Abstract
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) frequently causes skin and soft tissue infections, including impetigo, cellulitis, folliculitis, and infected wounds and ulcers. Uncomplicated CA-MRSA skin infections are typically managed in an outpatient setting with oral and topical antibiotics and/or incision and drainage, whereas complicated skin infections often require hospitalization, intravenous antibiotics, and sometimes surgery. The aim of this study was to develop a mouse model of CA-MRSA wound infection to compare the efficacy of commonly used systemic and topical antibiotics. A bioluminescent USA300 CA-MRSA strain was inoculated into full-thickness scalpel wounds on the backs of mice and digital photography/image analysis and in vivo bioluminescence imaging were used to measure wound healing and the bacterial burden. Subcutaneous vancomycin, daptomycin, and linezolid similarly reduced the lesion sizes and bacterial burden. Oral linezolid, clindamycin, and doxycycline all decreased the lesion sizes and bacterial burden. Oral trimethoprim-sulfamethoxazole decreased the bacterial burden but did not decrease the lesion size. Topical mupirocin and retapamulin ointments both reduced the bacterial burden. However, the petrolatum vehicle ointment for retapamulin, but not the polyethylene glycol vehicle ointment for mupirocin, promoted wound healing and initially increased the bacterial burden. Finally, in type 2 diabetic mice, subcutaneous linezolid and daptomycin had the most rapid therapeutic effect compared with vancomycin. Taken together, this mouse model of CA-MRSA wound infection, which utilizes in vivo bioluminescence imaging to monitor the bacterial burden, represents an alternative method to evaluate the preclinical in vivo efficacy of systemic and topical antimicrobial agents.
INTRODUCTION
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) skin and soft tissue infections (SSTIs), such as impetigo, folliculitis, cellulitis, and infected wounds and ulcers, have been increasing for more than a decade and are creating a serious public health concern (1, 2). In particular, outpatient and emergency room visits for SSTIs have been estimated to result in 11.6 to 14.2 million ambulatory care visits per year in the United States (3, 4). In 2004 and 2008, CA-MRSA was identified as the most common cause (59%) of all SSTIs presenting to emergency rooms across the United States (5, 6). In these and other studies, the USA300 clone has been isolated in up to 90% of all CA-MRSA SSTIs in the United States (5–8). USA300 causes severe and necrotic SSTIs and often causes infections in otherwise healthy individuals without any known risk factors for infection (1, 2, 9).
Uncomplicated CA-MRSA SSTIs, such as impetigo, infected abrasions, and folliculitis/furunculosis can be managed in an outpatient setting with oral antibiotics and/or incision and drainage (10–12). Typical oral antibiotic regimens used for CA-MRSA infections include trimethoprim-sulfamethoxazole (TMP/SMX), a tetracycline (doxycycline or minocycline), clindamycin or linezolid, and rifampin can be added as a second agent to these regimens (10–12). Complicated CA-MRSA SSTIs such as deeper soft tissue infections, surgical/traumatic wound infections, major abscesses, cellulitis, and infected ulcers and burns require intravenous antibiotics and sometimes surgical debridement (12, 13). Commonly used intravenous antibiotics with coverage against CA-MRSA include vancomycin, linezolid, daptomycin, telavancin, clindamycin, tigecycline, and quinupristin-dalfopristin (12–14). Topical antibiotics can also play an adjunctive role in CA-MRSA SSTIs, including impetigo, infected abrasions/lacerations, infections with poor blood supply (i.e., diabetic foot ulcers) and in the prevention of postsurgical wound infections (1, 10, 11). Mupirocin is a commonly used topical antibiotic for the treatment of CA-MRSA SSTIs (1, 10, 11) and is also used for decolonization of S. aureus and MRSA nasal carriage (15). Retapamulin is a newer prescription-strength topical antibiotic that can also be used to treat S. aureus and MRSA SSTIs (1, 10, 16).
A preclinical animal model system is an important step to evaluate the efficacy of an antimicrobial agent before more extensive studies are performed in human subjects. Previous animal models to evaluate S. aureus/MRSA SSTIs include a tape-stripping model (17, 18), a burned skin model (19–21), and a skin surgical/suture wound (22–25). However, in these models, large numbers of animals are required because animals need to be euthanized at various time points after infection to evaluate the ex vivo bacterial burden by performing traditional CFU counting. The aim of the present study was to develop a mouse model of CA-MRSA wound infection using a newly generated USA300 strain that possesses a stable bioluminescent construct to compare the efficacy of commonly used systemic and topical antibiotics.
MATERIALS AND METHODS
CA-MRSA bioluminescent strain.
The USA300 LAC::lux bioluminescent CA-MRSA strain, derived from the USA300 LAC parent strain, was used in all experiments (26). USA300 LAC::lux possesses a modified luxABCDE operon from the bacterial insect pathogen Photorhabdus luminescens, which was chromosomally transduced from the Xen29 bioluminescent S. aureus strain (Caliper, Perkin-Elmer Company, Alameda, CA) (27). This resulted in a bioluminescent USA300 LAC::lux strain that constitutively emits a blue-green light with a maximal emission wavelength of 490 nm (only live and metabolically active bacteria will emit light). The bioluminescent construct is stably integrated into the bacterial chromosome and is maintained in all progeny without selection.
Preparation of bacteria for inoculation.
USA300 LAC::lux bacteria were streaked onto tryptic soy agar plates (tryptic soy broth [TSB] plus 1.5% Bacto agar [BD Biosciences, Franklin Lakes, NJ]) and grown at 37°C overnight (28). Single bacterial colonies were cultured in TSB and grown overnight at 37°C in a shaking incubator (MaxQ 4450; Thermo Fisher Scientific, Waltham, MA). Mid-logarithmic-phase bacteria were obtained after a 2-h subculture of a 1/50 dilution of the overnight culture. Bacteria were pelleted, resuspended, and washed three times in phosphate-buffered saline (PBS). Bacterial inocula (2 × 105 CFU, 2 × 106 CFU, or 2 × 107 CFU in 10 μl of PBS) were estimated by measuring the absorbance at 600 nm (Biomate 3; Thermo Fisher Scientific). CFU were verified after an overnight culture.
Mice.
Six-week-old male C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). In some experiments, 12-week-old NONcNZO10/LtJ male mice (Jackson Laboratories) on a 10 to 11% (wt/wt) chow diet (LabDiet 5k20; PMI Nutrition International, St. Louis, MO) were used (29). NONcNZO10/LtJ male mice by 10 weeks of age develop a disease closely mimicking human type 2 diabetes with visceral obesity, hyperglycemia, dyslipidemia, moderate liver steatosis, and pancreatic islet atrophy (29) and were confirmed to have hyperglycemia (blood glucose levels > 300 mg/dl) before they were used in experiments. Mice were housed in one mouse per cage and in specific-pathogen-free conditions.
Mouse model of CA-MRSA skin wound infection using in vivo bioluminescence imaging.
All procedures were approved by the UCLA Animal Research Committee. Mice were anesthetized with inhalation isoflurane (2%), the posterior upper backs were shaved and three parallel 8-mm linear full-thickness scalpel cuts (#11 blade) were made through the dermis (28). The wounds were subsequently inoculated with USA300 LAC::lux (2 × 105 CFU, 2 × 106 CFU, or 2 × 107 CFU in 10 μl of PBS) using a micropipette. To obtain measurements of wound sizes, mice were anesthetized with inhalation isoflurane (2%) at several different time points after infection (e.g., days 0, 1, 3, 5, 7, and 10) and digital photographs of the infected-skin wounds were taken. The total lesion size (cm2) was quantified by using the image analysis software program ImageJ (NIH Research Services Branch [http://rsbweb.nih.gov/ij/]) and a millimeter ruler as a reference. A measurement of bacterial burden was obtained by performing in vivo bioluminescence imaging at the same time points using the Lumina II imaging system (Caliper). In vivo bioluminescence imaging data are presented on a color-scale overlaid on a grayscale photograph of mice and quantified as total flux (photons/s) within a circular region of interest using Living Image software (Caliper). In some experiments, to confirm that the in vivo bioluminescent signals accurately represented the bacterial burden in vivo, CFU counts were determined after overnight cultures of homogenized (Pro200 Series homogenizer; Pro Scientific, Oxford, CT) 8-mm punch biopsy specimens of lesional skin taken at 4 h and on days 1, 3, 5, and 7 after inoculation. Typically, 5 to 10 mice per group were used, and the numbers of mice used in each experiment are indicated in the figure legends.
Subcutaneous and oral antibiotic therapy.
Based on previously published studies, mice were administered subcutaneous (to the flank skin) or oral (via gavage) therapeutic doses of vancomycin (110 mg/kg administered subcutaneously twice daily) (30, 31) (Novaplus; Hospira, Inc., Lake Forest, IL), daptomycin (50 mg/kg administered subcutaneously daily) (32, 33) (Cubicin; Cubist Pharmaceuticals, Inc., Lexington, MA), linezolid (60 mg/kg administered subcutaneously and orally twice daily) (34) (Zyvox; Pfizer, Inc., New York, NY), clindamycin (100 mg/kg administered orally three times a day) (35, 36) (Cleocin phosphate; Pfizer, Inc.), doxycycline (100 mg/kg orally twice daily) (33), and TMP-SMX (320/1,600 mg/kg administered orally twice daily) (37, 38). Linezolid was used at the same dose for subcutaneous and oral administration because of equivalent bioavailability when given via either route (39). These doses were chosen to approximate the free-drug area under the curve (AUC) of typical human doses of intravenous vancomycin (440 μg·h/ml for 1 g twice daily) (40), intravenous daptomycin (598 μg·h/ml for 6 mg/kg daily) (41), intravenous/oral linezolid (138 μg·h/ml for 600 mg/kg twice daily) (42), clindamycin (116 μg·h/ml for 600 mg three times a day) (33), and doxycycline (55.7 μg·h/ml for 100 mg twice daily) (43). All mice were treated with the first dose of antibiotics administered at 4 h after CA-MRSA inoculation and continued at the aforementioned regimens for 7 days. For the USA300 LAC::lux strain, the following MICs were measured according to established guidelines and methods (44) using in-house prepared broth microdilution trays: oxacillin, 16 > μg/ml; vancomycin, 1.0 μg/ml; daptomycin, ≤0.25 μg/ml; linezolid, 2.0 μg/ml; clindamycin, ≤0.5 μg/ml; doxycycline, ≤1.0 μg/ml; and TMP/SMX, ≤0.5/≤9.5 μg/ml.
Topical antibiotic therapy.
CA-MRSA-infected skin wounds were treated topically by applying 100 μl of mupirocin 2% ointment (Bactroban; GlaxoSmithKline, Research Triangle Park, NC), retapamulin 1% ointment (Altabax; Stiefel/GlaxoSmithKline), or the corresponding vehicle ointments (polyethylene glycol [mupirocin] and white petrolatum [retapamulin]) at 4 h after CA-MRSA inoculation, followed by twice daily (every 12 h) thereafter for a total of 7 days.
Statistical analysis.
Data were compared using Student's t test (two-tailed). All data are expressed as mean ± the standard error of the mean (SEM). Values of P < 0.05 were considered statistically significant.
RESULTS
In vivo bioluminescence imaging to measure bacterial burden.
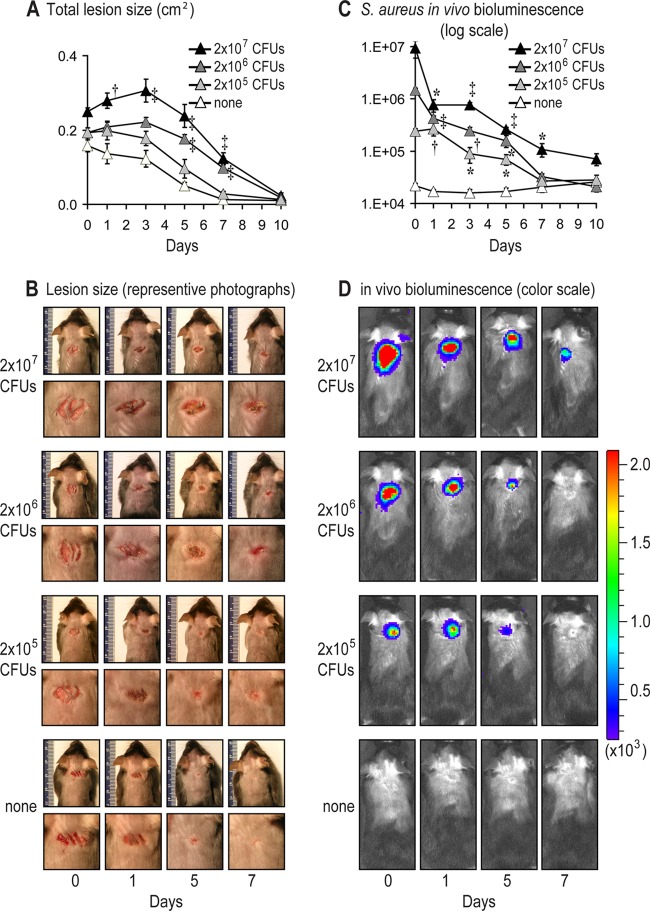
To model a CA-MRSA wound infection in mice, scalpel cuts on the shaved backs of mice were inoculated with the bioluminescent USA300 LAC::lux strain (26). The wound lesion sizes (Fig. 1A and B) and in vivo bacterial burden (Fig. 1C and D) of anesthetized mice were determined by analyzing digital photographs of the mice using image analysis (ImageJ; NIH Research Services Branch) and measuring the USA300 LAC::lux bioluminescent signals (Lumina II imaging system; Caliper), respectively. As a first step, the optimal bacterial inoculum that produced a consistent wound infection was determined by evaluating different inocula of USA300 LAC::lux (2 × 105, 2 × 106, and 2 × 107 CFU per 10 μl) or no bacterial inoculation (none) (Fig. 1). The inoculum of 2 × 107 CFU induced the largest lesions and the 2 × 106 CFU induced intermediate lesion sizes, which were both statistically greater than those of uninfected mice (Fig. 1A and B). In contrast, 2 × 105 CFU induced lesions that did not significantly differ from uninfected mice. The inoculum of 2 × 107 CFU induced higher bioluminescent signals than the 2 × 106 CFU, but the signals of both inocula decreased over time (Fig. 1C and D). The inoculum of 2 × 105 CFU resulted in bioluminescent signals that were below the bioluminescent signals of the other inocula and reached background levels by day 7. All three inocula had bioluminescent signals on days 1 through 5 that were statistically greater than the background bioluminescent signals of uninfected mice. Since our ultimate goal was to produce a CA-MRSA wound infection that induced relatively small lesion sizes and bioluminescent signals that were greater than the uninfected wounds, the intermediate inoculum of 2 × 106 CFU was used in all subsequent experiments.
Fig 1.
Mouse model of CA-MRSA wound infection. Three 8 mm in length, parallel, full-thickness scalpel wounds on the backs of C57BL/6 mice were inoculated with 2 × 105, 2 × 106, or 2 × 107 CFU of the bioluminescent CA-MRSA strain, USA300 LAC::lux, or no bacteria (none)/10 μl (n = 5 mice per group). (A) Mean total lesion size (cm2) ± the standard error of the mean (SEM). (B) Representative photographs of the lesions of the entire dorsal back (upper panels) and close-up photographs of the lesions (lower panels) are shown. (C) Bacterial counts as measured by in vivo bioluminescence imaging (mean total flux [photons/s] ± the SEM) (logarithmic scale). (D) Representative in vivo bioluminescent signals on a color scale overlaid on top of a grayscale image of mice. *, P < 0.05; †, P < 0.01; ‡, P < 0.001 (USA300 LAC::lux-infected mice versus none) (Student's t test [two-tailed]).
Correlation of in vivo bioluminescent signals with ex vivo bacterial burden.
To evaluate whether bioluminescent signals of USA300 LAC::lux accurately represented the bacterial burden, in vivo bioluminescence imaging and skin biopsies from the infected wounds were performed on the same mice after inoculation with 2 × 106 CFU of USA300 LAC::lux. At 4 h and on days 1, 3, 5, and 7 after inoculation, the bioluminescent signals were 9.56 ± 1.55 × 105, 1.72 ± 0.07 × 105, 1.05 ± 0.17 × 105, 5.0 ± 0.37 × 104, and 2.84 ± 0.18 × 104 photons/s (see Fig. S1A in the supplemental material) and the ex vivo bacterial burden was 1.29 ± 0.21 × 107, 2.95 ± 0.34 × 106, 2.29 ± 0.40 × 106, 2.7 ± 0.42 × 105, and 2.97 ± 0.78 × 104 CFU (see Fig. S1B in the supplemental material), respectively. These in vivo bioluminescent signals highly correlated with the ex vivo CFU values (correlation coefficient R2 = 0.9996) (Fig. 2). These data indicate that in vivo bioluminescence imaging of wounds infected with USA300 LAC::lux provides an accurate measurement of the in vivo bacterial burden that can be measured noninvasively and longitudinally during the course of an infection. It should be noted that the culture plates used to determine the CFU were imaged to detect the presence or absence of a bioluminescent signal. In every case, the CFU had a bioluminescent signal (data not shown), indicating that vast majority of bacteria present in the infected wounds were the bioluminescent USA300 LAC::lux strain that were inoculated into these wounds rather than other bacteria that may have contaminated the wounds. Furthermore, the numbers of CFU at 4 h increased 6.5-fold from the initial inoculum, demonstrating that the bacteria were multiplying within the wound and that the infection was established. We therefore chose to use the 4-h time point to initiate antibiotic treatment of the CA-MRSA-infected skin wounds. The 4-h time point was also the same time previously used to initiate treatment of a topically applied mupirocin cream formulation to treat a S. aureus surgical skin wound infection in mice (23).
Fig 2.
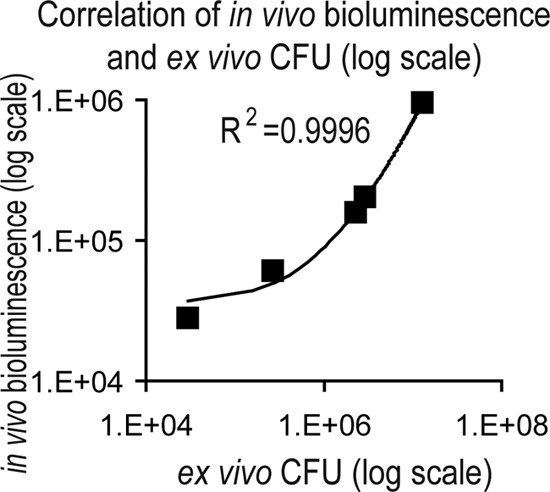
In vivo bioluminescent signals of CA-MRSA-infected wounds highly correlate with ex vivo bacterial CFU counts. Scalpel wounds on the backs of mice were inoculated with USA300 LAC::lux (2 × 106 CFU/10 μl). Correlation between in vivo bioluminescent signals (mean total flux [photons/s] ± the SEM) (logarithmic scale) from mice (n = 6 per group) imaged at 4 h and on days 1, 3, 5, and 7 and total ex vivo CFU (logarithmic scale) recovered from 8-mm lesional punch biopsies performed on euthanized mice (n = 6 per group) at the same time points. The trendline and the correlation coefficient of determination (R2) between in vivo bioluminescent signals and total CFU are shown.
Efficacy of systemic (subcutaneous and oral) antibiotic therapy.
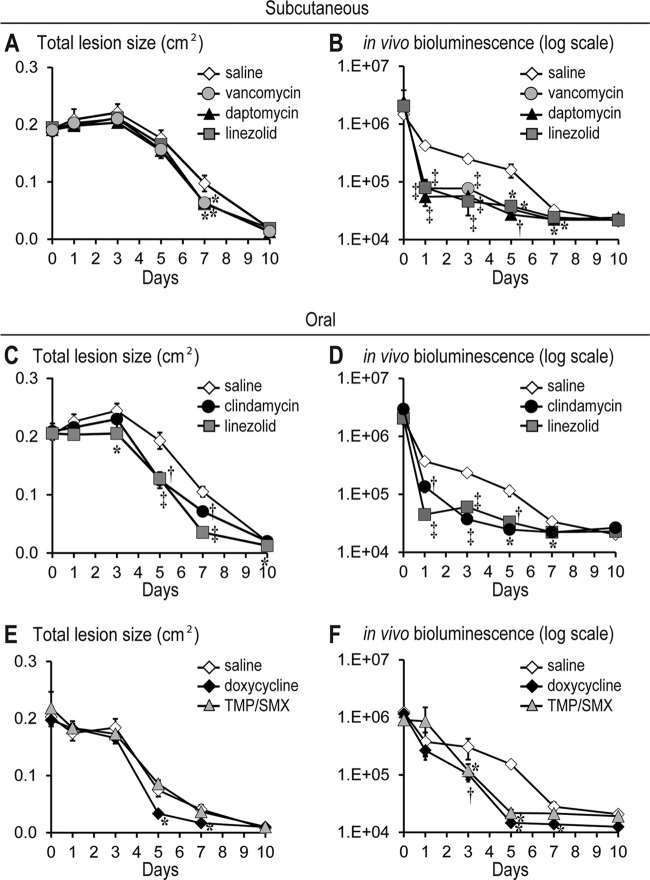
To evaluate the efficacy of commonly used intravenous antibiotics for hospitalized patients with complicated CA-MRSA SSTIs (12), therapeutic doses in mice of vancomycin (110 mg/kg twice daily) (30, 31), daptomycin (50 mg/kg daily) (32, 33), linezolid (60 mg/kg twice daily) (34), or sterile saline (sham control) were administered subcutaneously beginning at 4 h after USA300 LAC::lux inoculation and continued at the above regimens through day 7. We chose to evaluate daptomycin at a dose that simulated the 6-mg/kg human dose, which is used to treat MRSA bacteremia or complicated CA-MRSA SSTIs, rather than evaluating a dose that simulated the 4-mg/kg dose in humans, which is typically used to treat uncomplicated CA-MRSA SSTIs (45). Mice treated with vancomycin, daptomycin, or linezolid all had similar lesion sizes compared with control mice until day 5. However, by day 7, the antibiotic treatment groups had ∼40% significantly decreased lesion sizes than sham control mice, demonstrating enhanced wound healing (Fig. 3A). The enhanced would healing observed in the antibiotic treatment groups was associated with significantly lower bioluminescent signals beginning on day 1 (up to 7.6-fold decrease) and remained significantly below the signals of sham control mice through day 7 (Fig. 3B). There were no differences between the lesion sizes and bioluminescent signals between the various antibiotic treatment groups. Taken together, these data indicate that subcutaneously administered therapeutic doses of vancomycin, daptomycin, and linezolid all resulted in similar decreased lesion sizes and bioluminescent signals during a CA-MRSA wound infection in mice.
Fig 3.
Efficacy of systemic (subcutaneous and oral) antibiotics against CA-MRSA-infected wounds. Scalpel wounds on the backs of mice were inoculated with USA300 LAC::lux (2 × 106 CFU/10 μl). Mice (n = 5 to 10 mice per group) were treated with subcutaneous vancomycin (110 mg/kg twice daily), daptomycin (50 mg/kg once daily), linezolid (60 mg/kg twice daily), or sterile saline (sham control) (top panels) or oral (via gavage) linezolid (60 mg/kg twice daily), clindamycin (100 mg/kg three times a day), doxycycline (100 mg/kg twice daily), TMP/SMX (320/1600 mg/kg once daily), or sterile saline (sham control) (middle and bottom panels). Antibiotic treatment was initiated at 4 h after CA-MRSA inoculation and continued for the first 7 days. (A, C, and E) Mean total lesion size (cm2) ± the SEM. (B, D, and F) Bacterial counts as measured by in vivo bioluminescence imaging (mean total flux [photons/s] ± the SEM) (logarithmic scale). *, P < 0.05; †, P < 0.01; ‡, P < 0.001 (antibiotic treatment versus sham treatment [saline]) (Student's t test [two-tailed]).
To compare the efficacy of commonly used oral antibiotics that are used for outpatient therapy of CA-MRSA SSTIs (12), clindamycin (100 mg/kg three times a day) (35, 36), linezolid (60 mg/kg twice daily) (34), doxycycline (100 mg/kg twice daily) (33), TMP-SMX (320/1,600 mg/kg twice daily) (37, 38), or sterile saline (sham control) were administered orally beginning at 4 h after USA300 LAC::lux inoculation and continued as indicated through day 7. Mice treated with oral linezolid had significantly decreased lesion sizes compared with sham control mice beginning on day 3, whereas mice treated with clindamycin or doxycycline had significantly decreased lesion sizes beginning on day 5 (Fig. 3C and E). TMP/SMX was the only oral antibiotic evaluated that did not result in significantly decreased lesion sizes compared with sham control mice (Fig. 3E). Oral linezolid had the most rapid therapeutic effect because it resulted in the most substantial decrease in bioluminescent signals (8.3-fold on day 1), which was significantly lower compared with the decreased bioluminescent signals in mice treated with oral clindamycin (2.7-fold on day 1) (P < 0.05) (Fig. 3D). Oral TMP/SMX and doxycycline had bioluminescent signals that were decreased compared with sham control mice beginning on day 3 after inoculation (3.0- and 2.6-fold on day 3, respectively) (P < 0.05) (Fig. 3F). After day 3, all of the oral antibiotics resulted in bioluminescent signals that approached background levels by days 5 to 7. In summary, these data indicate that oral linezolid, clindamycin, and doxycycline, but not TMP/SMX, resulted in decreased lesion sizes. In addition, all of these oral antibiotics resulted in decreased bioluminescent signals with linezolid having the most rapid therapeutic effect.
Efficacy of topical antibiotic therapy.
Next, the efficacy of the two Food and Drug Administration (FDA)-approved topical prescription-strength ointments, mupirocin (1, 10, 11) and retapamulin (1, 10, 16), were compared in this mouse model of CA-MRSA wound infection. Mupirocin 2% ointment, retapamulin 1% ointment, or corresponding vehicle ointments (polyethylene glycol [PEG; mupirocin] and white petrolatum [retapamulin]) were topically applied (100-μl volume) to the infected wounds at 4 h after USA300 LAC::lux inoculation followed by twice daily (every 12 h) for the next 7 days. Mupirocin ointment resulted in 29 to 58% decreased lesion sizes beginning at day 5 after inoculation compared with the PEG vehicle ointment (Fig. 4B). In contrast, retapamulin ointment resulted in lesion sizes that did not differ from mice treated with petrolatum vehicle ointment alone. Compared with their respective vehicle ointments, between days 1 and 5, mupirocin ointment resulted in a 5- to 12-fold significant reduction in bioluminescent signals, whereas retapamulin ointment treatment resulted in a 10- to 41-fold significant reduction in bioluminescent signals (Fig. 4B). However, when the lesion sizes and bioluminescent signals for the mupirocin and retapamulin ointments were compared, they were not significantly different from each other. Interestingly, the observed differences in effectiveness of these ointments were impacted by changes induced by the vehicle ointment alone. In particular, the petrolatum vehicle ointment induced decreased lesion sizes that were comparable to those of mice treated with mupirocin or retapamulin ointment. This enhanced wound healing was unexpected because the petrolatum vehicle ointment also induced an increase in bioluminescent signals on day 1. In contrast, the PEG vehicle ointment had lesion sizes or bioluminescent signals that were similar to those observed without any topical treatment (saline control mice) in Fig. 3.
Fig 4.
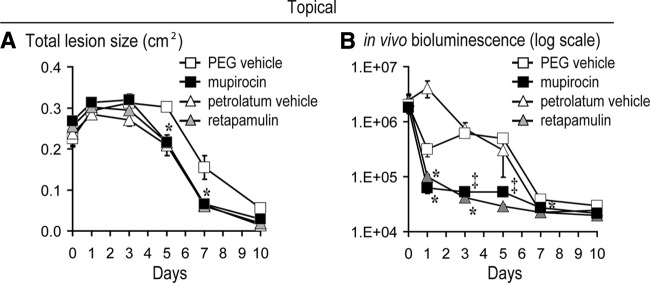
Efficacy of topical antibiotics against CA-MRSA-infected wounds. Scalpel wounds on the backs of mice were inoculated with USA300 LAC::lux (2 × 106 CFU/10 μl). Mice (n = 5 mice per group) were treated with topical mupirocin 2% ointment, retapamulin 1% ointment, or the corresponding vehicle ointment (polyethylene glycol [mupirocin] and white petrolatum [retapamulin]). Antibiotic treatment was initiated at 4 h after CA-MRSA inoculation and continued twice daily for the first 7 days. (A) Mean total lesion size (cm2) ± the SEM. (B) Bacterial counts as measured by in vivo bioluminescence imaging (mean total flux [photons/s] ± the SEM) (logarithmic scale). *, P < 0.05; †, P < 0.01; ‡, P < 0.001 (antibiotic treatment versus sham treatment [saline]) (Student's t test [two-tailed]).
Efficacy of systemic antibiotics in a mouse model of type 2 diabetes.
One advantage of developing a mouse model of CA-MRSA wound infection is that the efficacy of antibiotic therapy can be evaluated in genetically engineered mouse strains that mimic certain human diseases. One such disease is type II diabetes in which patients develop chronic wounds and ulcers that can become infected with CA-MRSA (46). To determine the efficacy of antibiotic therapy against a CA-MRSA wound infection in a mouse model of type II diabetes, the NONcNZO10/LtJ mouse strain, which expresses six known obesity-induced diabetes quantitative trait loci, was used (29). NONcNZO10/LtJ male mice on a high-fat diet develop many of the characteristics of type II diabetes when they become 10 weeks of age, including visceral obesity, hyperglycemia, dyslipidemia, moderate liver steatosis, and pancreatic islet atrophy (29). Vancomycin (110 mg/kg twice daily) (30, 31), daptomycin (50 mg/kg daily) (32, 33), linezolid (60 mg/kg twice daily) (34), or sterile saline (sham control) were administered subcutaneously to 12-week-old NONcNZO10/LtJ diabetic male mice beginning at 4-h after USA300 LAC::lux inoculation and continued at the above regimens through day 7. Although pharmacokinetic studies with vancomycin, daptomycin, and linezolid were not performed on these diabetic mice, these doses used were based on total body weight, which is how vancomycin and daptomycin are dosed in humans and levels of linezolid at normal dosing are decreased in obese patients (12, 47). As expected, sham control NONcNZO10/LtJ mice developed larger lesions and higher in vivo bioluminescent signals that persisted longer (up to 14 days) compared with wild-type C57BL/6 mice used in Fig. 3 (Fig. 5). In NONcNZO10/LtJ mice, subcutaneously administered vancomycin resulted in significantly decreased lesion sizes on day 7 (22% decrease [P < 0.05]), and subcutaneously administered daptomycin and linezolid resulted in significantly decreased lesion sizes on day 5 (19% [P < 0.05] and 23% [P < 0.05] decreases, respectively) and day 7 (38% [P < 0.01] and 44% [P < 0.001] decreases, respectively) compared with sham control mice (Fig. 5A). On day 1, vancomycin, daptomycin, and linezolid resulted in 3.2-, 7.9-, and 13.9-fold significantly decreased bioluminescent signals compared with sham control mice (Fig. 5B). The decreased bioluminescent signals in daptomycin- and linezolid-treated mice were not statistically different from each other. However, on day 1, the bioluminescent signals for both daptomycin and linezolid were both significantly lower than the bioluminescent signals of the vancomycin-treated mice (P < 0.05 and P < 0.001, respectively). After day 1, vancomycin-, daptomycin-, and linezolid-treated mice had similarly decreased bioluminescent signals compared with sham control mice. Taken together, in NONcNZO10/LtJ diabetic mice, subcutaneous linezolid and daptomycin treatment had a more rapid therapeutic effect compared with vancomycin, but after day 1, all three antibiotics had similar efficacy against this CA-MRSA wound infection.
Fig 5.
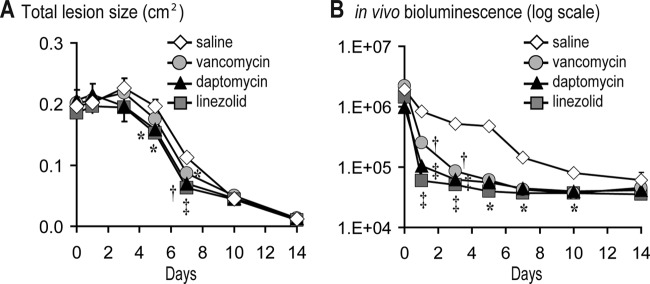
Efficacy of subcutaneous vancomycin, daptomycin, and linezolid against a CA-MRSA wound infection in diabetic mice. Scalpel wounds on the backs of NONcNZO10/LtJ diabetic mice were inoculated with USA300 LAC::lux (2 × 106 CFU/10 μl). Mice (n = 5 to 10 mice per group) were treated with subcutaneous vancomycin (110 mg/kg twice daily), daptomycin (50 mg/kg once daily), linezolid (60 mg/kg twice daily), or sterile saline (sham control) beginning at 4 h after CA-MRSA inoculation and continued for the first 7 days. (A) Mean total lesion size (cm2) ± the SEM. (B) Bacterial counts as measured by in vivo bioluminescence imaging (mean total flux [photons/s] ± the SEM) (logarithmic scale). *, P < 0.05; †, P < 0.01; ‡, P < 0.001 (antibiotic treatment versus sham treatment [saline]) (Student's t test [two-tailed]).
DISCUSSION
CA-MRSA SSTIs represent a major public health threat in the United States and are becoming an increasing problem worldwide (1, 2, 13). Antimicrobial resistance among CA-MRSA isolates has complicated the treatment of these infections. A rapid and cost-effective preclinical animal model of CA-MRSA SSTIs could provide an alternative system to evaluate the in vivo efficacy of existing and potential antimicrobial agents. In the present study, a mouse model of a CA-MRSA skin wound infection was developed in which a bioluminescent USA300 CA-MRSA strain (USA300 LAC::lux) was inoculated into skin wounds. Digital photography and in vivo bioluminescence imaging were used to obtain noninvasive and longitudinal measurements of wound healing and the bacterial burden.
The bioluminescent USA300 LAC::lux strain used in the present study has certain advantages compared with other available S. aureus and CA-MRSA bioluminescent strains. First, this strain was derived from the clinical USA300 LAC strain, which produces toxins associated with the increased virulence of CA-MRSA, including phenol soluble modulins, alpha-toxin, and Panton-Valentine leukocidin (48, 49). USA300 LAC::lux also possesses the bioluminescent construct stably integrated into the bacterial chromosome (26). Since the bioluminescent construct is maintained in all progeny without selection, it is thus not lost in vivo over time (26). We demonstrated that the in vivo bioluminescent signals of USA300 LAC::lux highly correlated with the numbers of ex vivo CFU harvested from the same infected skin wounds at various different time points (correlation coefficient R2 = 0.9996) (Fig. 2). Thus, in vivo USA300 LAC::lux bioluminescent signals provide an accurate measurement of bacterial burden that does not require euthanasia of animals for traditional CFU counting to determine the bacterial burden at each time point. For example, only 5 to 10 mice per group were used to monitor the bacterial burden with in vivo bioluminescence imaging. In contrast, to obtain CFU data from the infected skin, at least 5 mice per group would need to be euthanized at each time point (i.e., days 1, 3, 5, and 7), corresponding to 20 total mice per group. For future studies that seek to utilize techniques of in vivo bioluminescence imaging to monitor the bacteria burden in other animal models of infection, the bioluminescent construct containing the modified luxABCDE operon, which was chromosomally induced from Xen29 (Caliper, a Perkin-Elmer Company, Alameda, CA) (27), could be transduced into other S. aureus and MRSA strains (50, 51). Although, the in vivo bioluminescent signals correlate with bacterial burden, they are also dependent upon the transcription/translation of the bioluminescent construct as well as the metabolic activity of the bacteria (50, 51). However, we and others have demonstrated that in vivo bioluminescence imaging can be used to monitor the bacterial burden in various in vivo models of biofilm formation (26, 27, 52), demonstrating that this technology is sensitive enough to detect low levels of light produced by bacteria in biofilms.
This mouse model was used to compare the efficacy of commonly used systemic antibiotics against CA-MRSA SSTIs. We found that subcutaneous administration of therapeutic doses of vancomycin, daptomycin, or linezolid were all effective in treating the CA-MRSA infection in mice, suggesting that these antibiotics would have similar efficacy against CA-MRSA SSTIs in humans. In addition, both subcutaneous linezolid and daptomycin had a more rapid therapeutic effect in type II diabetic mice compared to vancomycin, suggesting that linezolid and daptomycin might have an additional clinical benefit in the context of diabetes. However, these preliminary results with these diabetic mice should be interpreted with caution since detailed pharmacokinetics studies were not performed on these mice, and the dosages used were based on total body weight. Nonetheless, there have been studies in humans that evaluated the efficacy of intravenous daptomycin or linezolid as single agents compared with intravenous vancomycin in the treatment of CA-MRSA SSTIs. In general, daptomycin has been shown to have increased efficacy compared with vancomycin (53). However, one study demonstrated that daptomycin had similar efficacy compared with vancomycin in the treatment of diabetic foot ulcers (54). Most studies have found that linezolid has equivalent or superior efficacy compared with vancomycin (55–60) and similar efficacy as vancomycin in diabetic patients (57). Given these findings in humans, this mouse model was able to confirm the efficacy of these antibiotics against a CA-MRSA wound infection, but it was not able to recapitulate the potentially increased efficacy of linezolid or daptomycin compared with vancomycin in nondiabetic mice. The reason for this may be due to different pharmacokinetics (i.e., absorption, distribution, metabolism, and elimination) between the species, which is a limitation of preclinical animal models. Despite this drawback, we believe that the ability to monitor wound healing and bacterial burden longitudinally over time provides valuable preclinical information about overall efficacy, which is necessary to establish before the commencement of more comprehensive studies in humans.
Our findings involving the comparison of orally administered antibiotics to treat the CA-MRSA wound infections in mice indicated that linezolid, clindamycin, doxycycline, and TMP/SMX were all effective in reducing the bacterial burden, but linezolid had a more rapid therapeutic effect. The reason that the linezolid resulted in a more dramatic decrease in bacterial burden than clindamycin at day 1 after infection is not clear. As mentioned above, this result could be due to different pharmacokinetics of these antibiotics in mice. Nonetheless, it should be mentioned that inducible clindamycin resistance is found in up to 8.4% of CA-MRSA strains, and this should be taken into account when treating patients (61). In this mouse model, TMP/SMX was effective at reducing the bioluminescent signals at a high dose (320/1,600 mg/kg twice daily). However, TMP/SMX was the only oral antibiotic evaluated that had no efficacy in decreasing the size of the lesions. It should be mentioned that TMP/SMX at a lower dose (160/800 mg twice daily) had no efficacy in decreasing the lesion size or bioluminescent signals (data not shown), even though this strain was highly sensitive to this drug (MIC ≤ 0.5 μg/ml). The high dose of TMP/SMX that was required for efficacy in this mouse model may be due the increased content of thymidine in mouse sera and tissues, which interferes with the activity of TMP (62). Since infected human tissues may also have increased thymidine levels, some studies have used high doses of TMP/SMX in treating CA-MRSA SSTIs in humans (37, 38).
Since this model of CA-MRSA SSTI involved the infection of open skin wounds, it provided an opportunity to evaluate the efficacy of topically applied antimicrobial agents. We compared two FDA-approved prescription-strength topical ointments, mupirocin 2% ointment and retapamulin 1% ointment. We found that both mupirocin and retapamulin ointments were equally effective in reducing the bacterial burden to levels seen with the subcutaneously and orally administered antibiotics. Interestingly, the white petrolatum ointment, the vehicle for retapamulin, initially induced an increase in the bacterial burden, which was not observed with PEG vehicle ointment for mupirocin. Despite this higher bacterial burden, the petrolatum ointment resulted in faster wound healing and the wound sizes were virtually identical to those treated with the mupirocin or retapamulin ointments. Therefore, the petrolatum vehicle ointment may provide a therapeutic benefit compared with the PEG vehicle ointment because it promoted wound healing. Clearly, the choice of vehicle is an important consideration in the development of future topical antimicrobial therapies. It could be that a vehicle that enhances wound healing without affecting bacterial growth may be even more efficacious.
One limitation of our study is that we studied a single isolate of USA300 CA-MRSA and the virulence and antibiotic therapeutic effects may be different with other MRSA strains. Moreover, since we only evaluated the USA300 strain, we were not able to describe variability between strains, including differences between USA300 and USA100 strains, which are likely very different because USA100 strains produce fewer toxins and may be less virulent than USA300 strains. These limitations will require additional studies to resolve.
Taken together, the mouse model of CA-MRSA wound infection developed here utilized digital photography/image analysis and in vivo bioluminescence imaging to monitor wound healing and the bacterial burden longitudinally over time. Since this model does not require euthanasia to determine the bacterial burden, fewer numbers of animals are required to evaluate the efficacy of antimicrobial agents, which is an important consideration for the reduction, refinement, and replacement of animals used in research and testing. For this particular model, since the bioluminescent signals for the antibiotic treatment and the sham control groups were nearly identical by day 7, future experiments may not need to be extended beyond day 5, providing additional labor and experimental cost savings. This model could serve as an alternative or complementary noninvasive, cost-effective, and accurate preclinical animal model to investigate in vivo efficacy of certain systemic and topical antimicrobial agents before extensive studies in human subjects. Our results using this model indicate that there are several viable options for intravenous and oral antibiotic therapy for the treatment of CA-MRSA SSTIs in humans and topical antibiotic therapy may provide an additional therapeutic benefit.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by an Investigator-Initiated Research Grant from Pfizer, Inc. (grant WS751303 to L.S.M.), and National Institutes of Health grants R01-AI078910 (to L.S.M.), T32-AR058921 (to J.S.C.), and R24-CA92865 (to the UCLA Small Animal Imaging Resource Program).
We thank Tammy Kielian, Kenneth W. Bayles, and Jennifer Endres at the University of Nebraska Medical Center for providing the USA300 LAC::lux bioluminescent CA-MRSA strain. We also thank Michael Lewinski and Kevin Ward at the UCLA Clinical Microbiology Laboratory for performing the antibiotic MICs for the bacterial strains used in this study.
Footnotes
Published ahead of print 3 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01003-12.
REFERENCES
- 1. David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23:616–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deleo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCaig LF, McDonald LC, Mandal S, Jernigan DB. 2006. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 12:1715–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 168:1585–1591 [DOI] [PubMed] [Google Scholar]
- 5. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 6. Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ. 2011. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin. Infect. Dis. 53:144–149 [DOI] [PubMed] [Google Scholar]
- 7. King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317 [DOI] [PubMed] [Google Scholar]
- 8. Jones RN, Nilius AM, Akinlade BK, Deshpande LM, Notario GF. 2007. Molecular characterization of Staphylococcus aureus isolates from a 2005 clinical trial of uncomplicated skin and skin structure infections. Antimicrob. Agents Chemother. 51:3381–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J. Antimicrob. Chemother. 64:441–446 [DOI] [PubMed] [Google Scholar]
- 10. Daum RS. 2007. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 357:380–390 [DOI] [PubMed] [Google Scholar]
- 11. Elston DM. 2007. Community-acquired methicillin-resistant Staphylococcus aureus. J. Am. Acad. Dermatol. 56:1–16 [DOI] [PubMed] [Google Scholar]
- 12. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak J, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 13. Dryden MS. 2010. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 65(Suppl 3):iii35–iii44 [DOI] [PubMed] [Google Scholar]
- 14. Curcio D. 2011. Skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus: role of tigecycline. Clin. Infect. Dis. 52:1468–1469 [DOI] [PubMed] [Google Scholar]
- 15. Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box AT, Voss A, d, Tvan IBAvan Verbrugh HA, Vos MC. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362:9–17 [DOI] [PubMed] [Google Scholar]
- 16. Yang LP, Keam SJ. 2008. Retapamulin: a review of its use in the management of impetigo and other uncomplicated superficial skin infections. Drugs 68:855–873 [DOI] [PubMed] [Google Scholar]
- 17. Hahn BL, Onunkwo CC, Watts CJ, Sohnle PG. 2009. Systemic dissemination and cutaneous damage in a mouse model of staphylococcal skin infections. Microb. Pathog. 47:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kugelberg E, Norstrom T, Petersen TK, Duvold T, Andersson DI, Hughes D. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 49:3435–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heggers JP, McHugh T, Zoellner S, Boertman JA, Niu XT, Robson MC, Velanovich V. 1989. Therapeutic efficacy of timentin and augmentin versus silvadene in burn wound infections. J. Burn Care Rehabil. 10:421–424 [DOI] [PubMed] [Google Scholar]
- 20. Rode H, de Wet PM, Millar AJ, Cywes S. 1988. Bactericidal efficacy of mupirocin in multi-antibiotic resistant Staphylococcus aureus burn wound infection. J. Antimicrob. Chemother. 21:589–595 [DOI] [PubMed] [Google Scholar]
- 21. Yamakawa T, Mitsuyama J, Hayashi K. 2002. In vitro and in vivo antibacterial activity of T-3912, a novel non-fluorinated topical quinolone. J. Antimicrob. Chemother. 49:455–465 [DOI] [PubMed] [Google Scholar]
- 22. Boon RJ, Beale AS. 1987. Response of Streptococcus pyogenes to therapy with amoxicillin or amoxicillin-clavulanic acid in a mouse model of mixed infection caused by Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 31:1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gisby J, Bryant J. 2000. Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob. Agents Chemother. 44:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McRipley RJ, Whitney RR. 1976. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob. Agents Chemother. 10:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rittenhouse S, Singley C, Hoover J, Page R, Payne D. 2006. Use of the surgical wound infection model to determine the efficacious dosing regimen of retapamulin, a novel topical antibiotic. Antimicrob. Agents Chemother. 50:3886–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 186:6585–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, DeBoer M, Rubin M, Bellinger-Kawahara C, Parr TRJ, Jr, Contag PR. 2003. Direct continuous method for monitoring biofilm infection in a mouse model. Infect. Immun. 71:882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho JS, Zussman J, Donegan NP, Ramos RI, Garcia NC, Uslan DZ, Iwakura Y, Simon SI, Cheung AL, Modlin RL, Kim J, Miller LS. 2011. Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against Staphylococcus aureus and USA300 MRSA skin infections. J. Invest. Dermatol. 131:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang RC, Kryger ZB, Buck DW, De la Garza M, Galiano RD, Mustoe TA. 2010. Limitations of the db/db mouse in translational wound healing research: is the NONcNZO10 polygenic mouse model superior? Wound. Repair Regen. 18:605–613 [DOI] [PubMed] [Google Scholar]
- 30. Crandon JL, Kuti JL, Nicolau DP. 2010. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob. Agents Chemother. 54:5115–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reyes N, Skinner R, Benton BM, Krause KM, Shelton J, Obedencio GP, Hegde SS. 2006. Efficacy of telavancin in a murine model of bacteraemia induced by methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:462–465 [DOI] [PubMed] [Google Scholar]
- 32. Dandekar PK, Tessier PR, Williams P, Nightingale CH, Nicolau DP. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405–411 [DOI] [PubMed] [Google Scholar]
- 33. LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob. Agents Chemother. 52:2156–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob. Agents Chemother. 55:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Girard AE, Girard D, Gootz TD, Faiella JA, Cimochowski CR. 1995. In vivo efficacy of trovafloxacin (CP-99,219), a new quinolone with extended activities against gram-positive pathogens, Streptococcus pneumoniae, and Bacteroides fragilis. Antimicrob. Agents Chemother. 39:2210–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azeh I, Gerber J, Wellmer A, Wellhausen M, Koenig B, Eiffert H, Nau R. 2002. Protein synthesis inhibiting clindamycin improves outcome in a mouse model of Staphylococcus aureus sepsis compared with the cell wall active ceftriaxone. Crit. Care Med. 30:1560–1564 [DOI] [PubMed] [Google Scholar]
- 37. Cadena J, Nair S, Henao-Martinez AF, Jorgensen JH, Patterson JE, Sreeramoju PV. 2011. Dose of trimethoprim-sulfamethoxazole to treat skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 55:5430–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitz GR, Bruner D, Pitotti R, Olderog C, Livengood T, Williams J, Huebner K, Lightfoot J, Ritz B, Bates C, Schmitz M, Mete M, Deye G. 2010. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann. Emerg. Med. 56:283–287 [DOI] [PubMed] [Google Scholar]
- 39. Hilliard JJ, Fernandez J, Melton J, Macielag MJ, Goldschmidt R, Bush K, Abbanat D. 2009. In vivo activity of the pyrrolopyrazolyl-substituted oxazolidinone RWJ-416457. Antimicrob. Agents Chemother. 53:2028–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Healy DP, Polk RE, Garson ML, Rock DT, Comstock TJ. 1987. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 31:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woodworth, Nyhart EH, Jr, Brier GL, Wolny JD, Black HR. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burkhardt O, Borner K, HNvon der, Koppe P, Pletz MW, Nord CE, Lode H. 2002. Single- and multiple-dose pharmacokinetics of linezolid and co-amoxiclav in healthy human volunteers. J. Antimicrob. Chemother. 50:707–712 [DOI] [PubMed] [Google Scholar]
- 43. Saano V, Paronen P, Peura P. 1990. Bioavailability of doxycycline from dissolved doxycycline hydrochloride tablets: comparison to solid form hydrochloride tablets and dissolved monohydrate tablets. Int. J. Clin. Pharmacol. Ther. Toxicol. 28:471–474 [PubMed] [Google Scholar]
- 44. Clinical and Laboratory Standards Institute (CLSI) 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9. CLSI, Wayne, PA [Google Scholar]
- 45. Seaton RA. 2008. Daptomycin: rationale and role in the management of skin and soft tissue infections. J. Antimicrob. Chemother. 62(Suppl 3):iii15–iii23 [DOI] [PubMed] [Google Scholar]
- 46. Lipsky BA, Tabak YP, Johannes RS, Vo L, Hyde L, Weigelt JA. 2010. Skin and soft tissue infections in hospitalized patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia 53:914–923 [DOI] [PubMed] [Google Scholar]
- 47. Dryden MS. 2011. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J. Antimicrob. Chemother. 66(Suppl 4):iv7–iv15 [DOI] [PubMed] [Google Scholar]
- 48. Otto M. 2010. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64:143–162 [DOI] [PubMed] [Google Scholar]
- 49. Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 [DOI] [PubMed] [Google Scholar]
- 50. Andreu N, Zelmer A, Wiles S. 2011. Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol. Rev. 35:360–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hutchens M, Luker GD. 2007. Applications of bioluminescence imaging to the study of infectious diseases. Cell Microbiol. 9:2315–2322 [DOI] [PubMed] [Google Scholar]
- 52. Pribaz JR, Bernthal NM, Billi F, Cho JS, Ramos RI, Guo Y, Cheung AL, Francis KP, Miller LS. 2012. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J. Orthop. Res. 30:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davis SL, McKinnon PS, Hall LM, Delgado G, Jr, Rose W, Wilson RF, Rybak MJ. 2007. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy 27:1611–1618 [DOI] [PubMed] [Google Scholar]
- 54. Lipsky BA, Stoutenburgh U. 2005. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J. Antimicrob. Chemother. 55:240–245 [DOI] [PubMed] [Google Scholar]
- 55. McKinnon PS, Sorensen SV, Liu LZ, Itani KM. 2006. Impact of linezolid on economic outcomes and determinants of cost in a clinical trial evaluating patients with MRSA complicated skin and soft-tissue infections. Ann. Pharmacother. 40:1017–1023 [DOI] [PubMed] [Google Scholar]
- 56. Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C. 2005. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 49:2260–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lipsky BA, Itani KM, Weigelt JA, Joseph W, Paap CM, Reisman A, Myers DE, Huang DB. 2011. The role of diabetes mellitus in the treatment of skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus: results from three randomized controlled trials. Int. J. Infect. Dis. 15:e140–e146 [DOI] [PubMed] [Google Scholar]
- 58. Itani KM, Weigelt J, Li JZ, Duttagupta S. 2005. Linezolid reduces length of stay and duration of intravenous treatment compared with vancomycin for complicated skin and soft tissue infections due to suspected or proven methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 26:442–448 [DOI] [PubMed] [Google Scholar]
- 59. Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. 2010. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am. J. Surg. 199:804–816 [DOI] [PubMed] [Google Scholar]
- 60. Sharpe JN, Shively EH, Polk HC., Jr 2005. Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am. J. Surg. 189:425–428 [DOI] [PubMed] [Google Scholar]
- 61. LaPlante KL, Rybak MJ, Amjad M, Kaatz GW. 2007. Antimicrobial susceptibility and staphylococcal chromosomal cassette mec type in community- and hospital-associated methicillin-resistant Staphylococcus aureus. Pharmacotherapy 27:3–10 [DOI] [PubMed] [Google Scholar]
- 62. Tokunaga T, Oka K, Takemoto A, Ohtsubo Y, Gotoh N, Nishino T. 1997. Efficacy of trimethoprim in murine experimental infection with a thymidine kinase-deficient mutant of Escherichia coli. Antimicrob. Agents Chemother. 41:1042–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.