Abstract
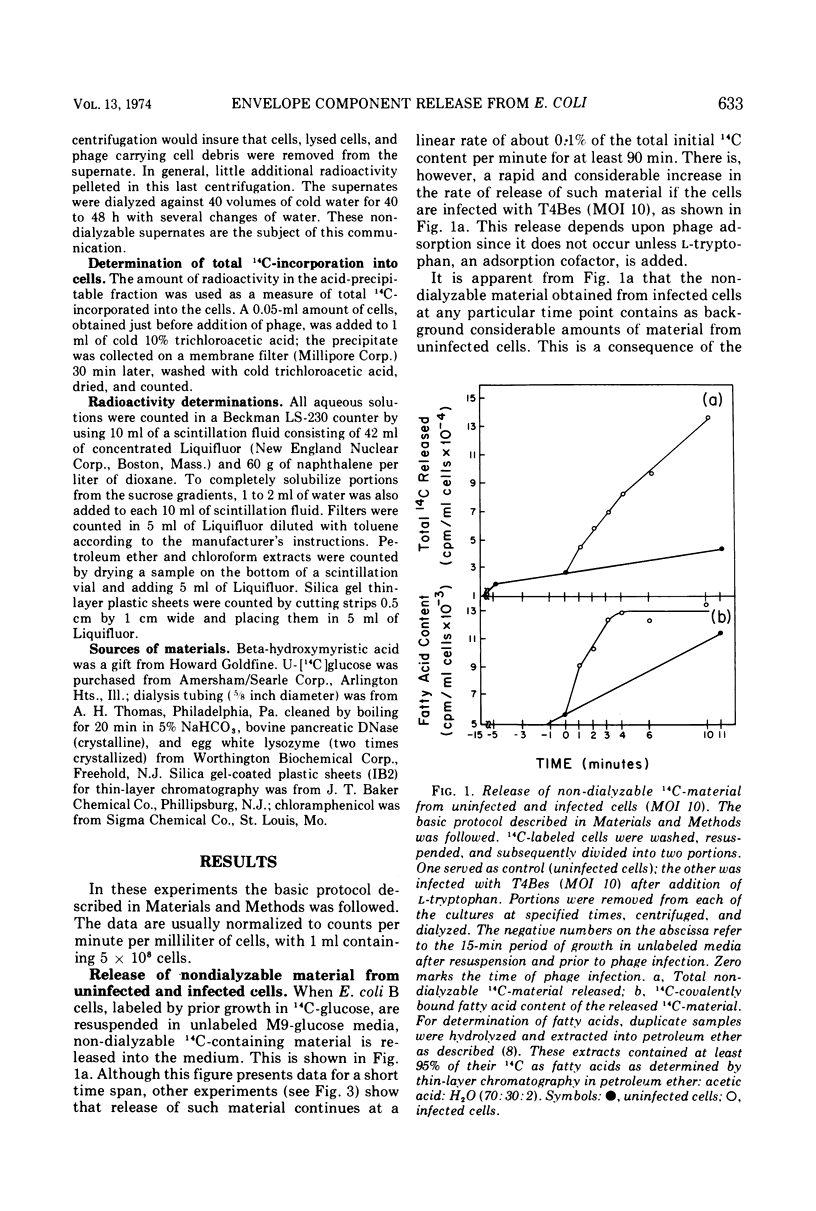
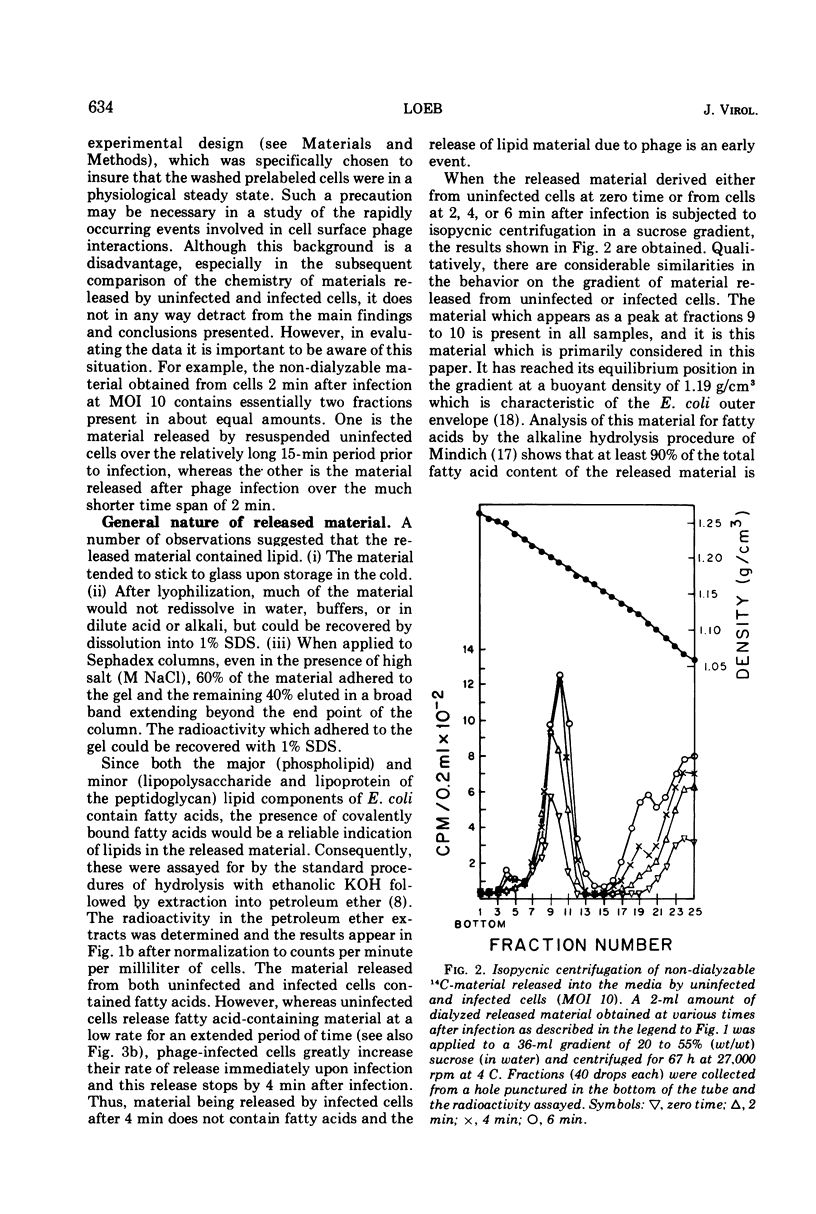
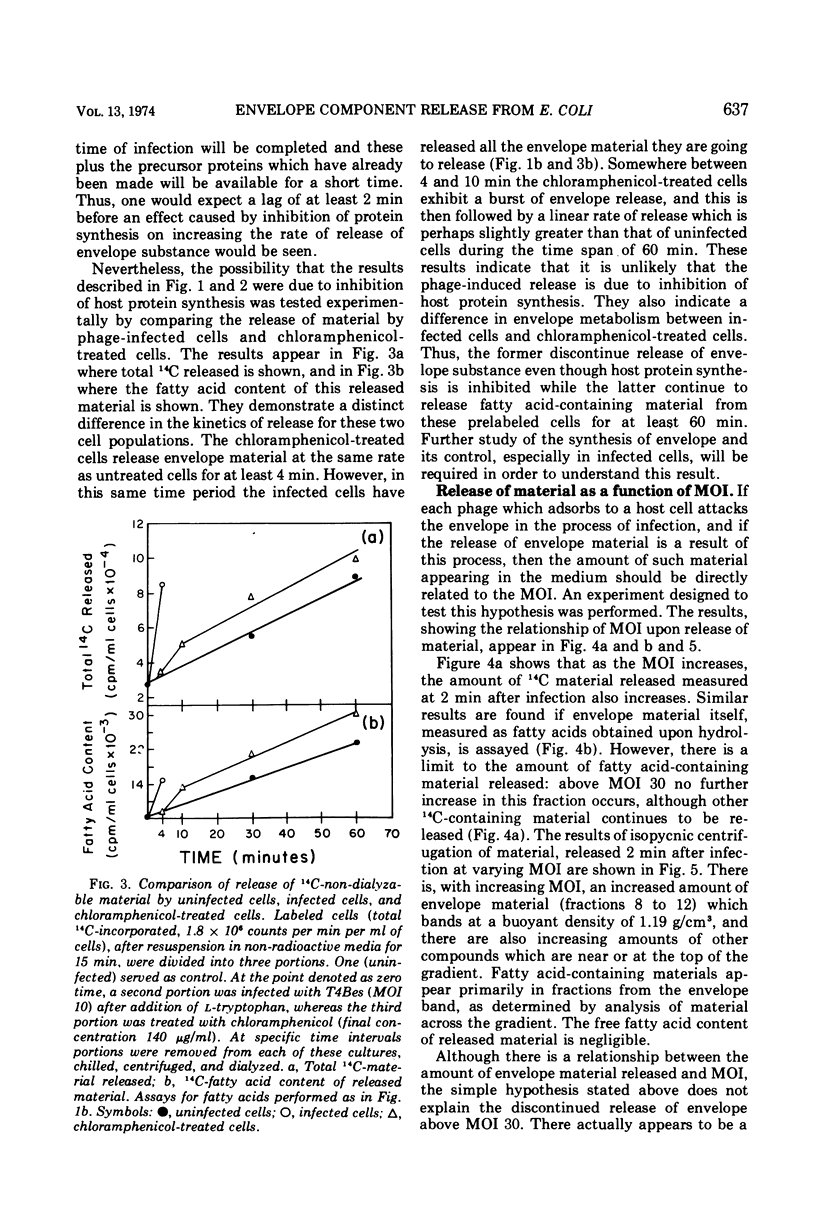
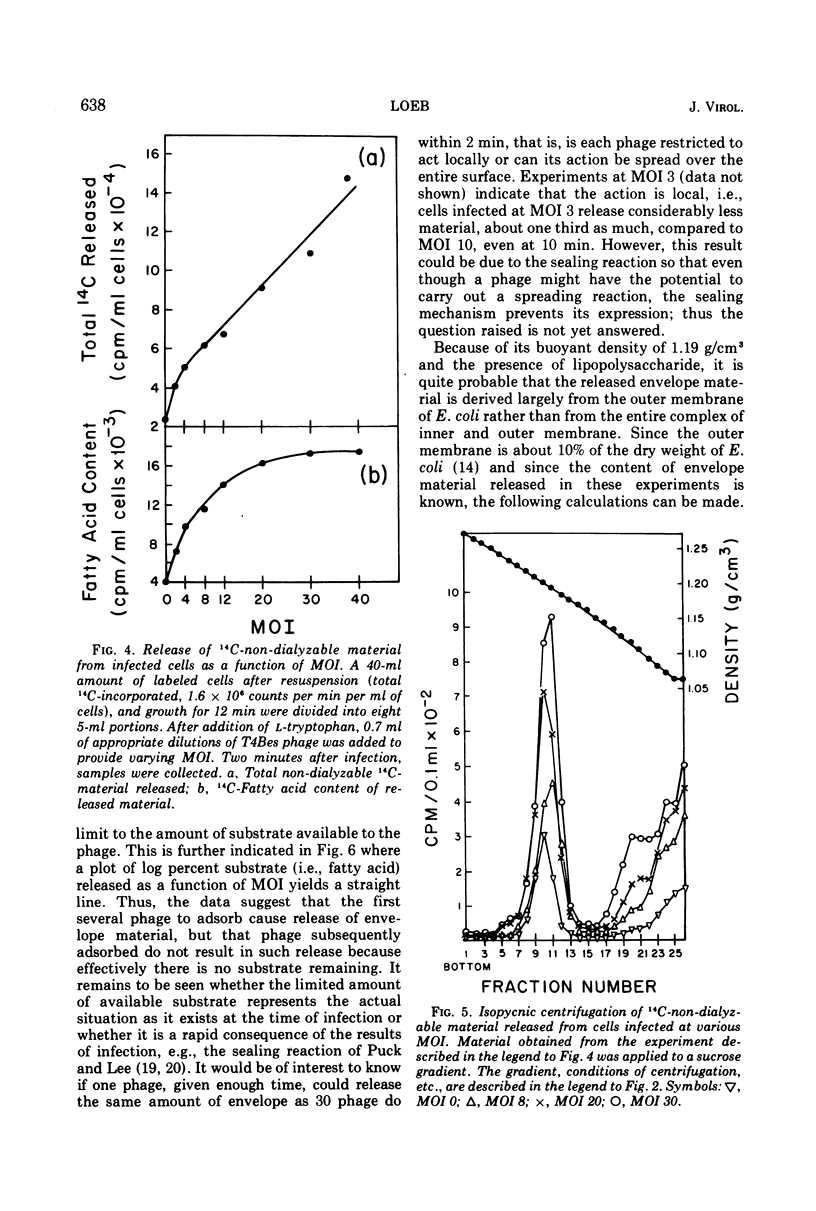
When Escherichia coli B, labeled by prior growth in 14C-glucose, are infected with T4 phage there is a rapid release of 14C-nondialyzable material into the medium. About half of this material is derived from the cell envelope as evidenced by its content of phospholipid and lipopolysaccharide and its buoyant density upon isopycnic ultracentrifugation of 1.19 g/cm3. It is similar in its gross chemical and physical properties to envelope material released at a lower rate from growing uninfected cells or from cells whose protein synthesis is inhibited by chloramphenicol (22). The rate of release of this envelope material at a multiplicity of infection (MOI) of 10 is greatest in the first minute after infection, and release is completed by 4 min. The rate of its release, as a function of MOI at 2 min after infection, is greatest at low MOI (e.g., MOI 2 and 4); in addition, the release does not continue above MOI 30. The main conclusion derived from the data is that phage, as part of the process of adsorption and injection of DNA, cause an increased release of envelope substance from the cells. With the assumption that all of the envelope material released is derived from the outer envelope, it is estimated that uninfected cells release 20 to 30% of their outer envelope per hour, whereas infected cells release 30% in 2 min at MOI 30. Further, because release does not continue at high MOI, this phenomenon is not considered to be a direct cause of lysis from without. Data are also presented on the amounts of other non-dialyzable 14C-components released and on the differences in the kinetics of release from chloramphenicol-treated cells compared to phage-infected cells. To avoid the possibility that the release is due to phage lysozyme which is an adventitious “contaminant” of wild-type phage, a phage mutant (T4BeG59s) devoid of this enzyme was used in these experiments.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRINGTON L. F., KOZLOFF L. M. Action of bacteriophage on isolated host cell walls. J Biol Chem. 1956 Dec;223(2):615–627. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BROWN D. D., KOZLOFF L. M. Morphological localization of the bacteriophage tail enzyme. J Biol Chem. 1957 Mar;225(1):1–11. [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Emrich J. Lysis of T4-infected bacteria in the absence of lysozyme. Virology. 1968 May;35(1):158–165. doi: 10.1016/0042-6822(68)90315-2. [DOI] [PubMed] [Google Scholar]
- Emrich J., Streisinger G. The role of phage lysozyme in the life cycle of phage T4. Virology. 1968 Nov;36(3):387–391. doi: 10.1016/0042-6822(68)90163-3. [DOI] [PubMed] [Google Scholar]
- Goldman E., Lodish H. F. T4 phage and T4 ghosts inhibit f2 phage replication by different mechanisms. J Mol Biol. 1973 Feb 25;74(2):151–161. doi: 10.1016/0022-2836(73)90104-6. [DOI] [PubMed] [Google Scholar]
- Israeli M., Artman M. Leakage of beta-galactosidase from phage-infected Escherichia coli: a re-evaluation. J Gen Virol. 1970;7(2):137–142. doi: 10.1099/0022-1317-7-2-137. [DOI] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. II. Induction of host messenger ribonucleic acid and its exclusion from polysomes. J Virol. 1970 Aug;6(2):208–217. doi: 10.1128/jvi.6.2.208-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male C. J., Kozloff L. M. Function of T4D structural dihydrofolate reductase in bacteriophage infection. J Virol. 1973 Jun;11(6):840–847. doi: 10.1128/jvi.11.6.840-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. I. An increase in host cell permeability induced by bacteriophage infection. J Exp Med. 1954 May 1;99(5):481–494. doi: 10.1084/jem.99.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. II. Demonstration of cyclic permeability change accompanying virus infection of Escherichia coli B cells. J Exp Med. 1955 Feb 1;101(2):151–175. doi: 10.1084/jem.101.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H., Sweeley C. C. The identification of trans-2-tetradecenoic acid in hydrolysates of lipid A from Escherichia coli. Biochim Biophys Acta. 1972 Jul 7;270(3):289–295. doi: 10.1016/0005-2760(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI M., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. II. SYNTHESIS OF PHAGE-INDUCED RNA AND SEQUENTIAL ENZYME PRODUCTION. J Mol Biol. 1964 May;8:638–659. doi: 10.1016/s0022-2836(64)80114-5. [DOI] [PubMed] [Google Scholar]
- Silver S. Acridine sensitivity of bacteriophage T2: a virus gene affecting cell permeability. J Mol Biol. 1967 Oct 14;29(1):191–202. doi: 10.1016/0022-2836(67)90190-8. [DOI] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Reeves P. Physiology of Escherichia coli K-12 during conjugation: altered recipient cell functions associated with lethal zygosis. J Bacteriol. 1973 Apr;114(1):11–17. doi: 10.1128/jb.114.1.11-17.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D. The properties of x-ray inactivated bacteriophage. I. Inactivation by direct effect. J Bacteriol. 1950 Dec;60(6):697–718. doi: 10.1128/jb.60.6.697-718.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Luftig R. B., Wood W. B. Interaction of bacteriophage T4 tail fiber components with a lipopolysaccharide fraction from Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):423–434. doi: 10.1016/0022-2836(70)90152-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y. Enzymatic activities on cell walls in bacteriophage T4. Biochim Biophys Acta. 1969 May 27;178(3):542–550. doi: 10.1016/0005-2744(69)90223-x. [DOI] [PubMed] [Google Scholar]


