition by vemurafenib is reduced in BRAF(V600E) mutant A375 cells. Taken together, our findings indicate that ABCG2 confers resistance to vemurafenib in A375 cells, suggesting involvement of this transporter in acquired resistance to vemurafenib. Thus, combination chemotherapy targeting multiple pathways could be an effective therapeutic strategy to overcome acquired resistance to vemurafenib for cancers harboring the BRAF(V600E) mutation.
Keywords: ABC proteins, ABCG2, multidrug resistance, BRAF(V600E) mutant, vemurafenib
1. Introduction
Melanoma is the most serious type of skin cancer and one of the most common cancers in the world [1]. Melanoma has a high potential for metastasis, and metastatic melanoma has a poor prognosis and very low survival rates [2]. The RAS activated BRAF kinase plays a central role in the regulation of mitogen-activated protein kinase (MAPK) signaling pathway, which regulates cell division, proliferation and differentiation in melanoma, and thus is considered a promising target for anti-melanoma therapies [3, 4]. Moreover, since constitutive activation of the BRAF kinase and downstream MAPK signaling caused by activating BRAF(V600E) kinase mutations is common in melanomas, inhibiting BRAF(V600E) signaling can significantly improve outcomes for melanoma patients [4–6].
Vemurafenib (PLX4032, Zelboraf) is a potent inhibitor of mutant BRAF(V600E) kinase that was recently approved by the US Food and Drug Administration for treatment of metastatic and unresectable melanomas that carry an activating BRAF(V600E) mutation [7–9]. As a single therapeutic agent, vemurafenib exhibited robust efficacy with an excellent anti-tumor response rate and improved overall survival in patients with metastatic melanoma [10, 11]. Although vemurafenib is currently approved to treat unresectable BRAF(V600E) mutant melanomas [7], other types of cancer such as colorectal cancer [12, 13] and papillary thyroid cancer [14] harboring the same BRAF(V600E) mutation, can also benefit from the same therapy. Unfortunately, the success of vemurafenib was short-lived. Acquired drug resistance and relapse among patients were reported frequently within months of therapy [10, 11]. Like most cancer chemotherapies, overcoming acquired clinical resistance to vemurafenib presents a significant therapeutic challenge. Recent studies have identified multiple mechanisms that reactivate the MAPK pathway in vemurafenib resistant BRAF(V600E) mutant cancer cells. These mechanisms include CRAF upregulation [15, 16], Tpl2/COT overexpression [15], Ras activation [17, 18], enhanced activation of FGFR3/Ras pathway [19], and pathways that lead to reactivation of ERK signaling [20]. In addition, activation of RTK signaling pathways such as IGF-1R or PDGFRβ was identified to play a role in vemurafenib resistance as well [17, 21, 22]. Interestingly, the response rate and mechanism of acquired resistance to vemurafenib appear to vary widely dependent on cancer type [14, 23].
Drug resistance has long been a serious problem associated with chronic treatment with anti-cancer drugs. The most common mechanism for acquired resistance in cancer chemotherapy is associated with the overexpression of three members of the ATP-binding cassette (ABC) transporter family: ABCB1, ABCC1 and ABCG2 [24]. Collectively, these drug transporters efflux a wide range of anticancer drug directly out of cancer cells, rendering chemotherapy ineffective [24]. Therefore, it is crucial to study the potential pharmacological and biochemical interactions between vemurafenib and these three major ABC drug transporters. Moreover, we explored whether ectopic overexpression of ABC drug transporters could lead to acquired resistance to vemurafenib in cancer cells harboring the BRAF(V600E) mutation.
In the present study, we showed that interaction of vemurafenib with the substrate binding pockets of ABCB1 and ABCG2 led to inhibition of drug transport. We discovered that vemurafenib has a higher binding affinity towards ABCG2, and it was able to reverse resistance to topotecan and mitoxantrone in ABCG2-overexpressing cell lines. Furthermore, by using BRAF(V600E) mutant A375 melanoma cell line transfected with wild-type human ABCG2, we demonstrated that ABCG2 confers resistance to vemurafenib and consequently reduces the inhibition of BRAF(V600E) kinase. Together, these results provide new insights into the pharmacological interactions between vemurafenib and ABC drug transporters, as well as reveal an additional resistance mechanism in cancer cells harboring a BRAF(V600E) mutation.
2. Materials and Methods
2.1. Cell cultures and compounds
pcDNA3.1-HEK293, R482-HEK293, MDR19-HEK293, MRP1-HEK293, KB-3-1, KB-V1, MCF7, MCF7-AdVp3000, MCF7-FLV1000, A375 and A375-G2 cells were all cultured in DMEM (Gibco, Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine and 100 units of penicillin/streptomycin/mL at 37°C in 5% CO2 humidified air. All HEK293 and HEK293 transfected lines were maintained in 2 mg/mL G418 [25]; MCF7-AdVp3000 (T482-ABCG2) cells were maintained in the presence of 3 µg/mL doxorubicin and 5 µg/ml verapamil, and MCF7-FLV1000 (R482-ABCG2) cells were cultured in the presence of 1 µg/mL flavopiridol [26, 27], whereas vinblastine was added to KB-V1 cells [28]. CORL-23/P, CORL-23/R, S1 and S1-M1-80 cells were cultured in RPMI-1640 (Gibco, Invitrogen), supplemented with 10% FCS, 2 mM L-glutamine and 100 units of penicillin/streptomycin/mL at 37°C in 5% CO2 humidified air. The CORL-23/R cells were maintained in the presence of 0.2 µg/mL of doxorubicin, whereas S1-M1-80 cells were cultured in 80 µM of mitoxantrone as described previously [25]. Mitoxantrone, MTT dye, FTC, doxorubicin, etoposide and all other chemicals were purchased from Sigma (St. Louis, MO, USA). Tariquidar was purchased from MedKoo Biosciences Inc (Chapel Hill, NC). Vemurafenib (PLX4032) was purchased from LC Laboratories (Woburn, MA, USA). [125I]-Iodoarylazidoprazosin (IAAP) (2200 Ci/mmol) was from Perkin-Elmer Life Sciences.
2.2. Generation of A375-G2 cell line
ABCG2-expressing A375 human malignant melanoma (denoted A375-G2) cells were generated by transfection with plasmid containing human wild-type ABCG2, the cDNA from A549 Bec cells were PCR amplified with primers 5’-GCGGGATCCATGTCTTCCAGTAATGTCGAAG-3’ and 5’-ATCAAGCTTCTGAATTAAGGGGAAATTTAAG-3’, then the DNA fragments were digested with BamHI and HindIII, cloned into the BglII/ HindIII sites of pEGFP-C1 vector (Clontech, Mountain View, CA, USA). Transfection was done with the lipofectamine 2000 (Invitrogen) according the instruction of manufacturer. The transfected A375 cells were subjected to FASCAria (Becton Dickinson, Franklin Lakes, NJ, USA) sorting for GFP positive cells, confirmed by drug sensitivity and Western blotting. Stably transfected cells were selected at 2 mg/ml G418 and maintained in complete medium containing with 0.5 mg/ml G418.
2.3. Fluorescent drug accumulation assay
Efflux assays were carried out using a FACSort flow cytometer equipped with Cell Quest software (Becton-Dickinson) as described previously [29]. The effect of vemurafenib and FTC on ABCG2-mediated efflux of pheophorbide A (PhA) and mitoxantrone was measured and analyzed as described previously [25]
2.4. ATPase assay and photoaffinity labeling of ABCB1 and ABCG2 with [125I]IAAP
ATPase activity of ABCB1 and ABCG2 in High-Five cell crude membranes was measured by endpoint Pi assay as described previously [30]. ABCB1- and ABCG2-specific ATPase activities were recorded as vanadate (Vi)-sensitive ATPase activity. For photoaffinity labeling assays, crude membranes (240 µg protein/mL) made from the MCF7-FLV1000 cells (ABCG2-overexpressing cells) or (500 µg protein/mL) membrane vesicles of High-Five cells expressing ABCB1 were prepared as described previously [30]. Crude membranes were incubated with varying concentrations of vemurafenib for 10 min at room temperature in 50 mM Tris-HCl, pH 7.5, and then 3–6 nmol/L [125I]IAAP (2200 Ci/mmole) was added. The samples were then processed as described previously [25].
2.5. Cytotoxicity assay
Cell Counting Kit-8 (CCK) and MTT assays were used to determine the general sensitivities of cells to the tested chemicals as described previously [25].
2.6. Immunoblotting
The following antibodies were used for Western blot immunoassay: C219 (1:1000), anti-MRP1 (1:1000), BXP-21 (1:500) and anti-α-tubulin (1:2000). The secondary antibody used was the Horseradish peroxidase-conjugated goat anti-mouse IgG (1:10,000). Signals were detected as described previously [25].
2.7. Measurement of BRAF kinase activity in BRAF(V600E) mutant cells
A375 and A375-G2 cells were treated with increasing concentrations (0.2 – 5.0 µM) of vemurafenib for 4 h. BRAF kinase activity was measured in total cell lysates by detecting the levels of total MEK1/2, p-MEK1/2, total ERK1/2 or p-ERK1/2 using MEK1/2, p-MEK1/2(S217/221), ERK1/2 or p-ERK1/2(T202/Y204) antibodies, respectively (Cell Signaling Technologies (Danvers, MA, USA) according to the manufacturer's recommendations.
2.8. Statistical analysis
Data are presented as mean ± S.E.M, whereas IC50 values were calculated as mean ± SD from at least three independent experiments. Differences between any mean values were analyzed by two-sided Student’s t-test and results were considered statistically significant at P < 0.05.
3. Results
3.1 Vemurafenib inhibits substrate transport mediated by ABCB1 and ABCG2
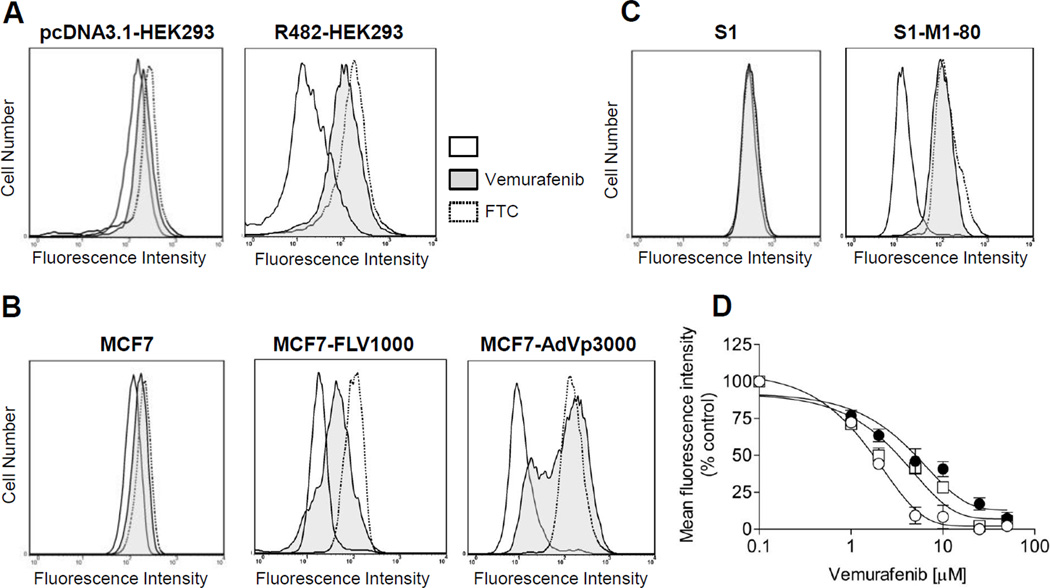
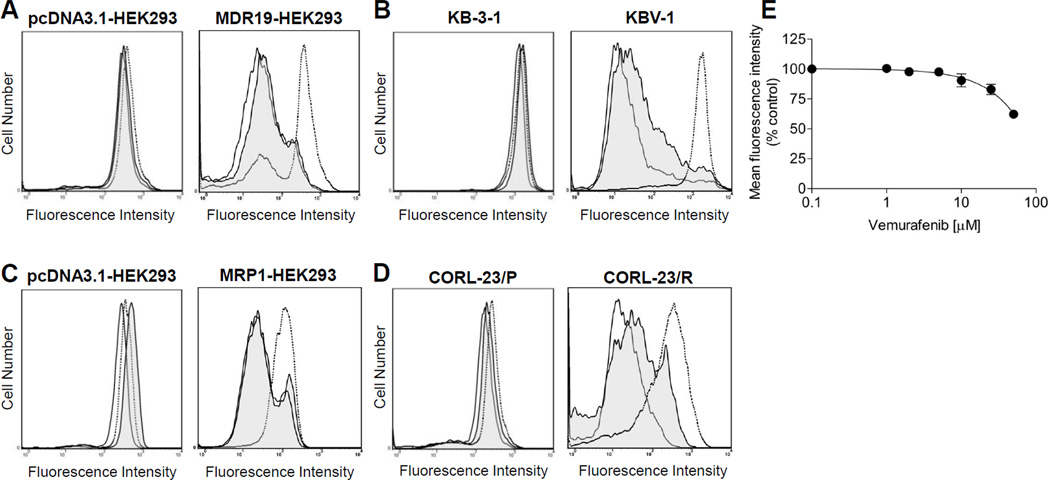
To determine whether vemurafenib interacts with major ABC transporters, we evaluated the inhibitory effect of vemurafenib on ABCB1, ABCC1 and ABCG2-mediated drug efflux in short-term drug accumulation assays. The efflux of fluorescent substrates (calcein-AM for ABCB1 and ABCC1, PhA and mitoxantrone for ABCG2) from cells overexpressing each respective ABC transporter was measured as detailed in Materials and Methods. ABCG2-mediated efflux of PhA was inhibited significantly by vemurafenib (2 µM) in ABCG2-overexpressing HEK293 (Fig. 1A), MCF7-FLV1000 (wild type R482) (Fig. 1B, middle panel), MCF7-AdVp3000 (R482T) (Fig 1B, right panel) and S1-M1-80 (R482G) (Fig. 1C) MDR cancer cell lines. In addition, we examined the effect of vemurafenib on ABCG2-mediated transport of anti-cancer drug mitoxantrone. Vemurafenib inhibited ABCG2-mediated efflux of mitoxantrone from MCF7-FLV1000, MCF7-AdVp3000 and S1-M1-80 cells in a concentration dependent manner, with calculated IC50 values of 1.24, 4.91 and 2.60 µM, respectively. (Fig. 1D). In contrast, vemurafenib at 2 µM had no significant effect on drug efflux mediated by either ABCB1 in MDR19-HEK293 (Fig. 2A) and KBV-1 (Fig.2B) or by ABCC1 in MRP1-HEK293 (Fig. 2C) and CORL-23/R (Fig. 2D). At higher concentrations, vemurafenib did inhibit ABCB1-mediated transport of calcein-AM, but with low affinity of IC50 > 50 µM (Fig. 2E). FTC (5 µM), tariquidar (3 µM) and MK-571 (25 µM) were used in the assay as specific inhibitors for ABCG2, ABCB1 and ABCC1, respectively. Moreover, vemurafenib (up to 50 µM) had no effect on the accumulation of fluorescent probes in any of the drug sensitive parental cells tested.
Fig. 1.
Effect of vemurafenib on ABCG2-mediated transport of PhA or mitoxantrone. The levels of accumulated fluorescent PhA in ABCG2-overexpressing (A) R482-HEK293, (B) MCF7-FLV1000 (wild-type), MCF7-AdVp3000 (R482T), (C) S1-M1-80 (R482G) cells and drug-sensitive parental cells or (D) concentration-dependent inhibition of ABCG2-mediated efflux of mitoxantrone by increasing concentrations of vemurafenib in MCF7-FLV1000 (○), MCF7-AdVp3000 (●) and S1-M1-80 (□) cells. Solid lines represent cells in the absence of inhibitor, shaded solid lines represent cells in the presence of 2 µM vemurafenib, whereas dotted lines represent cells in the presence of 5 µM FTC. Representative histograms of three independent experiments are shown. Data points represent the mean ± SEM from three independent experiments..
Fig. 2.
Effect of vemurafenib on the fluorescent substrate transport mediated by ABCB1 or ABCC1. The levels of accumulated fluorescent substrate calcein-AM in (A) ABCB1-transfected MDR19-HEK293, (B) ABCB1-overexpressing KBV-1, as well as (C) ABCC1-transfected MRP1-HEK293 cells and (D) ABCC1-overexpressing CORL-23/R and their respective drug-sensitive parental cells were measured in the presence or absence of vemurafenib or known inhibitors. Cells were first resuspended in IMDM supplemented with 5% FBS before addition of 0.25 µM calcein-AM in the presence or absence of vemurafenib or known inhibitors. ABCB1- and ABCC1-overexpressing cells were incubated at 37°C in the dark for 10 minutes before pelleted by centrifugation at 500×g, resuspended in 300 µL PBS containing 0.1% FBS and analyzed immediately by flow cytometry as described in Materials and Methods. Solid lines represent cells in the absence of vemurafenib or inhibitors, shaded solid lines represent cells in the presence of 2 µM vemurafenib, whereas dotted lines represent cells in the presence of known inhibitors (3 µM tariquidar for ABCB1 cells and 25 µM MK-571 for ABCC1 cells). Representative histograms of three independent experiments are shown. (E) Concentration-dependent inhibition of ABCB1-mediated efflux calcein-AM by vemurafenib in ABCB1-overexpressing KBV-1 cells. Data points represent the mean ± SEM from three independent experiments. The IC50 values were calculated as the concentration that inhibited the efflux to 50% of the control values.
3.2 Vemurafenib inhibits [125I]IAAP photoaffinity labeling and stimulates ATPase activity of ABCB1 and ABCG2
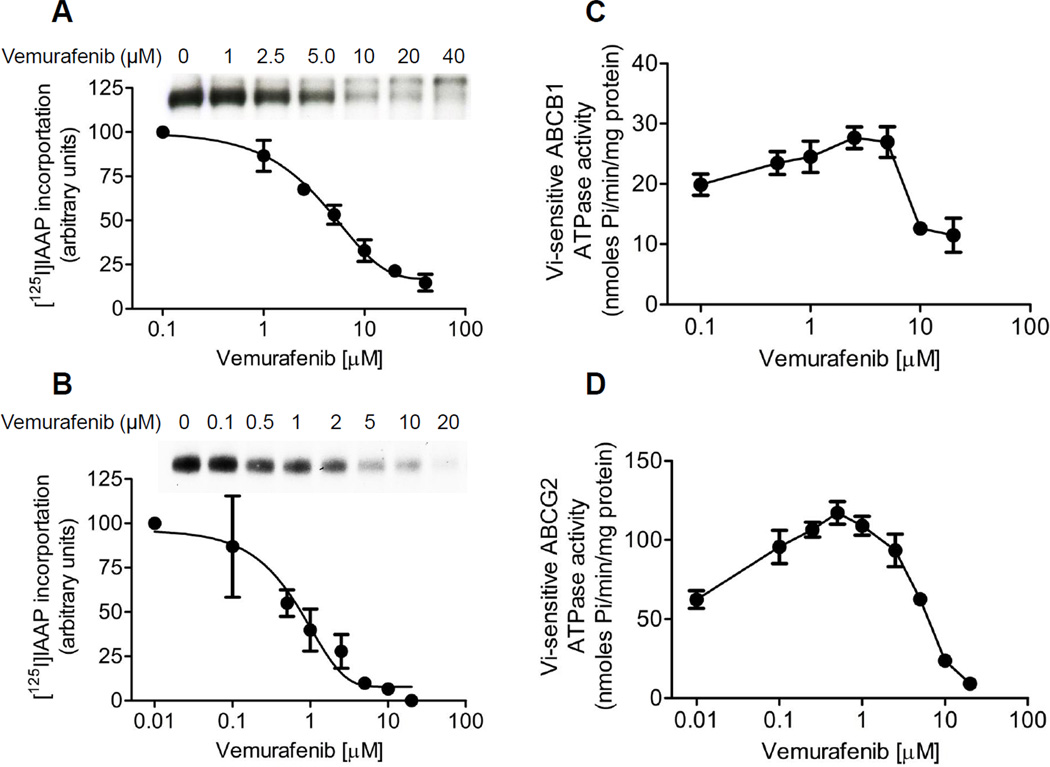
Next, we examined the effect of vemurafenib on [125I]IAAP photoaffinity labeling and ATPase activities of ABCB1 and ABCG2 as described in Materials and Methods. [125I]IAAP is a transport substrate that binds directly to the substrate binding sites of both ABCB1 and ABCG2 [31]. Therefore, any substrate or inhibitor that binds to the same site will inhibit the photolabeling [25, 32]. As seen in Fig. 3, the photoaffinity labeling of [125I]IAAP to both ABCB1 and ABCG2 was inhibited by vemurafenib in a concentration dependent manner, with calculated IC50 values of 4.51 µM for ABCB1 and 0.57 µM for ABCG2, respectively (Fig. 3A and 3B). This data confirmed that vemurafenib inhibited ABCB1- and ABCG2-mediated transport (Fig. 1 and 2) by binding at the substrate-binding sites of ABCB1 and ABCG2. Furthermore, we examined the effect of vemurafenib on the Vi-sensitive ATPase activity in crude membranes isolated from cells expressing either ABCB1 or ABCG2, as described in Materials and Methods. Vemurafenib had a biphasic effect on both ABCB1 and ABCG2 ATP hydrolysis, as it stimulated the ATPase activity at lower concentrations, but inhibited the activity at higher concentrations. At lower concentrations, vemurafenib stimulated the ATPase activity of ABCB1 and ABCG2 by ~ 40% and ~ 95% higher than the respective basal levels, and inhibited by ~ 50% and ~ 90% at higher concentrations (Fig. 3C and 3D). The data here confirmed a considerably stronger interaction between vemurafenib and ABCG2, which is in agreement with data of the drug efflux assays (Fig. 1).
Fig. 3.
Effect of vemurafenib on [125I]IAAP photoaffinity labeling and ATPase activity of (A) ABCB1 and (B) ABCG2 was carried out and quantified as previously described. [25] Representative autoradiograms from one of the three independent experiments were shown. Panels, position of the ABCB1 (A) and ABCG2 (B) bands. Vemurafenib-stimulated ATPase activity of (C) ABCB1or (D) ABCG2 was recorded as Vi-sensitive ATPase activity, as described previously [25]. Pointsmean from at least three independent experiments; bars, SE.
3.3 Overexpression of ABC drug transporters does not confer resistance to vemurafenib in BRAF wild-type cancer cells
Next, we examined the cytotoxicity of vemurafenib in various drug-sensitive, MDR, BRAF wild-type and BRAF(V600E) mutant cancer cell lines. The IC50 values of vemurafenib in these human cancer cell lines were calculated and summarized in Table 1. The fold-resistance is represented by the resistance factor (R.F), which is the degree of reduced drug sensitivity caused by the overexpression of a particular ABC transporter. This value was calculated by dividing the IC50 value of respective drug resistant subline by the IC50 value of the drug sensitive parental line. We found no significant differences in R.F values in ABC drug transporter-positive BRAF wild-type MDR cancer cell lines in respect to their parental drug sensitive lines. On the other hand, BRAF(V600E) mutant Colo-205 and A375 cancer cells were much more sensitive to vemurafenib than BRAF wild-type cancer cells, as expected. It is also worth noting that breast adenocarcinoma MCF7 cells appeared to be intrinsically less sensitive to vemurafenib than all other cancer cell lines expressing wild-type BRAF.
Table 1.
Sensitivity of various human cancer cells to BRAF(V600E) kinase inhibitor vemurafenib.
| Cell line | Cancer origin | Transporter expressed | BRAF V600E mutation status | IC50 (µM)† | R.F‡ |
|---|---|---|---|---|---|
| KB-3–1 | epidermal | - | negative | 14.77 ± 4.19 | - |
| KBV-1 | epidermal | ABCB1 | negative | 16.92 ± 4.97 | 1.15 |
| CORL-23/P | lung | - | negative | 8.52 ± 1.39 | - |
| CORL-23/R | lung | ABCC1 | negative | 8.54 ± 2.25 | 1.00 |
| MCF7 | breast | - | negative | 53.55 ± 9.42 | - |
| MCF7-FLV1000 | breast | ABCG2 (R) | negative | 58.70 ± 15.78 | 1.10 |
| MCF7-AdVp3000 | breast | ABCG2 (T) | negative | 69.99 ± 27.35 | 1.31 |
| S1 | colon | - | negative | 13.65 ± 3.84 | - |
| S1-M1-80 | colon | ABCG2 (G) | negative | 14.70 ± 3.02 | 1.08 |
| Colo-205 | colon | - | positive | 0.118 ± 0.011 | - |
| A375 | melanoma | - | positive | 0.069 ± 0.010 | - |
Abbreviation: RF, resistance factor.
IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and Methods.
RF were calculated by dividing IC50 values of ABC transporter overexpressing cells by IC50 values of respective parental cells.
3.4 Vemurafenib reverses MDR in cells overexpressing ABCG2
We next evaluated the ability of vemurafenib to restore drug sensitivity in cells overexpressing either ABCB1, ABCC1 or ABCG2. The relative resistance (R.R) was calculated by dividing the IC50 value of HEK293 cells transfected with respective ABC transporter by the IC50 value of the parental HEK293 cells in the presence of a particular inhibitor. A maximum non-toxic re-sensitization concentration of 5 µM vemurafenib was chosen based on the cytotoxicity curves, and the MDR reversal effect of vemurafenib in HEK293 cells expressing either ABCB1, ABCC1 or ABCG2 is shown in Table 2. Tariquidar, verapamil and FTC were used as positive controls to reverse drug resistance conferred by ABCB1 or ABCC1 or ABCG2, respectively [33]. Vemurafenib had no significant effect on either ABCB1-mediated doxorubicin resistance or ABCC1-mediated etoposide resistance. In contrast, vemurafenib was able to restore sensitivity of ABCG2-expressing R482-HEK293 cells to mitoxantrone and topotecan, two well-established anticancer drug substrates of ABCG2, in a concentration-dependent manner. At 5 µM, vemurafenib re-sensitized ABCG2-overexpressing R482-HEK293 cells to mitoxantrone and topotecan by ~6-fold and ~9-fold, respectively. The reversal effect by vemurafenib was comparable to the benchmark ABCG2 inhibitor FTC at 3 µM.
Table 2.
Chemosensitizing effect of vemurafenib on ABC transporter-mediated drug resistance in HEK293 cells and BRAF(V600E) mutant A375 human malignant melanoma cells.
| Treatment | Concentration (µM) | IC50 (nM)† |
R.R‡ | |
|---|---|---|---|---|
| pcDNA-HEK293 | R482-HEK293 (ABCG2) | |||
| Vemurafenib | - | 28.14 ± 6.93 | 27.91 ± 2.15 | 1 |
| Mitoxantrone | - | 5.30 ± 1.24 | 177.59 ± 19.03 | 34 |
| +Vemurafenib | 1 | 5.73 ± 1.33 | 161.62± 41.38 | 28 |
| +Vemurafenib | 2 | 5.27 ± 0.96 | 116.06 ± 37.66 | 22 |
| +Vemurafenib | 5 | 5.13 ± 1.56 | 30.18 ± 15.20*** | 6 |
| +FTC | 3 | 5.82± 1.32 | 29.37 ± 11.52*** | 5 |
| Topotecan | - | 18.27 ± 4.48 | 510.36 ± 94.45 | 28 |
| + Vemurafenib | 1 | 14.88 ± 4.13 | 288.67 ± 79.57* | 19 |
| + Vemurafenib | 2 | 13.59± 3.38 | 206.58 ± 56.25** | 15 |
| + Vemurafenib | 5 | 18.70 ± 5.72 | 56.24 ± 27.75** | 3 |
| +FTC | 3 | 13.83 ± 4.02 | 39.41 ± 9.83** | 3 |
|
IC50 (nM)† |
||||
| A375 | A375-G2 (ABCG2) | |||
| Mitoxantrone | - | 2.35 ± 0.64 | 14.99 ± 3.48 | 6 |
| + FTC | 3 | 2.30 ± 0.36 | 5.42 ± 2.41* | 2 |
| + Vemurafenib | 0.02 | 3.30± 1.29 | 6.58 ± 2.00* | 2 |
| Vemurafenib | - | 69.31 ± 10.09 | 214.63 ± 32.84 | 3 |
| + FTC | 3 | 71.66± 19.35 | 70.31 ± 17.88** | 1 |
|
IC50 (nM)† |
||||
| pcDNA-HEK293 | MDR19-HEK293 (ABCB1) | |||
| Vemurafenib | - | 28.14 ± 6.93 | 37.97 ± 4.97 | 1 |
| Doxorubicin | - | 18.46 ± 8.31 | 593.89 ± 78.02 | 32 |
| +Vemurafenib | 1 | 23.99 ± 9.10 | 561.87 ± 113.06 | 23 |
| +Vemurafenib | 2 | 20.88 ± 9.69 | 470.11 ± 98.48 | 23 |
| +Vemurafenib | 5 | 18.87 ± 7.59 | 411.19 ± 143.67 | 22 |
| +Tariquidar | 1 | 17.24 ± 8.71 | 49.86 ± 16.53 | 3 |
|
IC50 (µM)† |
||||
| pcDNA-HEK293 | MRP1-HEK293 (ABCC1) | |||
| Vemurafenib | - | 28.14 ± 6.93 | 21.76 ± 2.56 | 0.8 |
| Etoposide | - | 0.217 ± 0.072 | 34.87 ± 4.03 | 161 |
| +Vemurafenib | 1 | 0.215 ± 0.038 | 34.29 ± 4.33 | 159 |
| +Vemurafenib | 2 | 0.216 ± 0.047 | 35.73 ± 6.87 | 165 |
| +Vemurafenib | 5 | 0.224 ± 0.049 | 25.88 ± 8.52 | 116 |
| +Verapamil | 5 | 0.121 ± 0.024 | 8.68 ± 1.86*** | 72 |
Abbreviation: RR, relative resistance.
IC50 values are mean ± SD in the presence and absence of vemurafenib or other tested compounds. The IC50 values were calculated from dose-response curves obtained from three independent experiments.
Relative resistance (R.R) values were obtained by dividing IC50 values of ABC transporter-overexpressing cancer cells by IC50 values of respective sensitive cells.
P < 0.05;
P < 0.01;
P < 0.001
3.5 Vemurafenib has no effect on ABCB1, ABCC1 or ABCG2 protein expression level
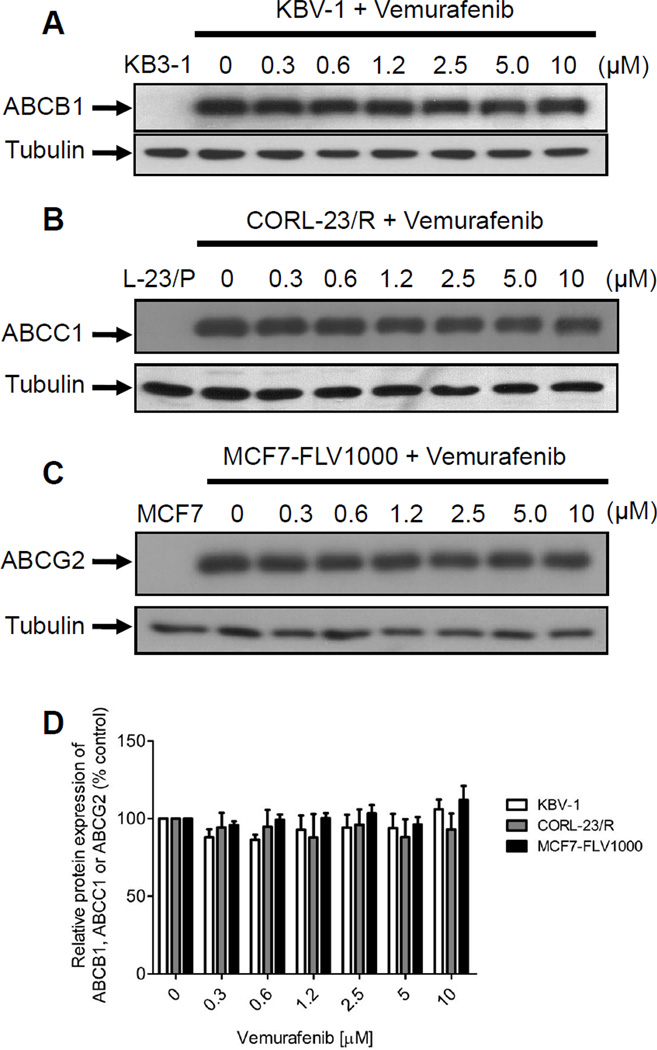
Drug-induced up-regulation or down-regulation of ABC drug transporters can potentially affect the drug sensitivity of cancer cells to chemotherapeutics. Therefore, we examined whether vemurafenib can affect the protein expression of ABCB1, ABCC1 or ABCG2 in MDR cancer cells by interfering with transcriptional or translational processes. Briefly, ABCB1-overexpressing KBV-1 (Fig. 4A), ABCC1-overexpressing CORL-23/R (Fig. 4B) and ABCG2-overexpressing MCF7-FLV1000 (Fig. 4C) cancer cells were incubated in increasing concentrations of vemurafenib (0.3 µM-10 µM) for 72 h and processed by immunoblotting as described in Materials and Methods. Results showed that vemurafenib has no significant effect on the protein expression level of either ABC drug transporters in these MDR cancer cell lines. This data also confirmed that vemurafenib reversed ABCG2-mediated MDR by inhibiting the function of ABCG2, and not by downregulating the protein level of this transporter.
Fig. 4.
Effect of vemurafenib on protein expression of ABCB1, ABCC1 and ABCG2 in human MDR cancer cells. Immunoblot detection of total cell lysate (10 µg) of (A) ABCB1 in parental KB3-1 (lane 1) and ABCB1-overexpressing subline KBV-1 (lane 2-7) cells, (B) ABCC1 in parental CORL-23/P (lane 1) and its ABCC1-overexpressing subline CORL-23/R (lane 2–7) cells, and (C) ABCG2 in parental MCF7 (lane 1) and ABCG2-overexpressing subline MCF7-FLV1000 (lane 2–7) cells, treated with increasing concentrations (0–10 µM) of vemurafenib for 72 h, as described previously[25]. α-tubulin was used as an internal control for equal loading. Representative blots from three independent experiments were shown. (D) The averaged relative expression levels of ABCB1 in KBV-1 cells (open bars), ABCC1 in CORL-23/R cells (shaded bars) and ABCG2 in MCF7-FLV1000 cells (closed bars) in the absence and presence of increasing concentrations of vemurafenib. Values are presented as mean ± SD calculated from three independent experiments.
3.6 Functional ABCG2 confers resistance to mitoxantrone and vemurafenib in BRAF(V600E) mutant human A375 melanoma cells
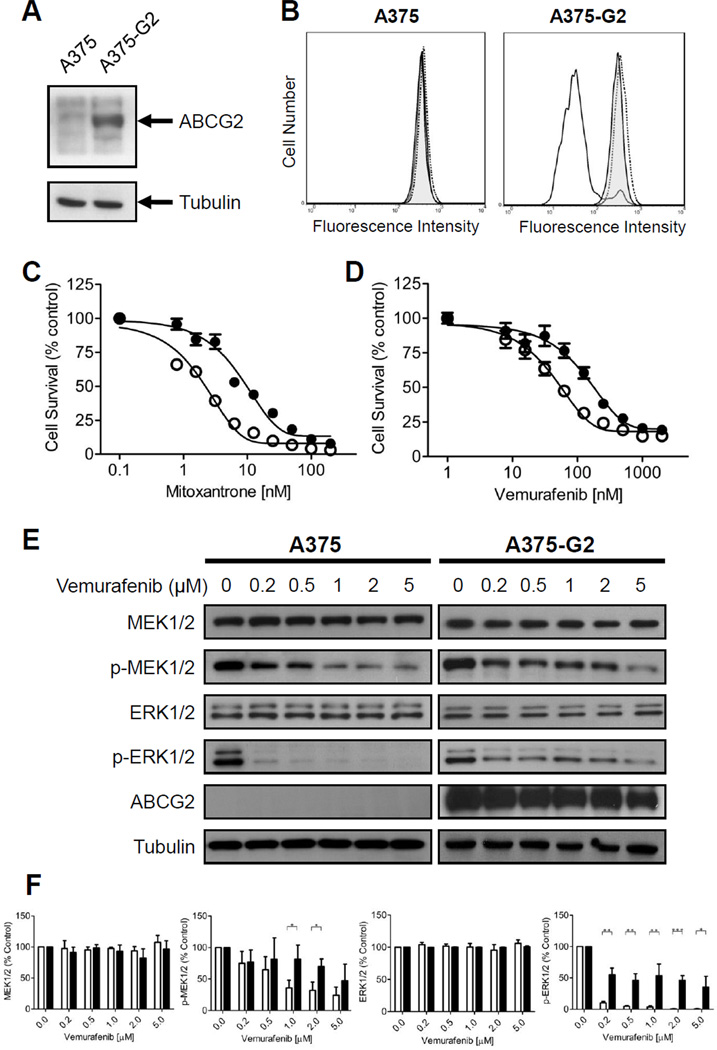
Collectively, our data indicates that vemurafenib interacts with ABCG2 with high affinity and stimulates ABCG2 ATP hydrolysis in the same manner as other well-established ABCG2 substrates. Therefore, we examined whether the overexpression of ABCG2 can lead to vemurafenib resistance in BRAF(V600E) mutant cancer cells. In order to test our hypothesis, we transfected human BRAF(V600E) mutant A375 melanoma cells with human wild-type R482 ABCG2 protein (denoted A375-G2) as detailed in Materials and Methods. Western blotting showed significantly higher ABCG2 protein expression in A375-G2 than in parental A375 cells (Fig. 5A), and flow cytometry experiment showed that ABCG2-mediated efflux of pheophorbide A was inhibited completely by both FTC and vemurafenib (Fig. 5B). Next, the IC50 values of mitoxantrone and vemurafenib in A375 and A375-G2 were determined by cytotoxicity assays. Mitoxantrone was used to confirm that ABCG2 in A375-G2 cells is capable of conferring resistance to ABCG2 drug substrate in a standard cytotoxicity assay (Fig. 5C). As expected, A375-G2 cells are over 6-fold more resistant to mitoxantrone than parental A375 cells, and can be re-sensitized by either FTC or vemurafenib (Table 2). Surprisingly, in contrast to ABCG2-positive BRAF wild-type cancer cells, we discovered that ABCG2-positive BRAF(V600E) mutant human A375-G2 melanoma cells were 3-fold more resistant to vemurafenib than the parental A375 cells (Fig. 5D). Moreover, the ABCG2-mediated resistance to vemurafenib can be reversed completely by FTC (Table 2).
Fig. 5.
Effect of human ABCG2 protein expression in BRAF(V600E) mutant A375 human malignant melanoma cells. (A) Immunoblot detection of human ABCG2 in A375 (lane 1) and ABCG2-expressing A375-G2 melanoma cells (lane 2). (B) Effect of vemurafenib on PhA accumulation in A375 (left panel) or A375-G2 (right panel) cells in the absence (solid lines) or presence of 25 µM vemurafenib (shaded solid lines) or 5 µM FTC (dotted lines). Sensitivity of A375 (open circles) and A375-G2 (filled circles) to (C) mitoxantrone or (D) vemurafenib was determined as described in Materials and Methods. Points, mean from at least three independent experiments; bars, SE. (E) Inhibitory effect of vemurafenib on BRAF kinase activity is reduced by ABCG2 in A375 cells. A375 and A375-G2 cells were treated with increasing concentration of vemurafenib for 4 h. The effects of vemurafenib on MAPK signaling were measured by monitoring the levels of p-MEK1/2 and p-ERK1/2 as described in Materials and Methods. Representative immunoblots of total MEK1/2, p-MEK1/2, total ERK1/2, p-ERK1/2, ABCG2 and loading control tubulin, are showed here. (F) The averaged relative expression levels of total MEK1/2, p-MEK1/2, total ERK1/2 and p-ERK1/2 treated with increasing concentrations of vemurafenib in parental A375 cells (open bars) and A375-G2 cells (closed bars). Values are presented as mean ± SD calculated from three independent experiments. * P < 0.05 ; ** P < 0.01 ; *** P < 0.001
3.7 ABCG2 reduces BRAF kinase inhibition in human A375 melanoma cells
In order to examine whether ABCG2-mediated vemurafenib resistance can directly affect BRAF kinase inhibition by vemurafenib, we monitored the phosphorylation of the RAF downstream effectors, MEK1/2 and ERK1/2 in parental BRAF(V600E) mutant A375 and ABCG2-expressing A375-G2 melanoma cells, in the presence or absence of vemurafenib. While the levels of total MEK1/2 and ERK1/2 remained unchanged, vemurafenib reduced the levels of p-MEK1/2 and p-ERK1/2 in a concentration-dependent manner in both cell lines. Although the inhibitory effect of vemurafenib on p-MEK1/2 appeared to be comparable in both A375 and A375-G2 cell lines, the inhibition of p-ERK1/2 was considerably less in ABCG2-expressing A375-G2 cells than in parental A375 cells (Fig. 5E).
4. Discussion
The rapid development of acquired resistance presents a significant therapeutic challenge to cancer patients receiving vemurafenib [10, 11]. There is an urgent need to identify potential mechanisms of vemurafenib resistance in order to develop new therapeutic strategies. Recent studies have shown that RAF isoform switch and activation of compensatory survival pathways contribute significantly to acquired resistance to vemurafenib in BRAF(V600E) mutant cancer cells [15–22]. However, the potential role of ABC drug transporters in the development of acquired resistance to vemurafenib, has not been explored. Given that the overexpression of ABC drug transporters is one of the most common mechanisms of acquired resistance to anticancer agents, we sought to evaluate if overexpression of ABCB1, ABCC1 or ABCG2 could mediate resistance to vemurafenib. In the present study, we characterized the biochemical and pharmacological interactions of vemurafenib with these three major ABC transporters. We showed that vemurafenib binds to the drug binding pockets of ABCB1 and ABCG2 directly, stimulates ATP hydrolysis and inhibits the efflux function of both ABCB1 and ABCG2, but was only able to re-sensitize ABCG2-expressing cells to mitoxantrone and topotecan. Moreover, we demonstrated that ectopic expression of ABCG2 can confer resistance to vemurafenib in BRAF(V600E) mutant A375 melanoma cells, implicating ABCG2 as a mechanism of resistance for vemurafenib. To our knowledge, this is the first report to link ABCG2 overexpression to acquire resistance to vemurafenib and consequently reduced BRAF kinase inhibition in BRAF(V600E) mutant cancer cells.
We first studied the effect of vemurafenib on drug efflux mediated by ABCB1, ABCC1 or ABCG2. At a low concentrations, vemurafenib significantly inhibited ABCG2-mediated transport of PhA and mitoxantrone (Fig. 1). In contrast, the inhibition of ABCB1-mediated efflux by vemurafenib was considerably weaker, and almost no inhibition was observed in ABCC1-mediated drug efflux (Fig. 2). Next, we examined whether vemurafenib inhibited ABCB1- and ABCG2-mediated transport by binding directly to ABCB1 and ABCG2. Photoaffinity labeling assays showed that vemurafenib inhibited the IAAP photolabeling of both ABCB1 and ABCG2 at the substrate binding pockets, but with significantly higher affinity to ABCG2 compared to ABCB1 (Fig 3A and 3B). Our data indicated that strong inhibition of ABCG2-mediated drug efflux by vemurafenib was the result of binding of vemurafenib with high affinity to the substrate binding pocket of ABCG2. In order to explore whether vemurafenib behaves as a transported substrate of ABCB1 and ABCG2, we measured the effect of vemurafenib on the vanadate-sensitive ABCB1 and ABCG2 ATPase activity (Fig. 3C and 3D). The stimulation of ATP hydrolysis is known to be coupled with substrate transport by ABC transporters [34]. Therefore, rapid stimulation of ATPase activity is associated with the presence of a substrate, while inhibition of ATPase activity is associated with the presence of an inhibitor or substrate with a much lower transport rate [35]. At low concentrations, vemurafenib produced a sharp stimulation of the ABCG2 ATPase activity, suggests vemurafenib as a high affinity substrate of ABCG2 (Fig. 3D). Similar result was observed in ABCB1 ATPase activity, however, the degree of stimulation and inhibition induced by vemurafenib suggested that vemurafenib could be a low-affinity substrate of ABCB1 (Fig. 3C). Our finding is in agreement with a recent report showing that the penetration of vemurafenib to the central nervous system is restricted at the blood-brain barrier, highlighting the importance of ABCB1 and/or ABCG2 in the distribution of vemurafenib [36]. However, interactions between vemurafenib and ABC drug transporters or the potential role of ABC drug transporters in acquired vemurafenib resistance in cancer cells, was not investigated.
Next, we examined if inhibition of ABC transporter-mediated drug efflux by vemurafenib lead to resensitization of ABC drug transporter-overexpressing cells to chemotherapeutic drugs. Interestingly, the search for specific inhibitor of ABC drug transporters has been ongoing for years without much success [37], but the recent burst of research interest on studying the interactions between tyrosine kinase inhibitors (TKIs) and ABC drug transporters showed some promising results [38]. The high affinity interactions of many TKIs and ABCG2 were translated into in vitro and in vivo inhibition of the function and restoration of drug sensitivity in MDR cancer cells overexpressing ABCG2 [39–42]. Consistent with these findings, vemurafenib inhibited ABCG2-mediated transport with high affinity, re-sensitized ABCG2-overexpressing cells to mitoxantrone and topotecan (Table 2), implicating that in combination with other anticancer drugs, vemurafenib could increase oral bioavailability or antitumor responses in cancer patients [43]. In addition to inhibiting the function of ABC drug transporter, an alternative way to re-sensitize ABC transporter-positive MDR cancer cells is to transiently reduce the expression of ABC drug transporter mediated by a particular drug [44]. Therefore, we also examined the effect of vemurafenib on the protein expression of major ABC transporters in the respective ABC transporter-overexpressing MDR cancer cells over a period of 72 h (Fig. 4). Our data showed vemurafenib has no significant effect on the protein expression of any ABC drug transporters, confirming that vemurafenib reversed drug resistance in ABCG2 expressing cells via direct interaction at the drug substrate-binding site(s). Knowing how vemurafenib interacts with ABCG2 in MDR cancer cells, we decided to investigate whether ectopic expression of ABCG2 was sufficient to confer resistance to vemurafenib in BRAF(V600E) mutant cancer cells. We transiently transfected human A375 melanoma cells with human wild-type ABCG2 and tested the drug sensitivity and BRAF kinase activity of these cells (Fig. 5). We chose BRAF(V600E) mutant human A375 melanoma cell line for this experiment because it has very low endogenous levels of EGFR [23] and ABCG2 (Fig. 5A). The presence of endogenous EGFR or ABCG2 (such as in colon cancer Colo-205 cells) could potentially interfere with our study [45–48]. Since ABCG2 has been detected in subpopulation of CD133-positive melanoma cells [49], colorectal cancer cells [45, 46] and in thyroid carcinoma ARO and WRO cells [47], the upregulation or overexpression of ABCG2 becomes more likely when patients who were treated chronically with a drug that is a substrate of ABCG2, causing serious therapeutic problems.
In our study, we were surprised to discover that ABCG2 conferred resistance to vemurafenib in ABCG2-positive BRAF(V600E) mutant melanoma cells (Fig. 5D), but not in ABCG2-positive wild-type BRAF cancer cells (Table 1). A possible explanation for this is that ABCG2 is only capable of conferring resistance when intracellular concentration of vemurafenib is low. It is known that depending on the substrate being transported, ABCG2 can mediate either a high affinity, low capacity transport that is easily saturated, or a low affinity, high capacity transport that does not saturate easily [50]. In our case, the IC50 values of vemurafenib in wild-type BRAF cancer cells were 100–1000 fold higher than in BRAF(V600E) mutant cells (Table 1), suggesting that a high affinity, low capacity transport system would not be efficient enough to remove high concentrations of vemurafenib from ABCG2-positive wild-type BRAF cancer cells to confer drug resistance. This finding is consistent with our ABCG2 photoaffinity labeling and ATPase assays (Fig. 3) showing high affinity binding of vemurafenib to ABCG2. Nevertheless, the detailed pharmacokinetics of vemurafenib in these cancer cells remains to be determined. As we confirmed that ABCG2 is over-expressed, functional and confers resistance to mitoxantrone and vemurafenib in BRAF(V600E) mutant A375-G2 cells (Fig. 5), we monitored the downstream phosphorylation of MEK1/2 and ERK 1/2. We observed that BRAF kinase inhibition by vemurafenib is reduced in ABCG2-overexpressing BRAF(V600E) mutant A375-G2 melanoma cells when compared to parental BRAF(V600E) mutant A375 melanoma cells (Fig. 5E). Our data suggested that ABCG2 overexpression could have significant impact on the treatment of BRAF(V600E)-positive melanoma, colorectal cancer and thyroid cancer by vemurafenib.
In summary, our data suggest that vemurafenib could potentially be used as a potent modulator of ABCG2 for reversing drug resistance in ABCG2-overexpressing MDR cancer cells. Moreover, we conclude that ABCG2-mediated vemurafenib resistance could have serious clinical implications in the treatment of cancers harboring BRAF(V600E) mutation, and drug combinations targeting multiple pathways, including ABCG2, could be an effective therapeutic strategy to overcome acquired resistance to vemurafenib.
Acknowledgments
We thank Dr Chih-Chien Lin (Providence University, Taiwan) for A375 cells. This work was supported by funds from National Science Council of Taiwan (grant no. NSC100-2320-B-182-002, C-P. Wu, and NSC-99-2311-B-182-005-MY3, S.-C. Hsu), the Chang Gung Medical Research Program CMRPD190652 (C-P. Wu) and grant to Chang Gung University from the Ministry of Education EMRPD1B0271 (C-P. Wu and S.-C. Hsu). Drs H-M. Sim and S.V. Ambudkar were supported by the Intramural Research Program of the National Cancer Institute, NIH, Center for Cancer Research.
Abbreviations
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- EGFR
epidermal growth factor receptor family 1
- TKI
tyrosine kinase inhibitor
- PBS
phosphate-buffered saline
- FTC
Fumitremorgin C
- IAAP
Iodoarylazidoprazosin
- MTT
3-(4,5-dimethylthiazol-yl)-2,5-diphenyllapatinibrazolium bromide
- Vi
sodium orthovanadate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None declared.
Contributor Information
Chung-Pu Wu, Email: wuchung@mail.cgu.edu.tw.
Sheng-Chieh Hsu, Email: schsu@mail.cgu.edu.tw.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Arkenau HT, Kefford R, Long GV. Targeting BRAF for patients with melanoma. Br J Cancer. 2011;104:392–398. doi: 10.1038/sj.bjc.6606030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K, et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820–827. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 14.Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 15.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su F, Bradley WD, Wang Q, Yang H, Xu L, Higgins B, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969–978. doi: 10.1158/0008-5472.CAN-11-1875. [DOI] [PubMed] [Google Scholar]
- 19.Yadav V, Zhang X, Liu J, Estrem S, Li S, Gong XQ, et al. Reactivation of Mitogen-Activated Protein Kinase (MAPK) Pathway by FGF Receptor 3 (FGFR3)/Ras Mediates Resistance to Vemurafenib in Human B-RAF V600E Mutant Melanoma. J Biol Chem. 2012;287:28087–28098. doi: 10.1074/jbc.M112.377218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H, Kong X, Ribas A, Lo RS. Combinatorial treatments that overcome PDGFRbeta-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 2011;71:5067–5074. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 24.Wu CP, Hsieh CH, Wu YS. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm. 2011;8:1996–2011. doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- 25.Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol Cancer Ther. 2007;6:3287–3296. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- 27.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, et al. Overexpression of the ATP-binding Cassette Half-Transporter, ABCG2 (MXR/BCRP/ABCP1), in Flavopiridol-resistant Human Breast Cancer Cells. Clin Cancer Res. 2001;7:145–152. [PubMed] [Google Scholar]
- 28.Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, Pastan I, et al. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986;232:643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- 29.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 30.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 31.Shukla S, Rai V, Banerjee D, Prasad R. Characterization of Cdr1p, a major multidrug efflux protein of Candida albicans: purified protein is amenable to intrinsic fluorescence analysis. Biochemistry. 2006;45:2425–2435. doi: 10.1021/bi0519147. [DOI] [PubMed] [Google Scholar]
- 32.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci U S A. 2000;97:2515–2520. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) FEBS J. 2005;272:4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambudkar SV, Cardarelli CO, Pashinsky I, Stein WD. Relation between the turnover number for vinblastine transport and for vinblastine-stimulated ATP hydrolysis by human P-glycoprotein. J Biol Chem. 1997;272:21160–21166. doi: 10.1074/jbc.272.34.21160. [DOI] [PubMed] [Google Scholar]
- 35.Hegedus C, Ozvegy-Laczka C, Szakacs G, Sarkadi B. Interaction of ABC multidrug transporters with anticancer protein kinase inhibitors: substrates and/or inhibitors? Curr Cancer Drug Targets. 2009;9:252–272. doi: 10.2174/156800909788166565. [DOI] [PubMed] [Google Scholar]
- 36.Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J Pharmacol Exp Ther. 2012;342:33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 38.Shukla S, Chen ZS, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat. 2012;15:70–80. doi: 10.1016/j.drup.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat. 2005;8:15–26. doi: 10.1016/j.drup.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Hegedus C, Ozvegy-Laczka C, Apati A, Magocsi M, Nemet K, Orfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–7991. doi: 10.1158/0008-5472.CAN-10-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–7499. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, et al. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–4815. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004;109:848–854. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 46.Liu HG, Pan YF, You J, Wang OC, Huang KT, Zhang XH. Expression of ABCG2 and its significance in colorectal cancer. Asian Pac J Cancer Prev. 2010;11:845–848. [PubMed] [Google Scholar]
- 47.Lopez JP, Wang-Rodriguez J, Chang C, Chen JS, Pardo FS, Aguilera J, et al. Gefitinib inhibition of drug resistance to doxorubicin by inactivating ABCG2 in thyroid cancer cell lines. Arch Otolaryngol Head Neck Surg. 2007;133:1022–1027. doi: 10.1001/archotol.133.10.1022. [DOI] [PubMed] [Google Scholar]
- 48.Zheng X, Cui D, Xu S, Brabant G, Derwahl M. Doxorubicin fails to eradicate cancer stem cells derived from anaplastic thyroid carcinoma cells: characterization of resistant cells. Int J Oncol. 2010;37:307–315. doi: 10.3892/ijo_00000679. [DOI] [PubMed] [Google Scholar]
- 49.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 50.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. Aaps J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]