Abstract
Changes in plasma, extracellular and intracellular calcium can affect renin secretion from the renal juxtaglomerular (JG) cells. Elevated intracellular calcium directly inhibits renin release from JG cells by decreasing the dominant second messenger intracellular cyclic adenosine monophosphate (cAMP) via actions on calcium-inhibitable adenylyl cyclases and calcium-activated phosphodiesterases. Increased extracellular calcium also directly inhibits renin release by stimulating the calcium-sensing receptor (CaSR) on JG cells, resulting in parallel changes in the intracellular environment and decreasing intracellular cAMP. In vivo, acutely elevated plasma calcium inhibits plasma renin activity (PRA) via parathyroid hormone-mediated elevations in renal cortical interstitial calcium that stimulate the JG cell CaSR. However, chronically elevated plasma calcium or CaSR activation may actually stimulate PRA. This elevation in PRA may be a compensatory mechanism resulting from calcium-mediated polyuria. Thus, changing the extracellular calcium in vitro or in vivo results in inversely related acute changes in cAMP, and therefore renin release, but chronic changes in calcium may result in more complex interactions dependent upon the duration of changes and the integration of the body’s response to these changes.
Keywords: calcium sensing receptor, hypercalcemia, parathyroid hormone, cAMP, juxtaglomerular cell, adenylyl cyclase
Calcium
The ubiquitous cation calcium has an important role in signal transduction pathways as a second messenger involved in a variety of cellular functions in almost all mammalian cells. Calcium also acts as critical cofactor for most enzymes, as well as the process of secretion [82]. Extracellular calcium, and particularly the plasma level of calcium is closely regulated with a normal concentration of 2.2–2.6 mM of total calcium (complexing with various other ions and binding to plasma proteins), and an ionized free calcium of 1.1–1.4 mM. These levels are normally maintained within a tight range and rarely fluctuate more than 5% over time.
Within most cells, the concentration of ionized calcium is roughly 100 nM, but can increase as much as 1–2 orders of magnitude as part of a stimulation of a cell signaling cascade. Thus, the intracellular calcium concentrations are five orders of magnitude less than that of the extracellular concentration, creating a steep diffusion gradient which is maintained by limited permeability, calcium extrusion pumps and efficient binding by intracellular stores [82].
Juxtaglomerular cells and the calcium paradox. In vitro studies
While increased calcium usually a positive factor in secretory processes [82], there are two cells in which increased extracellular calcium has be characterized as a negative regulator. These include the chief cells of the parathyroid gland [83] in which increased circulating calcium suppresses parathyroid hormone (PTH) secretion, and the renin-containing juxtaglomerular (JG) cells [90] at the glomerular pole of the afferent arteriole of the kidney.
Classic stimuli for the secretion of renin are mediated by various second messengers, including cAMP, cGMP and intracellular calcium [25]. However, the dominant second messenger for renin secretion is cAMP [25,31], synthesized by adenylyl cyclases within the JG cell. Activation of adenylyl cyclase is the target for all known classical stimuli for renin secretion, such as β-adrenergic activation [46,70] and macula densa-mediated PGE2 stimulation via the EP-4 receptors [30,40,106]. The question has always been how do the other second messengers interact either directly with renin secretion (in parallel with cAMP), or perhaps by mediating the synthesis or degradation of cAMP [25]. As for cGMP, its interaction seems to be indirect, either by inhibiting phosphodiesterase-3 degradation of cAMP [11,37,40,67], or by via inhibition through cGMP-dependent protein kinase II [41,122]. The exact role of calcium as a second messenger has been more elusive. Renin secretion from the JG cells has a somewhat unique interaction with calcium, in that while in most secretory cells calcium is a regulatory co-factory for stimulation, both increased intracellular and extracellular calcium inhibits renin secretion [9,10,25,36]. It was Park and Malvin [90] who first suggested there was an inverse relationship between intracellular calcium concentration in the JG cell and its release of renin. Because of this unusual and seemingly paradoxical relationship between intracellular calcium and renin secretion, it has been referred to as the “calcium paradox” [46]. Numerous in vitro studies have reinforced this observation, including increasing extracellular calcium [9,24,87,107], chelating intracellular [79,85] or extracellular calcium [9], increasing intracellular calcium using calcium channel agonists [27] or various receptor-mediated hormones such as angiotensin II, endothelin and vasopressin [71,98,116] known to increase intracellular calcium, activating release of intracellular stored calcium [108], or using calcium ionophores or calcium channel blockers [24,29,38]. While the phenomenon has been observed for over 25 years, recently two laboratories presented a mechanistic explanation of how changes in intracellular calcium influenced renin secretion [45,85,86]. The first key was that manipulating intracellular calcium resulted in coincident changes in both JG cell cAMP and renin release [45,85], suggesting that the effects of calcium were “upstream” from the production of the dominant second messenger cAMP. Next, both laboratories reported multiple lines of evidence for the calcium-inhibitable isoforms of adenylyl cyclase (AC), AC-5 and/or AC-6 in their JG cells. Further, while AC-6 is generally regarded as a cell membrane-bound isoform fluorescent immunohistochemistry of AC-5 found it co-localized on the renin containing granules within the JG cell cytoplasm [85,86]. Interestingly, while the mechanism for either isoform is presumably similar, the laboratories in Regensburg find both AC-5 and AC-6 in their JG cells, with a dominant role for AC-6 [6,45], while the laboratories in Detroit did not find AC-6 in their JG cells, and could account for the inhibitory effect of intracellular calcium completely through AC-5 [86]. So, what is the resolution to this discrepancy? Both laboratories used a similar technique with isolated JG cells in primary culture from mice. While it has not yet been tested, it is highly likely that these differences are due to the huge variability in the phenotype of mice as a laboratory animal, even in similar strains [35]. Regardless of the isoform(s), the key to the calcium paradox seems to be that when calcium increases, the activity of calcium-inhibited adenylyl cyclase is reduced, attenuating the basal or stimulated synthesis of the second messenger cAMP, resulting in decreased renin secretion. This pathway is illustrated in figure 1. It should be noted that while the predominance of the literature suggests renin secretion is highly cAMP dependent, at least one study [66] suggests under certain circumstances renin may be released without changes in cAMP.
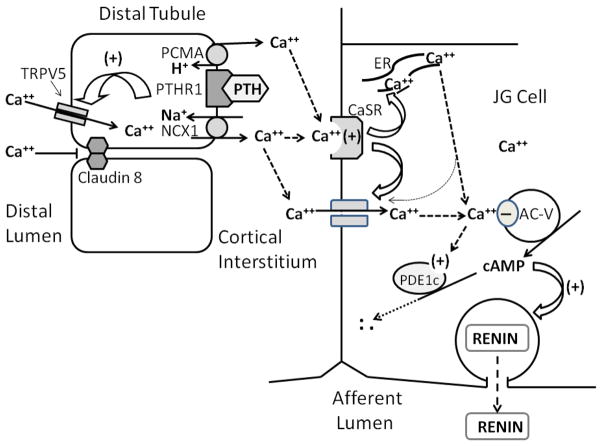
Figure 1.
Schematic summary of the calcium-regulated pathway from PTH-mediated calcium reabsorption in the distal tubule to cAMP-regulated renin secretion into the afferent arteriolar lumen. Abbreviations and acronyms: AC-V = adenylyl cyclase isoform 5, Ca++ = (ionized free) calcium, cAMP = cyclic adenine monophosphate, CaSR = calcium-sensing receptor, ER = endoplasmic reticulum, H+ = hydrogen ion, JG = juxtaglomerular, NCX1= sodium/calcium exchanger- member 1, PMCA= plasma membrane Ca++ ATPase, PTH = Parathyroid hormone, PTH-R1 = parathyroid hormone type 1 receptor, PDE1c = phosphodiesterase isoform 1c, TRPV5= Transient receptor potential cation channel subfamily V member 5, (+) = stimulatory, and (−) = inhibitory action.
Calcium and phosphodiesterase
In addition to the inhibition of cAMP synthesis by calcium, it has also been reported that the JG cells contain the calcium-activated phosphodiesterase (PDE) isoform, PDE-1C [87]; the only calcium-stimulated PDE-1 isoform that targets cAMP. These studies suggested that at normal extracellular calcium concentrations, this PDE was relatively dormant. However, as extracellular (and intracellular) calcium concentrations increased, its degradation of cAMP became more prominent. Such an interaction between calcium-inhibited AC and calcium-stimulated PDE-1 are usually found co localized in the same tissues. PDE1c has been localized in various tissues, including brain [21], testis [127] and smooth muscle [101] in association with calcium inhibitable adenylyl cyclases, isoforms 5 and 6 [44]. These data would suggest that with increases in intracellular calcium, there is a symbiotic interaction between decreased synthesis (via AC) and increased degradation (via PDE) of cAMP, and thus an amplified inhibition of the signal for renin secretion.
Calcium-calmodulin
Cyclic nucleotide hydrolysis by the PDE1 family is calcium-calmodulin dependent [44]. The isoforms of the PDE1 family are characterized as the “calcium- and calmodulin-dependent phosphodiesterases” [22]. Their activity is regulated by binding with the calcium-calmodulin complex. Calcium-calmodulin binding stimulates the PDE1 activity by increasing its Vmax [57]. The calcium calmodulin complex may actually inactivate an inhibitory domain on the PDE, increasing its activity [21,109], and there is a [intracellular] calcium threshold at which this activation may be initiated. Calcium-mediated inhibition of renin secretion has been shown to be calmodulin-dependent. Inhibitors of calmodulin increase renin release [9,28,65,91,108] in vitro, and reverse high extracellular calcium-mediated inhibition of renin [9]. However, how calmodulin fits into the calcium-mediated inhibition of renin has remained unclear. The Ca-calmodulin dependent PDE1 family comprises three isoforms; PDE1A, PDE1B and PDE1C {44]. PDE1 is regulated by calcium, and is tightly associated with calmodulin. Calmodulin inhibitors do not change basal cAMP content in JG cells [65,87]. However, either activating the CaSR or increasing extracellular calcium lead to a rise in intracellular calcium, and reduces both JG cell cAMP content and renin release [87], in part by increasing PDE1 activity. This is consistent with the activating threshold of calcium-calmodulin activation of PDE1. The PDE1A and PDE1B isoforms have a high affinity for cGMP, while PDE1C has an equally high affinity for both cAMP and cGMP [44]. In isolated JG cells in primary culture, both calmodulin inhibition and PDE1 inhibition reversed the suppression of cAMP formation and renin release in response to increased intracellular or media calcium. The similar reversals of calmodulin or PDE1 inhibition of renin were not additive suggesting PDE1 and calmodulin work in concert in the JG cell, as in other cell types [44].
The calcium-sensing receptor
If changes in the extracellular calcium concentration do effect similar changes in the cytosolic calcium concentration in order to influence calcium-sensitive AC’s and PDE’s, how might such a signal be transmitted? Similar to the parathyroid gland [99], the JG cells have been found to have calcium-sensing receptors (CaSR); G protein-coupled receptor whose activation increases intracellular calcium by activating phospholipase C (PLC) via Gq [117]. These membrane-bound receptors sense and translate micromolar changes in the extracellular calcium into parallel changes in intracellular calcium [99]. The half-maximal effective calcium concentration (EC50) for the CaSR is 1.2 mM, which is the normal concentration of interstitial free ionized calcium in the renal cortical interstitium [5,66]. Ortiz-Capisano et al [84] identified CaSR in primary cultures and freshly isolated mouse JG cells. They found that either increased extracellular calcium or activation of the CaSR using the calcimimetic Cinacalcet-HCl significantly decreased both JG cell cyclic AMP formation and renin release by approximately 45%. They concluded that JG cells express CaSR, and CaSR respond to changes in extracellular calcium to modify or activate calcium-mediated intracellular signaling [84] that regulates the secretion of renin. Their results have been corroborated by both in vitro [78] and in vivo [8,78] studies.
If the changes in intracellular calcium in the JG cells are linked to the activation of the CaSR, then what is the post-receptor signaling cascade? While the actual pathway from CaSR to renin secretion in the JG cell has not been described, in the parathyroid gland activation of the CaSR activates phospholipase C [16], with the generation of receptor inositol trisphosphate (IP3) and diacylglycerol. Subsequent activation of the IP3 on the endoplasmic reticulum causes release of calcium into the cytosol, and the depletion of endoplasmic reticulum calcium then stimulates store operated calcium channels [95]. Intracellular calcium is buffered by mitochondrial uptake and calcium sequestration in the endoplasmic reticulum [10]. Changes in cytoplasmic calcium can come from calcium release from sequestered intracellular stores or from the extracellular fluid through opening of calcium channels. Numerous studies of juxtaglomerular renin release in various preparations of renal cortical tissue have identified possible pathways which might lead to increased intracellular calcium, including activation of store-operated calcium entry channels [107,108], mobilization of calcium from intracellular stores in the endoplasmic reticulum [29,31,33], and activation of voltage-gated L-type calcium channels [24,26,27,38]. Renin secretion is coupled to the electrical potential of the JG cell, such that depolarization inhibits renin release [24,26] and hyperpolarization increases renin release. Numerous studies in vitro have shown KCl depolarization inhibit renin release [10,56], while patch-clamp studies [38] have shown renin release is characterized by changes in JG cell electrical potential [105]. Activation of these channels inhibits cAMP induced renin secretion [38]. Inhibition of the L-type voltage-gated calcium channels has been repeatedly shown to stimulate renin release [24,26,27]. Thus, either release of stored intracellular calcium, opening of store-operated calcium channels, or the opening of voltage gated calcium channels are all possible pathways which could be triggered by activation of the CaSR on the JG cell, but which of these is actually coupled to the CaSR has yet to be definitively shown.
Vasoconstrictors such as Angiotensin II and Endothelin [3,50,73] stimulate arteriolar contraction via the AT1 receptor or ETB receptor (3), respectively. In JG cells these G-protein coupled receptors stimulate calcium influx or the release of calcium from intracellular stores, increase cytosolic calcium and inhibit renin release (24,79,112). The membrane potential of the JG cell is a critical factor in regulating renin secretion. Vasoconstriction of the afferent arteriole is associated with JG cell depolarization [18,19] and the inhibition of renin secretion, and there is significant electrical coupling between JG cells and the afferent arteriolar smooth muscle cells (18,72,100). In vitro, potassium-induced depolarization of the JG cells also suppresses renin release [10,24,26,56,68]. The JG cells have calcium-activated chloride channels [20,55,72] that, when activated shift the chloride equilibrium potential of the JG cell to a depolarized state, inhibiting of renin release [72]. Patch-clamp studies have shown renin release is coupled to changes in JG cell electrical potential [38,102]. Numerous studies in various preparations of renal cortical tissue have identified possible pathways which might lead to increased intracellular calcium, including activation of store-operated calcium entry channels [107,108], mobilization of calcium from intracellular stores in the endoplasmic reticulum [29,31,33], and activation of voltage-gated L-type calcium channels [24,26,27,38]. JG cell depolarization may induce opening of L-type voltage-gated calcium channels, and these channels have been confirmed in JG cells [39], and a number of studies have shown blocking these channels results in increased renin release [24,26,40]. Scholz and Kurtz (104) reported that afferent vascular smooth muscle cells differed as they approached the JGA. While both populations contained angiotensin AT-1 receptors and calcium-activated chloride channels, in afferent smooth muscle cells distant from the JG cells, intracellular calcium was increased via voltage-gated channels, but in those closer to the JG cells (presumably more similar in phenotype), the increase in cytosolic calcium takes place through mobilization of intracellular stores. Consistent with this, recent studies have suggested the magnitude of depolarization needed to induce voltage-gated channels in JG cells is too great, and have suggested alternatively receptor-mediated activation of store-operated calcium channels may be the predominant pathway for increasing intracellular calcium [45,72,128] under normal physiologic conditions. All of these studies of calcium-mediated regulation of renin require the presence of extracellular calcium, suggesting ultimately the result of any pathway involves calcium entry. It is not clear, however, if the inhibition of renin secretion by vasoconstriction or depolarization is mediated just by increasing intracellular calcium, as additional indirect pathways are probably also involved, especially in vivo.
In contrast, vasodilation results in JG cell hyperpolarization and stimulates renin secretion. In vitro, potassium-induced JG cell hyperpolarization or chloride channel inhibition stimulates renin release [26,34,39,50,54,80]. In vitro, furosemide inhibition of the Na.K/2Cl co-transporter NKCC1 results in JG cell hyperpolarization and stimulates renin release from isolated JG cells [20]. Presumably, this works in the opposite direction as depolarization, reversing calcium-mediated renin inhibition. In vivo, this would work in concert with furosemide inhibition of NKCC2, provoking macula densa stimulation of renin secretion via PGE2 targeting the JG cell adenylyl cyclase. In vivo, the integration of the multiple signals associated with the hemodynamic changes (contraction, dilation) obviously extend well beyond a simple calcium-mediated signaling pathway in the JG cell. It is important to consider that the calcium effect in the JG cell appears to modify the activity of the target enzymes (AC 5/6 and PDE1), modifying the renin response to the typical renin-stimulating pathways (renal baroreceptor, macula densa or sympathetic stimulation) rather than directly causing renin secretion or inhibition.
Propagation of a calcium wave
An alternative calcium-mediated pathway involves the initiation and propagation of calcium waves through the juxtaglomerular region. JG cells contain gap junctions linking them to the adjacent endothelial cells, and these intercellular channels have been suggested as possible conduit pathways for propagating a calcium-mediated signal through the endothelium and into JG cells [64,113,128]. Connexins are transmembrane proteins that combine to form hemichannels in the plasma membranes of the gap junction, linking the cytoplasm of two cells. The sizes of these channels are large enough to permit the diffusional movement of calcium. Mechanical distortion of a renal endothelial monolayer was found to generate a wave of increased intracellular calcium which was mediated by connexin 40 [113], a major connexin involved in the JG cells [121]. Wagner et al [121] found connexin 40 deletion eliminated the calcium-mediated negative feedback response of renin to both angiotensin and renal baroreceptor inhibition of renin by increased renal perfusion. Peti-Peterdi [94] found that changing tubular flow rate lead to propagation of a calcium wave, which increased intracellular calcium in the JG cells. These data suggest Cx40-dependent gap junctions of the JG cells may mediate inhibition of renin by calcium-dependent factors such as angiotensin and renal perfusion, and possibly the macula densa via a unique endothelial transmitted pathway [64,121]. The end result would be a separate integrated pathway in the juxtaglomerular apparatus to influence the intracellular calcium in the JG cell, independent of the signaling through the CaSR. Importantly, this pathway would be characterized by a cell-to-cell, intracellular signal derived from the endothelium, perhaps in response to some initiating mechanical transmission. It is also possible that the direction of the transmission is not to the JG cell, but originating from the JG cell, secondary to the CaSR initiating changes in intracellular calcium
Summary, in vitro studies
Overall, the mystery of the calcium paradox in the JG cell has at least one tenable explanation, in that changes in calcium concentration of the extracellular environment can be transmitted to similar changes in the intracellular compartment via the CaSR. An increase in intracellular calcium, possibly mediated through any number of channels or stores, results in diminished activity of the calcium-inhibitable isoforms of adenylyl cyclase, AC-5 (and AC-6), as schematically summarized in figure 1. This results in decreased synthesis of the positive second messenger cAMP, and consequently diminished renin release. This response is supplemented by calcium activation of PDE1C to enhance cAMP degradation in concert with diminished synthesis. Changes in intracellular calcium can also be initiated by any number of receptor-mediated factors (such as angiotensin) that also stimulate increased intracellular calcium concentrations, or through calcium waves initiated by hemodynamic or tubular signals, propagated via the connexins in the gap junctions. The changes in intracellular calcium alter the activity of these regulatory enzymes, influencing both basal renin release as well as the classical renin stimuli that target activation of adenylyl cyclases. But in the whole kidney, what are the conditions that mimic the changes in extracellular calcium suggested by these many in vitro studies? The following section will address the extrapolation of this cellular regulatory pathway into the whole animal, and provide some perspective on how it may be important in clinical and pathological conditions.
Calcium and renin secretion In Vivo
Similar to its well-studied in vitro effects, calcium also has many effects on renin in vivo. Both acute and chronic changes in plasma calcium can affect renin. Chronic elevations in plasma calcium due to hyperparathyroidism or hypercalcemia of malignancy are encountered more often clinically, but acute changes in plasma calcium are also seen in rhabdomyolysis-related acute renal failure. We will review and describe the effects of acute and chronic experimental elevations of plasma calcium on renin and their assorted clinical correlates.
The acute in vivo effects of calcium on renin
The acute effects of calcium on renin in vivo are generally similar to those seen in vitro: calcium inhibits renin secretion. A strong body of literature demonstrates that acutely increasing plasma calcium inhibits plasma renin activity or renin secretion in rats, dogs and humans [1,7,8,51,61,62 121,124,125,126,130]. The literature is not completely unanimous, as some studies have shown equivocal or negligible effects of acute calcium changes on renin secretion [29,52,75]. The reasons for these discrepancies are not entirely clear, but it may pertain to the basal renin levels from these experiments. The acute in vivo inhibitory effects of calcium on renin are much stronger in studies that have renin secretion stimulated by sodium restriction or other means [7,58,92,125,126]. One could argue this illustrates that calcium acts as a brake on the traditional stimulatory pathways of renin secretion, rather than a major inhibitory pathway in its own right. Irrespectively, the amount of evidence demonstrating an inhibitory effect of acutely increased plasma calcium on renin greatly outnumbers those suggesting otherwise.
After initial studies demonstrated that acutely elevated plasma calcium inhibited renin secretion, research into a mechanistic explanation for this response failed to progress. However, this changed with the discovery and cloning of the calcium-sensing receptor (CaSR) [16]. The CaSR is a 7-transmembrane domain, G-protein-coupled receptor that transmits changes in plasma calcium into intracellular signaling [17]. While originally discovered in the parathyroid gland [16], the CaSR was also strongly detected in the kidney [96]. Based on this, Ortiz et al. [84] discovered that the CaSR was functionally expressed in primary cultures of mouse, renin-secreting JG cells. The expression of the CaSR in mouse JG cell primary cultures suggested that it could be the receptor responsible for the acute inhibitory effects of elevated plasma calcium on renin secretion.
Because of this, two separate groups tested whether the CaSR could inhibit renin in vivo. The group of Maillard et al. [78], tested whether stimulating the CaSR with pharmacological agonists could inhibit plasma renin activity (PRA) in vivo. They found that the acute administration of the calcimimetic (allosteric CaSR agonist) R-568 decreased isoproterenol-, enlapril- or furosemide-stimulated PRA in conscious rats. Similarly, Atchison et al. [8] tested whether the CaSR was expressed in JG cells in vivo and whether pharmacologically stimulating the CaSR could decrease PRA. In slices of renal cortex fixed in vivo, they demonstrated positive immunofluorescent staining of the CaSR in the renin-containing afferent arteriole. Pharmacologically stimulating the CaSR with intravenous calcimimetics acutely decreased both basal and furosemide-stimulated PRA in anesthetized rats. When combined with the in vitro data presented in the first part of this review, these data suggest that activation of the JG cell-expressed CaSR inhibits renin secretion in vivo.
The results from the preceding data led to the obvious question: is there a calcium paradox in vivo? If the CaSR is functionally expressed in vivo, can it regulate the inhibitory effects of acutely elevated plasma calcium on renin secretion? It has now been shown that pharmacologically inhibiting the CaSR in vivo completely eliminated the hypercalcemia-mediated inhibition of PRA, confirming that the CaSR regulates the inhibition of renin by high plasma calcium [7]. Additionally, PTH appears to permissively regulate the inhibition of PRA by hypercalcemia. Hypercalcemia results in acutely increased renal interstitial (extracellular) calcium, driven by PTH via the TRPV5 calcium transporter in the distal tubule, activating the CaSR, resulting in acute inhibition of PRA [7]. A schematic for the proposed pathway from PTH-mediated calcium reabsorption to renin secretion acute hypercalcemia on PRA is summarized in figure 1.
Further research questions on the inhibitory effects of renal cortical interstitial calcium on renin remain. Based on previous results, the TRPV5 knockout mouse should have an impaired inhibitory response of calcium on renin [48]. However, another possibility could be claudin-16, which is a tight-junction protein in the thick ascending limb of the loop of Henle, where it regulates the paracellular reabsorption of cations [49]. Genetic deletion of claudin-16 leads to hypomagnesaemia and hypercalciuria, suggesting that claudin-16 could also regulate interstitial calcium levels [13].
Finally, the acute inhibitory effect of elevated plasma calcium on renin secretion may have clinical implications as well. While pathologies with chronically elevated plasma calcium arise more often, acute changes in plasma Ca can occur in acute renal failure associated with rhabdomyolysis [4]. Concomitant and inverse changes in PRA occur with these changes in plasma Ca [114], but whether the changes in plasma Ca actually influence PRA is unknown.
Chronic effects of calcium on renin in vivo
Chronic changes in plasma calcium also affect renin. Plasma calcium is chronically manipulated via dietary means or by hormonal changes seen in various neoplastic syndromes. Chronically elevated plasma calcium tends to stimulate renin secretion. However, the effects of plasma calcium on renin in hypercalcemia models may be due to the specific way in which changes (or the extent of change) in plasma calcium are induced.
Increased Dietary calcium consumption
Chronically increasing dietary consumption of calcium does not affect PRA under normal conditions [60], but dietary CaCl2 decreases PRA stimulated by NaCl restriction [62]. However, this is likely due to chloride replenishment, as chronic calcium gluconate consumption has no effect on PRA stimulated by NaCl restriction [60]. This is consistent with the inhibitory effect of chloride at the macula densa [77]. Thus, outside of chloride-specific effects, changes in dietary calcium do not affect renin. It should be noted that plasma calcium did not change with the increase in dietary consumption. Thus, increased dietary calcium may not affect PRA because plasma calcium levels are very tightly controlled, and do not change much with increased dietary calcium intake.
Vitamin D-induced hypercalcemia
To test the effects of chronically elevated plasma calcium on PRA, some groups have employed models of vitamin D-induced toxicity and hypercalcemia. Spangler et al. [111] found that vitamin D-induced hypercalcemia significantly increased PRA as well as JG cell hypertrophy and hyperplasia. Peterson [93] found that Vitamin D-induced hypercalcemia caused polyuria with no changes in PRA, except when Vitamin D-treated rats were pair-watered with controls. Under these conditions, PRA increased slightly. Levi et al. [75] found that Vitamin D-induced hypercalcemia and polyuria had no effect on PRA. Thus, while the effects of Vitamin D-induced hypercalcemia on renin are somewhat ambiguous, the current data suggest that chronic, vitamin D-induced hypercalcemia may mildly stimulate PRA as a protective mechanism against hypercalcemia-induced polyuria and dehydration [93].
The likely reason these three studies found differing effects on PRA was that different levels of hypercalcemia were induced in each. Spangler induced a 60% increase in plasma calcium with their Vitamin D treatment, and this correlated with a 3-fold increase in PRA [111]. Peterson affected a 38% increase in plasma calcium, which caused a 51% increase in PRA in pair-watered rats [93]. Levi increased plasma calcium by 28%, and this had no effect on PRA [75]. Thus, Vitamin D-induced hypercalcemia may dose-dependently stimulate PRA.
Leydig cell tumor-induced hypercalcemia
Sowers et al.[83] tested the effects of Leydig cell tumor transplantation on PRA and plasma calcium in the Fischer rat. Leydig cell tumor transplantation significantly increased both PRA and plasma calcium. Whether the increase in plasma calcium contributed to the increase in PRA is unknown. However, tumorous Leydig cells express renin [89]. Whether the renin expressed in Leydig cells is contributing to the elevation in PRA is unknown.
Chronic CaSR stimulation
As previously mentioned, the CaSR mediates the inhibitory effects of acute hypercalcemia on renin. However, whether chronic CaSR stimulation affects renin is less clear. It has been shown that the oral administration of calcimimetics (CaSR agonists) did not affect either basal or stimulated PRA in rats [8]. This is in contrast to patients with type-V Bartter Syndrome, a heterogeneous disorder, defined by impaired NaCl reabsorption, hypokalemic metabolic acidosis and hyperreninemia [59]. Type-V Bartter syndrome is due to an extremely potent activating mutation of the CaSR [118,124]. Patients with type-V Bartter syndrome also have elevated PRA [90,91,96]. The elevated PRA in these patients is likely due to the severity of the activating mutation, as the CaSR is near maximally stimulated at physiological (1.0 mM) concentrations of ionized calcium in these patients [118,124]. Thus, similar to the dose-dependent effects of Vitamin D-induced hypercalcemia on renin, the ability of chronic CaSR stimulation to increase PRA is likely due to the strength of stimulation (or the severity of the activating mutation) of the CaSR. Since a large percentage of these patients develop renal dysfunction, further studies clarifying the role of renin in these patients are necessary [118,124].
Interestingly, the potential side effects of aminoglycoside antibiotic therapy include the development of transient hypoparathyroidism, hypocalcaemia and hypercalciuria, similar to a type V Bartter Syndrome phenotype [23]. These symptoms are presumably due to an aminoglycoside-induced stimulation of the CaSR [123]. Chou et al.[24] highlighted in a literature review that the “type-V Bartter-like” syndrome led to elevated PRA in some, but not all patients treated with aminoglycosides. Whether other CaSR agonists can stimulate renin chronically remains to be determined.
Primary hyperparathyroidism
The effects of primary hyperparathyroidism on renin are controversial. Some studies suggest that patients with primary hyperparathyroidism have elevated PRA [14,15,42,63,81,97] while other studies find no relationship [10,12,115]. The reason for this discrepancy is not clear. One possibility, similar to the effects of Vitamin D-induced hypercalcemia and CaSR activating mutations, is that the effects of hyperparathyroidism on renin could be dose dependant. Whether these proposed increases in PRA contribute to the elevated renal and cardiovascular morbidity rates in these patients is unknown [129]. Similarly, the effects of chronic parathyroid hormone-related protein (PTHrP) administration on renin are unknown. PTHrP is one of the causative factors of hypercalcemia of malignancy and acts on the same receptor as PTH to exert its hypercalcemic effects [2]. While it is known to acutely stimulate renin is isolated-perfused kidneys [103], it is not known if it stimulates renin secretion chronically in vivo. Further research is needed to determine if experimentally-induced hyperparathyroidism or related hypercalcemic, neoplastic syndromes stimulate PRA.
Conclusions
Calcium has a dichotomous relationship with renin in vivo. Acutely elevated plasma calcium inhibits renin via increasing the (extracellular) renal cortical interstitial calcium, consistent with the pathways described in the extensive in vitro literature. However, chronically elevated calcium appears to (indirectly) stimulate renin secretion. While we now have a better understanding of acute inhibitory mechanisms of calcium on renin, the explanation or resolution of the renin-stimulating pathway of chronically elevated plasma calcium remain to be determined. These are likely influenced by a plethora of chronic changes associated with elevated calcium that may influence renin indirectly. Future research should also determine whether elevations in renin activity cause or contribute to cardiorenal disease in these patients.
Overall, calcium acts as an inhibitory second messenger by directly influencing the activity of the enzymes that control the synthesis and degradation of the primary second messenger cAMP within the JG cell. Thus, it acts as a regulator “upstream” from cAMP. Importantly, while calcium can directly influence the basal release of renin, it also modifies the activity and therefore the magnitude of the response of adenylyl cyclase which is the target for the classical stimulatory pathways for renin, such as β-adrenergic innervation, the macula densa pathway and the renal baroreceptor (10).
Acknowledgments
Sources of Funding:
This research was supported by funding from the National Institutes of Health (NIH) from grants F30DK084654-03 and PPG 5PO1HL090550-03. Mr. Atchison is a member of the Wayne State University School of Medicine MD/PhD program.
Footnotes
Disclosures:
There are no conflicts or disclosures to report.
This article is published as part of a special issue on the renin-angiotensin system.
BIBLIOGRAPHY
- 1.Abe Y, Yukimura T, Iwao H, Mori N, Okahara T, Yamamoto K. Effects of EDTA and verapamil on renin release in dogs. Jpn J Pharmacol. 1983;33:627–33. doi: 10.1254/jjp.33.627. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT, Jr, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M, Ritthaler T, Riegger G, Kurtz A, Kramer BK. Endothelin inhibits cAMP-induced renin release from isolated renal juxtaglomerular cells. J Cardiovasc Pharm. 1995;26(Suppl 3):S135–S137. [PubMed] [Google Scholar]
- 4.Akmal M, Bishop JE, Telfer N, Norman AW, Massry SG. Hypocalcemia and hypercalcemia in patients with rhabdomyolysis with and without acute renal failure. J Clin Endocrinol Metab. 1986;63:137–142. doi: 10.1210/jcem-63-1-137. [DOI] [PubMed] [Google Scholar]
- 5.Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension. 2011;57(3):460–8. doi: 10.1161/HYPERTENSIONAHA.110.167130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclase 5 and 6. Hypertension. 2011;57:460–468. doi: 10.1161/HYPERTENSIONAHA.110.167130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atchison DK, Harding P, Beierwaltes WH. Hypercalcemia Reduces Plasma Renin via Parathyroid Hormone, Renal Interstitial Calcium and the Calcium-Sensing Receptor. Hypertension. 2011;58:604–610. doi: 10.1161/HYPERTENSIONAHA.111.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atchison DK, Ortiz-Capisano MC, Beierwaltes WH. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am J Physiol - Regul Integr Comp Physiol. 2010;299:R1020–1026. doi: 10.1152/ajpregu.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beierwaltes WH. Nitric oxide participates in calcium-mediated regulation of renin release. Hypertension. 1994;23:I40–I44. doi: 10.1161/01.hyp.23.1_suppl.i40. [DOI] [PubMed] [Google Scholar]
- 10.Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol - Renal Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. Am J Physiol - Renal Physiol. 2006;290:F1376–F1381. doi: 10.1152/ajprenal.00209.2005. [DOI] [PubMed] [Google Scholar]
- 12.Bernini G, Moretti A, Lonzi S, Bendinelli C, Miccoli P, Salvetti A. Renin-angiotensin-aldosterone system in primary hyperparathyroidism before and after surgery. Metabolism. 1999;48:298–300. doi: 10.1016/s0026-0495(99)90075-6. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001;59:2206–2215. doi: 10.1046/j.1523-1755.2001.00736.x. [DOI] [PubMed] [Google Scholar]
- 14.Brinton GS, Jubiz W, Lagerquist LD. Hypertension in primary hyperparathyroidism: the role of the renin-angiotensin system. J Clin Endocrinol Metab. 1975;41:1025–9. doi: 10.1210/jcem-41-6-1025. [DOI] [PubMed] [Google Scholar]
- 15.Broulik PD, Horký K, Pacovský V. Blood pressure in patients with primary hyperparathyroidism before and after parathyroidectomy. Exp Clin Endocrinol. 1985;86:346–352. doi: 10.1055/s-0029-1210507. [DOI] [PubMed] [Google Scholar]
- 16.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 17.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 18.Buhrle CP, Nobiling R, Taugner R. Intracellular recordings from renin-positive cells of the afferent glomerular arteriole. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;249:F272–F281. doi: 10.1152/ajprenal.1985.249.2.F272. [DOI] [PubMed] [Google Scholar]
- 19.Buhrle CP, Scholz H, Hackenthal E, Nobiling R, Taugner R. Epithelioid cells: membrane potential changes induced by substances influencing renin secretion. Mol Cell Endocrinol. 1986;45:37–47. doi: 10.1016/0303-7207(86)90080-8. [DOI] [PubMed] [Google Scholar]
- 20.Castrop H, Lorenz JN, Hansen PB, Friis U, Mizel D, Oppermann M, Jensen BL, Briggs J, Skott O, Schnermann J. Contribution of the basolateral isoform of the Na-K-2Cl cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol. 2005;289:F1185–F1192. doi: 10.1152/ajprenal.00455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charbonneau H, Kumar S, Novack P, Blumenthal K, Griffer PR, Shabanowitz J, Hunt DF, Beavo JA, Walsh KA. Evidence for domain organization within the 61-kDcalmodulin-dependent cycle nucleotide phosphodiesterase from bovine brain. Biochemistry. 1991;30:7931–3940. doi: 10.1021/bi00246a009. [DOI] [PubMed] [Google Scholar]
- 22.Cheung WY. Cyclic nucleotide 3_,5_-nucleotide phosphodiesterase: evidence for and properties of a protein activator. Biochem Biophys Res Commun. 1970;38:533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- 23.Chou CL, Chen YH, Chau T, Lin SH. Acquired Bartter-like syndrome associated with gentamicin administration. Am J Med Sci. 2005;329:144–149. doi: 10.1097/00000441-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Churchill PC. Effect of D-600 on inhibition of in vitro renin release in the rat by high extracellular potassium and angiotensin II. J Physiol. 1980;304:449–58. doi: 10.1113/jphysiol.1980.sp013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Churchill PC. Second messengers in renin secretion. Am J Physiol. 1985;249:F175–F184. doi: 10.1152/ajprenal.1985.249.2.F175. [DOI] [PubMed] [Google Scholar]
- 26.Churchill PC, Churchill MC. Ca-dependence of the inhibitory effect of K-depolarization on renin secretion from rat kidney slices. Arch Int Pharmacodyn Ther. 1982;258:300–312. [PubMed] [Google Scholar]
- 27.Churchill PC, Churchill MC. BAY K 8644, a calcium channel agonist, inhibits renin secretion in vitro. Arch Int Pharmacodyn Ther. 1987;285:87–97. [PubMed] [Google Scholar]
- 28.Della BR, Pinet F, Corvol P, Kurtz A. Calmodulin antagonists stimulate renin secretion and inhibit renin synthesis in vitro. Am J Physiol. 1992;262:F397–F402. doi: 10.1152/ajprenal.1992.262.3.F397. [DOI] [PubMed] [Google Scholar]
- 29.Epstein S, Sagel J, Brodocky H, Tuff S, Eales L. Absence of an acute effect of calcium or parathyroid hormone administration on plasma renin activity in man. Clin Sci Mol Med. 1976;50:79–81. doi: 10.1042/cs0500079. [DOI] [PubMed] [Google Scholar]
- 30.Facemire CS, Nguyen M, Jania L, Beierwaltes WH, Koller BH, Coffman TM. A major role for the EP4 receptor in regulation of renin. Am J Physiol – Renal Physiol. 2011;301:F1035–1041. doi: 10.1152/ajprenal.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol - Renal Physiol. 2007;292:F175–F184. doi: 10.1152/ajprenal.00050.2006. [DOI] [PubMed] [Google Scholar]
- 32.Fellner SK, Arendshorst WJ. Ryanodine receptor and capacitative Ca2+ entry in fresh preglomerular vascular smooth muscle cells. Kidney Int. 2000;58:1686–1694. doi: 10.1046/j.1523-1755.2000.00329.x. [DOI] [PubMed] [Google Scholar]
- 33.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol - Renal Physiol. 2005;288:F785–F791. doi: 10.1152/ajprenal.00372.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ferrier CP, Kurtz A, Lehner P, Shaw SG, Pusterla C, Saxenhofer H, Weidmann P. Stimulation of renin secretion by potassium-channel activation with cromakalim. Eur J Clin Pharmacol. 1989;36:443–447. doi: 10.1007/BF00558067. [DOI] [PubMed] [Google Scholar]
- 35.Fox RR, Witham BA. Handbook of genetically modified mice. 5. The Jackson Laboratory; Bar Harbor, ME: 1997. Chapter 2, Definitions of inbred strains, substrains, sublines and F1 hybrids. [Google Scholar]
- 36.Fray JCS, Park CS, Valentine AND. Calcium and the control of renin secretion. Endocrine Rev. 1987;8:53–93. doi: 10.1210/edrv-8-1-53. [DOI] [PubMed] [Google Scholar]
- 37.Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skott O. control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res. 2002;90:996–1003. doi: 10.1161/01.res.0000017622.25365.71. [DOI] [PubMed] [Google Scholar]
- 38.Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Membrane potential and cation channels in rat juxtaglomerular cells. Acta Physiol Scand. 2004;181:391–396. doi: 10.1111/j.1365-201X.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 39.Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Molecular and functional identification of cAMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res. 2003;93:213–220. doi: 10.1161/01.RES.0000085041.70276.3D. [DOI] [PubMed] [Google Scholar]
- 40.Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nusing RM, Skott O, Jensen BL. Prostaglandin E2 EP2 receptor activation mediates cAMP-dependent hyper polarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol. 2005;289:F989–F997. doi: 10.1152/ajprenal.00201.2005. [DOI] [PubMed] [Google Scholar]
- 41.Garnbaryan S, Wagner C, Smolenski A, Walter U, Poller W, Haase W, Kurtz A, Lohmann SM. Endogenous or over expressed cGMP-dependent protein kinases inhibit cAMP-dependent renin release from rat isolated perfused kidney, micro dissected glomeruli, and isolated juxtaglomerular cells. Proc Natl Acad Sci USA. 1998;95:9003–9008. doi: 10.1073/pnas.95.15.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gennari C, Nami R, Gonnelli S. Hypertension and primary hyperparathyroidism: the role of adrenergic and renin-angiotensin-aldosterone systems. Miner Electrolyte Metab 1995. 1995;21:77–81. [PubMed] [Google Scholar]
- 43.Ginesi LM, Munday KA, Noble AR. Secretion control for active and inactive renin: effects of calcium and potassium on rabbit kidney cortex slices. J Physiol. 1983;344:453–463. doi: 10.1113/jphysiol.1983.sp014951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goraya TA, Masada N, Ciruela A, Willoughby D, Clynes MA, Cooper DM. Kinetic properties of Ca2+/calmodulin-dependent phosphodiesterase isoforms dictate intracellular cAMP dynamics in response to elevation of cytosolic Ca2+ Cell Signal. 2008;20:359–374. doi: 10.1016/j.cellsig.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. the calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylyate cyclases AC5 and AC6. Circ Res. 2006;99:1197–1206. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 46.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 47.Hackenthal E, Schwertschlag U, Taugner R. Cellular mechanisms of renin release. Clin Exp Hypertens A. 1983;5:975–993. doi: 10.3109/10641968309048836. [DOI] [PubMed] [Google Scholar]
- 48.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Mérillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyper absorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 50.Ichihara A, Suzuki H, Murakami M, Naitoh M, Matsumoto A, Saruta T. Interactions between angiotensin II and norepinephrine on renin release by juxtaglomerular cells. Eur J Endocrinol. 1995;133:569–577. doi: 10.1530/eje.0.1330569. [DOI] [PubMed] [Google Scholar]
- 51.Isaac R, Raymond JP, Rainfray M, Ardaillou R. Effects of an acute calcium load on plasma ACTH, Cortisol, aldosterone and renin activity in man. Acta Endocrinol (Copenh) 1984;105:251–257. doi: 10.1530/acta.0.1050251. [DOI] [PubMed] [Google Scholar]
- 52.Iwao, Abe Y, Yamamoto K. Effect of intrarenal arterial infusion of calcium in renin release in dogs. Jpn J Pharmacol. 1974;24:482–484. doi: 10.1254/jjp.24.482. [DOI] [PubMed] [Google Scholar]
- 53.Jensen BL, Gambaryan S, Scholz H, Kurtz A. KATP channels are not essential for pressure-dependent control of renin secretion. Pflugers Arch. 1998;435:670–677. doi: 10.1007/s004240050568. [DOI] [PubMed] [Google Scholar]
- 54.Jensen BL, Skott O. Blockade of chloride channels by DIDS stimulates renin release and inhibits contraction of afferent arterioles. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F718–F727. doi: 10.1152/ajprenal.1996.270.5.F718. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest. 1996;98:723–730. doi: 10.1172/JCI118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 57.Kincaid RL, Stith-Coleman IE, Vaughan M. Proteolytic activation of calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biol Chem. 1985;260:9009–9015.6c. [PubMed] [Google Scholar]
- 58.Kisch ES, Dluhy RG, Williams GH. Regulation of renin release by calcium and ammonium ions in normal man. J Clin Endocrinol Metab. 1976;43:1343–1350. doi: 10.1210/jcem-43-6-1343. [DOI] [PubMed] [Google Scholar]
- 59.Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol. 2006;104:73–80. doi: 10.1159/000094001. [DOI] [PubMed] [Google Scholar]
- 60.Kotchen TA, Galla JH, Luke RG. Effects of calcium on renin and aldosterone in the rat. Am J Physiol. 1977;232:E388–E393. doi: 10.1152/ajpendo.1977.232.4.E388. [DOI] [PubMed] [Google Scholar]
- 61.Kotchen TA, Maull KI, Kotchen JM, Luke RG. Effect of calcium gluconate infusion in the dog. J Lab Clin Med. 1977;89:181–189. [PubMed] [Google Scholar]
- 62.Kotchen TA, Mauli KI, Luke R, Rees D, Flamenbaum W. Effect of acute and chronic calcium administration on plasma renin. J Clin Invest. 1974;54:1279–1286. doi: 10.1172/JCI107873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovács L, Góth MI, Szabolcs I, Dohán O, Ferencz A, Szilágyi G. The effect of surgical treatment on secondary hyperaldosteronism and relative hyperinsulinemia in primary hyperparathyroidism. Eur J Endocrinol. 1998;138:543–547. doi: 10.1530/eje.0.1380543. [DOI] [PubMed] [Google Scholar]
- 64.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin-40 regulates renin production and blood pressure. Kidney Intl. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 65.Kurtz A, Dela Bruna R. Determinants of renin secretion and renin synthesis in isolated mouse juxtaglomerular cells. Kidney Int Suppl. 1991;32:S13–S15. [PubMed] [Google Scholar]
- 66.Kurtz A, Della Bruna R, Kuhn K. Cyclosporine A enhances renin secretion and production in isolated juxtaglomerular cells. Kidney Int. 1988;33:947–953. doi: 10.1038/ki.1988.92. [DOI] [PubMed] [Google Scholar]
- 67.Kurtz A, Götz KH, Hamann M, Wagner C. Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci USA. 1998;95:4743–4747. doi: 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurtz A, Hamann M, Gotz K. Role of potassium channels in the control of renin secretion from isolated perfused rat kidneys. Pflugers Arch. 2000;440:889–895. doi: 10.1007/s004240000373. [DOI] [PubMed] [Google Scholar]
- 69.Kurtz A, Pfeilschifter J, Bauer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun. 1984;124:359–366. doi: 10.1016/0006-291x(84)91561-4. [DOI] [PubMed] [Google Scholar]
- 70.Kurtz A, Pfeilschifter J, Baurer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun. 1984;124:359–366. doi: 10.1016/0006-291x(84)91561-4. [DOI] [PubMed] [Google Scholar]
- 71.Kurtz A, Pfeilschifter J, Hutter A, Burhle C, Nobiling R, Taugner R, Hackenthal E, Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol – Cell Physiol. 1986;250:C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- 72.Kurtz A, Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci USA. 1989;86:3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurtz A, Pfeilschifter J, Hutter A, Buhrle C, Nobiling R, Taugner R, Hackenthal E, Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol Cell Physiol. 1986;250:C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- 74.Kurtz A, Skott O, Chegini S, Penner R. Lack of direct evidence for a functional role of voltage-operated calcium channels in juxtaglomerular cells. Pflugers Arch. 1990;416:281–287. doi: 10.1007/BF00392064. [DOI] [PubMed] [Google Scholar]
- 75.Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin-angiotensin system, and calcium. J Clin Invest. 1983;71:1624–1632. doi: 10.1172/JCI110918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Llach F, Weidmann P, Reinhart R, Maxwell MH, Coburn JW, Massry SG. Effect of acute and long-standing hypocalcemia on blood pressure and plasma renin activity in man. J Clin Endocrinol Metab. 1974;38:841–847. doi: 10.1210/jcem-38-5-841. [DOI] [PubMed] [Google Scholar]
- 77.Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol. 1991;260:F486–493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 78.Maillard MP, Tedjani A, Perregaux C, Burnier M. Calcium-sensing receptors modulate renin release in vivo and in vitro in the rat. J Hypertens. 2009;27:1980–1987. doi: 10.1097/HJH.0b013e32832f0d22. [DOI] [PubMed] [Google Scholar]
- 79.Moe O, Tejedor A, Campbell WB, Alpern RJ, Henrich WL. Effects of endothelin on in vitro renin secretion. Am J Physiol Endocrine Metab. 1991;260:E521–E525. doi: 10.1152/ajpendo.1991.260.4.E521. [DOI] [PubMed] [Google Scholar]
- 80.Nabel C, Schweda F, Riegger GA, Kramer BK, Kurtz A. Chloride channel blockers attenuate the inhibition of renin secretion by angiotensin II. Pflugers Arch. 1999;438:694–699. doi: 10.1007/s004249900095. [DOI] [PubMed] [Google Scholar]
- 81.Naomi S, Umeda T, Iwaoka T, Sato T, Uemura K. A case of functioning parathyroid carcinoma with hypereninemic hypertension. Jpn J Med. 1983;22:129–133. doi: 10.2169/internalmedicine1962.22.129. [DOI] [PubMed] [Google Scholar]
- 82.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 4. WH Freeman and Co; New York: 2005. Chapter 12: Biosignalling. [Google Scholar]
- 83.Nemeth EF, Scarpa A. Rapid mobilization of cellular Ca2+ in bovine parathyroid cells evoked by extracellular divalent cations. Evidence for a cell surface calcium receptor. J Biol Chem. 1987;202:5188–5196. [PubMed] [Google Scholar]
- 84.Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and Function of the Calcium-sensing Receptor in Juxtaglomerular Cells. Hypertension. 2007;50:738–744. doi: 10.1161/HYPERTENSIONAHA.107.095158. [DOI] [PubMed] [Google Scholar]
- 85.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates cAMP and renin release via calcium-inhibitable adenylyl cyclase. Hypertension. 2007;49:162–169. doi: 10.1161/01.HYP.0000250708.04205.d4. [DOI] [PubMed] [Google Scholar]
- 86.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform V mediates renin release from juxtaglomerular cells. Hypertension. 2007;49:618–624. doi: 10.1161/01.HYP.0000255172.84842.d2. [DOI] [PubMed] [Google Scholar]
- 87.Ortiz-Capisano MC, Liao T-D, Ortiz PA, Beierwaltes WH. Calcium-dependent phosphodiesterase 1C mediates renin release from isolated juxtaglomerular cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1469–R1476. doi: 10.1152/ajpregu.00121.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palmer CE, Rudd MA, Bukoski RD. Renal interstitial Ca2+ during sodium loading of normotensive and Dahl-salt hypertensive rats. Am J Hypertens. 2003;16:771–776. doi: 10.1016/s0895-7061(03)00914-2. [DOI] [PubMed] [Google Scholar]
- 89.Pandey KN, Ascoli M, Inagami T. Induction of renin activity by gonadotropic hormones in cultured Leydig tumor cells. Endocrinology. 1985;117:2120–2126. doi: 10.1210/endo-117-5-2120. [DOI] [PubMed] [Google Scholar]
- 90.Park CS, Malvin RL. Calcium in the control of renin release. Am J Physiol. 1978;235:F22–F25. doi: 10.1152/ajprenal.1978.235.1.F22. [DOI] [PubMed] [Google Scholar]
- 91.Park CS, Honeyman TW, Chung ES, Lee JS, Sigmon DH, Fray JC. Involvement of calmodulin in mediating inhibitory action of intracellular Ca2+ on renin secretion. Am J Physiol. 1986;251:F1055–F1062. doi: 10.1152/ajprenal.1986.251.6.F1055. [DOI] [PubMed] [Google Scholar]
- 92.Peart WS, Roddis SA, Unwin RJ. Renal electrolyte excretion and renin release during calcium and parathormone infusions in conscious rabbits. J Physiol. 1986;373:329–341. doi: 10.1113/jphysiol.1986.sp016050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterson LN. Vitamin D-induced chronic hypercalcemia inhibits thick ascending limb NaCl reabsorption in vivo. Am J Physiol. 1990;259:F122–F129. doi: 10.1152/ajprenal.1990.259.1.F122. [DOI] [PubMed] [Google Scholar]
- 94.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol – Renal Physiol. 2006;291:F473–F480. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 95.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 96.Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA. 1995;92:131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richards AM, Espiner EA, Nicholls MG, Ikram H, Hamilton EJ, Maslowski AH. Hormone, calcium and blood pressure relationships in primary hyperparathyroidism. J Hypertens. 1988;6:747–752. doi: 10.1097/00004872-198809000-00009. [DOI] [PubMed] [Google Scholar]
- 98.Ritthaler T, Della-Bruna R, Kramer BK, Kurtz A. Endothelin inhibits cAMP induced renin gene expression in cultured mouse juxtaglomerular cells. Kidney Int. 1996;50:108–115. doi: 10.1038/ki.1996.293. [DOI] [PubMed] [Google Scholar]
- 99.Ruan X, Arendshorst WJ. Calcium entry and mobilization signaling pathways in ANG II-induced renal vasoconstriction in vivo. Am J Physio – Renal Physiol. 1996;270:F398–F405. doi: 10.1152/ajprenal.1996.270.3.F398. [DOI] [PubMed] [Google Scholar]
- 100.Russ U, Rauch U, Quast U. Pharmacological evidence for a KATP channel in renin-secreting cells from rat kidney. J Physiol. 1999;517:781–790. doi: 10.1111/j.1469-7793.1999.0781s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003b;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 102.Salahudeen AK, Thomas TH, Sellars L, Tapster S, Keavey P, Farndon JR, Johnston ID, Wilkinson R. Hypertension and renal dysfunction in primary hyperparathyroidism: effect of parathyroidectomy. Clin Sci (Lond) 1989;76:289–296. doi: 10.1042/cs0760289. [DOI] [PubMed] [Google Scholar]
- 103.Saussine C, Massfelder T, Parnin F, Judes C, Simeoni U, Helwig JJ. Renin stimulating properties of parathyroid hormone-related peptide in the isolated perfused rat kidney. Kidney Int. 1993;44:764–773. doi: 10.1038/ki.1993.311. [DOI] [PubMed] [Google Scholar]
- 104.Scholz H, Kurtz A. Differential regulation of cytosolic calcium between affterent arteriol or smooth muscle cells from mouse kidney. Pflugers Arch – Europ J Physiol. 1995;43:46–51. doi: 10.1007/BF00374376. [DOI] [PubMed] [Google Scholar]
- 105.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology. 2007;22:310–319. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 106.Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol - Renal Physiol. 2004;287:F427–F433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 107.Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand. 2004;181:383–390. doi: 10.1111/j.1365-201X.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 108.Schweda F, Riegger GA, Kurtz A, Kramer BK. Store-operated calcium influx inhibits renin secretion. Am J Physiol - Renal Physiol. 2000;279:F170–F176. doi: 10.1152/ajprenal.2000.279.1.F170. [DOI] [PubMed] [Google Scholar]
- 109.Sonnenburg WK, Seger D, Kwak KS, Huang J, Charbonneau H, Beavo JA. Identification of inhibitory and calmodulin-binding domains of the PDE1A1 and PDE1A2 calmodulin-stimulated cyclic nucleotide phosphodiesterases. J Biol Chem. 1995;270:989–1000. doi: 10.1074/jbc.270.52.30989. [DOI] [PubMed] [Google Scholar]
- 110.Sowers JR, Barrett JD. Hormonal changes associated with hypertension in neoplasia-induced hypercalcemia. Am J Physiol. 1982;242:E330–E334. doi: 10.1152/ajpendo.1982.242.5.E330. [DOI] [PubMed] [Google Scholar]
- 111.Spangler WL, Gribble DH, Lee TC. Vitamin D intoxication and the pathogenesis of vitamin D nephropathy in the dog. Am J Vet Res. 1979;40:73–83. [PubMed] [Google Scholar]
- 112.Takagi M, Matsuoka H, Atarashi K, Yagi S. Endothelin: a new inhibitor of renin release. Biochem Biophys Res Commun. 1988;157:1164–1168. doi: 10.1016/s0006-291x(88)80996-3. [DOI] [PubMed] [Google Scholar]
- 113.Toma I, Bansal E, Meer E, Kang J, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP dependent calcium wave in renal glomerular endothelial cells. Am J Physiol – Reg Integ Comp Physiol. 2008;294:R1769–R1776. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tu WH. Plasma renin activity in acute tubular necrosis and other renal diseases associated with hypertension. Circulation. 1965;31:686–695. doi: 10.1161/01.cir.31.5.686. [DOI] [PubMed] [Google Scholar]
- 115.Valvo E, Bedogna V, Gammaro L, Casagrande P, Ortalda V, Maschio G. Systemic hemodynamic pattern in primary hyperparathyroidism and its changes after parathyroidectomy. Miner Electrolyte Metab. 1991;17:147–152. [PubMed] [Google Scholar]
- 116.VanDongen R, Pert WS. Calcium dependence of the inhibitory effect of angiotensin on renin secretion in the isolated perfused kidney of the rat. Brit J Pharmacol. 1974;50:125–129. doi: 10.1111/j.1476-5381.1974.tb09599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varault A, Pena MS, Goldsmith PK, Mithal A, Brown EM, Spiegel AM. Expression of G protein alpha-subunits in bovine parathyroid. Endocrinol. 1995;136:4390–4396. doi: 10.1210/endo.136.10.7664659. [DOI] [PubMed] [Google Scholar]
- 118.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–2266. doi: 10.1097/01.asn.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 119.Vezzoli G, Arcidiacono T, Paloschi V, Terranegra A, Biasion R, Weber G, Mora S, Syren ML, Coviello D, Cusi D, Bianchi G, Soldati L. Autosomal dominant hypocalcemia with mild type 5 Bartter syndrome. J Nephrol. 2006;19:525–528. [PubMed] [Google Scholar]
- 120.Wagner C. Function of connexins in the renal circulation. Kidney Intl. 2008;73:547–555. doi: 10.1038/sj.ki.5002720. [DOI] [PubMed] [Google Scholar]
- 121.Wagner C, deWit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin 40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 122.Wagner C, Pfeifer A, Ruth P, Hoffmann F, Kurtz A. Role of cGMP kinase II in the control of renin secretion and renin expression. J Clin Invest. 1998;102:1576–1582. doi: 10.1172/JCI4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ward DT, Maldonado-Pérez D, Hollins L, Riccardi D. Aminoglycosides induce acute cell signaling and chronic cell death in renal cells that express the calcium-sensing receptor. J Am Soc Nephrol. 2005;16:1236–1244. doi: 10.1681/ASN.2004080631. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 125.Watkins BE, Davis JO, Lohmeier TE, Freeman RH. Intrarenal site of action on calcium on renin secretion in dogs. Circ Res. 1976;39:847–853. doi: 10.1161/01.res.39.6.847. [DOI] [PubMed] [Google Scholar]
- 126.Wilcox CS. The effect of increasing the plasma magnesium concentration on renin release from the dog’s kidney: interactions with calcium and sodium. J Physiol. 1978;284:203–217. doi: 10.1113/jphysiol.1978.sp012536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan C, Zhao AZ, Sonnenburg WK, Beavo JA. Stage and cell-specific expression of calmodulin-dependent phosphodiesterases in mouse testis. Biol Reprod. 2001;64:1746–1754. doi: 10.1095/biolreprod64.6.1746. [DOI] [PubMed] [Google Scholar]
- 128.Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATP-dependent mechanisms for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res. 2003;93:338–345. doi: 10.1161/01.RES.0000086802.21850.5D. [DOI] [PubMed] [Google Scholar]
- 129.Yu N, Donnan PT, Leese GP. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: the Parathyroid Epidemiology and Audit Research Study (PEARS) Clin Endocrinol (Oxf) 2011;75:169–176. doi: 10.1111/j.1365-2265.2010.03958.x. [DOI] [PubMed] [Google Scholar]
- 130.Zawada ET, Jr, Johnson M. Effects of changes in serum calcium and parathyroid hormone on plasma renin in intact mongrel dogs. Nephron. 1985;40:368–371. doi: 10.1159/000183495. [DOI] [PubMed] [Google Scholar]