Abstract
Bemisia tabaci (Hemiptera: Aleyrodidae) is a species complex containing >28 cryptic species, some of which are important crop pests worldwide. Like many other sap-sucking insects, whiteflies harbor an obligatory symbiont, “Candidatus Portiera aleyrodidarum,” and a number of secondary symbionts. So far, six genera of secondary symbionts have been identified in B. tabaci. In this study, we report and describe the finding of an additional bacterium in the indigenous B. tabaci cryptic species China 1 (formerly known as B. tabaci biotype ZHJ3). Phylogenetic analysis based on the 16S rRNA and gltA genes showed that the bacterium belongs to the Alphaproteobacteria subdivision of the Proteobacteria and has a close relationship with human pathogens of the genus Orientia. Consequently, we temporarily named it Orientia-like organism (OLO). OLO was found in six of eight wild populations of B. tabaci China 1, with the infection rate ranging from 46.2% to 76.8%. Fluorescence in situ hybridization (FISH) of B. tabaci China 1 in nymphs and adults revealed that OLOs are confined to the bacteriome and co-occur with “Ca. Portiera aleyrodidarum.” The vertical transmission of OLO was demonstrated by detection of OLO at the anterior pole end of the oocytes through FISH. Quantitative PCR analysis of population dynamics suggested a complex interaction between “Ca. Portiera aleyrodidarum” and OLO. Based on these results, we propose “Candidatus Hemipteriphilus asiaticus” for the classification of this symbiont from B. tabaci.
INTRODUCTION
Symbiotic bacteria (symbionts) are ubiquitous in animal hosts and particularly prevalent in arthropods. Among these bacteria, some coevolve with their hosts and are strictly vertically transmitted. These bacteria provide essential nutrients to supplement the asymmetric diet of hosts and are thus called primary symbionts (1). Other symbionts are not necessary for all hosts but may have important effects on host biology and are thus called secondary or facultative symbionts (2). These secondary symbionts may confer resistance to parasitoid wasps, pathogens, and heat stress (2, 3) or manipulate hosts' reproduction (4).
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodoidea) is a cryptic species complex containing at least 28 morphologically indistinguishable putative species (referred to here as “species”) (5–7), some of which have caused considerable losses to agricultural crops by sucking plant phloem sap and transmitting plant viruses (8). Whiteflies, like other phloem-feeding hemipterans, require bacteria for balancing their diet. “Candidatus Portiera aleyrodidarum” (Oceanospirillales), the primary symbiont of B. tabaci, was recently reported to provide its whitefly host with essential amino acids as well as carotenoids (9). That symbiont is localized in the bacteriome, which comprises a number of bacteriocytes. “Ca. Portiera aleyrodidarum” can be vertically transmitted through bacteriocytes during reproduction (10). In addition to “Ca. Portiera aleyrodidarum,” six genera of secondary symbionts have so far been found in B. tabaci, including “Candidatus Cardinium hertigii” (Bacteroidales) (11), “Candidatus Fritschea bemisiae” (Chlamydiales) (12), “Candidatus Hamiltonella defensa” (Enterobacteriales) (13), Arsenophonus spp. (Enterobacteriales) (13), Wolbachia spp. (Rickettsiales) (14), and Rickettsia spp. (Rickettsiales) (10).
In a previous study, we detected a novel Orientia-like organism (OLO) in laboratory specimens of B. tabaci (15). In this study, we investigated the natural distribution, taxonomic position, in vivo localization, and population dynamics of this novel OLO in the B. tabaci species China 1 (formerly known as B. tabaci biotype ZHJ3), and we describe our findings here. Based on the data, we propose the provisional name “Candidatus Hemipteriphilus asiaticus” for this secondary symbiont newly discovered from B. tabaci.
MATERIALS AND METHODS
Whitefly rearing and collection.
Bemisia tabaci China 1 (mitochondrial cytochrome oxidase 1 [mtCOI] gene; GenBank accession no. GQ303180) was obtained from Hangzhou, Zhejiang, China, in November 2009 and maintained on cotton [Gossypium hirsutum (Malvaceae) cv. Zhe-Mian 1793] in climate chambers at 27 ± 1°C with 14 h of light and 10 h of dark and 70% ± 10% relative humidity (16). Other than the primary symbiont “Ca. Portiera aleyrodidarum,” Wolbachia and OLO were detected in this population (15). All analyses of OLO were done with this laboratory population. Samples of wild B. tabaci China 1 were collected from different localities covering 5 provinces of China from June to October 2009 (Table 1). Whiteflies used for molecular analysis were initially immersed in 95% ethanol and subsequently kept at −20°C until DNA extraction.
Table 1.
Detection of Rickettsia and OLO in Chinese B. tabaci China 1 populations
| Whitefly population | Locality in China | Latitude | Longitude | Host plant | Collection date | Whiteflies tested |
GenBank accession no. of bacterium 16S rRNA | GenBank accession no. of whitefly mtCOI genea | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | % with OLO | % with Rickettsia | ||||||||
| JGS-JX | Jinggangshan, Jiangxi | 26°74′N | 114°30′E | Ipomoea batatas (sweet potato) | Oct. 2009 | 24 | 58.3 | JX042437 | HM137328 | |
| ZY1-GZ | Zunyi, Guizhou | 27°39′N | 107°70′E | Ipomoea batatas (sweet potato) | Jul. 2009 | 27 | 77.8 | JX042441 | HM137355 | |
| ZY2-GZ | Zunyi, Guizhou | 27°39′N | 107°70′E | Solanum melongena (eggplant) | Jul. 2009 | 30 | 76.7 | JX042440 | ||
| BB1-CQ | Beibei, Chongqing | 29°46′N | 106°22′E | Glycine max (soybean) | Jul. 2009 | 27 | 74.1 | JX042434 | HM137341 | |
| BB2-CQ | Beibei, Chongqing | 29°81′N | 106°41′E | Ipomoea batatas (sweet potato) | Jul. 2009 | 27 | 55.6 | JX042435 | HM137350 | |
| CD-SC | Chengdu, Sichuan | 30°44′N | 103°54′E | Glycine max (soybean) | Jul. 2009 | 26 | 46.2 | JX042436 | HM137315 | |
| LS-SC | Leshan, Sichuan | 29°36′N | 103°45′E | Glycine max (soybean) | Jul. 2009 | 28 | 3.6 | JX042438 | HM137316 | |
| MY-SC | Mianyang, Sichuan | 31°39′N | 104°51′E | Ipomoea batatas (sweet potato) | Jul. 2009 | 12 | 25.0 | JX042439 | HM137318 | |
GenBank accession numbers of mtCOI genes were all obtained from reference 6.
DNA extraction, cloning, and sequencing.
For all samples, total DNA was extracted from individual adult specimens following the method of Frohlich et al. (17). The bacterial 16S rRNA gene was amplified using the universal primers 27F and 1494R (18). The bacterial citrate synthase-encoding gene (gltA) was amplified using the primers gltAF3 (5′-ACATGCAGACCATGAGCAGA-3′) and gltAR11 (5′-CATTTCATTCCATTGTGCCATC-3′) (19). The groEL gene was amplified using the newly designed primers OR-groEL-F (5′-CACCWAAAATTACTAAAGATGG-3′) and OR-groEL-R (5′-TAGAARTCCATWCCKCCCATWC-3′). All PCR analyses were conducted with Taq polymerase (TaKaRa, Dalian, China) in a PTC-200 thermocycler (Bio-Rad, CA). The cycling conditions were an initial denaturation at 94°C for 3 min, followed by 34 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 2 min and a final extension of 72°C for 10 min. The PCR products were cloned into the pMD-18T plasmid vector (TaKaRa, Dalian, China) and sequenced in an ABI 3730 DNA analyzer.
Diagnostic PCR and RFLP analysis.
To detect the presence of OLO in whitefly populations, primers OLO-F (5′-GCTCAGAACGAACGCTRKC-3′) and OLO-R (5′-TTCGCCACTGGTGTTCCTC-3′) were developed to amplify a product of about 670 bp from OLO's 16S rRNA gene. The PCR procedures were 94°C for 4 min, followed by 35 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 2 min. An endonuclease, HphI, was found to digest the PCR products into different bands and distinguish OLO and Rickettsia of B. tabaci. The DNA cloning and sequencing methods described above were used to confirm the restriction fragment length polymorphism (RFLP) results. The quality of DNA samples was confirmed by PCR amplification of the partial mitochondrial cytochrome oxidase 1 (mtCOI) gene of the whitefly. Primers used to amplify the mtCOI gene fragment were C1-J-2195 and L2-N-3014 (17). All PCRs included sterile water as a negative control and a positive control.
FISH.
We followed the method of Sakurai et al. (20) and Gottlieb et al. (10) for the fluorescence in situ hybridization (FISH) experiments, with slight modifications (for details, see Appendix S1 in the supplemental material). The probe BTP1-Cy3 (5′-Cy3-TGTCAGTGTCAGCCCAGAAG-3′) was used to target the 16S rRNA of the primary symbiont “Ca. Portiera aleyrodidarum” (10). On the basis of the sequence of OLO 16S rRNA, a probe, OLO-Cy5 (5′-Cy5-CTCACCCGTTTGCCACTAAT-3′), was designed using Primer3 software (http://fokker.wi.mit.edu/primer3/). The specificity of the detection was confirmed using the following controls: a no-probe control, an unlabeled competitive suppression control, and OLO-free whiteflies (samples of the B. tabaci Mediterranean species [5]).
Molecular phylogenetic analysis.
DNA sequences were aligned using the Clustal W (version 1.6) program in MEGA (version 5.05) software (21). The alignments were then inspected and corrected manually. A pairwise distance matrix for aligned 16S rRNA genes was constructed by Kimura's two-parameter method (22). Phylogenetic trees were constructed with the Bayesian inference by the program MrBayes (version 3.1) (23). The best-fit substitution model for each aligned sequence was selected with the jModelTest (version 0.1.1) program using the Bayesian information criterion (24). The Bayesian trees were constructed with a TIM3+G model for the 16S rRNA gene, an HKY+G model for the gltA gene, and a GTR+G model for the groEL gene. For these gene data, 1 million generations were run; 10,000 trees were obtained, and the first 25% of these were discarded as burn-in.
Quantitative PCR.
Bacterial density was quantified by the SYBR green and Bio-Rad CFX96 real-time system (for details, see Appendix S2 in the supplemental material). “Ca. Portiera aleyrodidarum” was quantified in terms of the number of 16S rRNA gene copies using primers Port73-F (5′-GTGGGGAATAACGTACGG-3′) and Port266-R (5′-CTCAGTCCCAGTGTGGCTG-3′) (25). OLO was quantified in terms of the number of 16S rRNA gene copies using newly designed primers OLO-16S-F (5′-TAGTGGCAAACGGGTGAGTA-3′) and OLO-16S-R (5′-GCTCATCCATCAGCGATAAA-3′). For normalization, the B. tabaci β-actin gene was also quantified as an internal standard using primers Actin-F (5′-TCTTCCAGCCATCCTTCTTG-3′) and Actin-R (5′-CGGTGATTTCCTTCTGCATT-3′) (26). For analysis of the densities of the two symbionts in female and male whiteflies at different times of development, a three-factor analysis of variance (ANOVA) was performed using Data Processing System (DPS) statistical software (27). Means were compared using a least-significant-difference (LSD) test (P < 0.05).
Nucleotide sequence accession numbers.
The 16S rRNA gene, gltA gene, and groEL gene sequences obtained in this study were deposited in the GenBank database under accession numbers JX042442, JX042443, and JX042444, respectively. The GenBank accession numbers for the 16S rRNA gene sequences of OLO from field-collected whiteflies are JX042434 to JX042441.
RESULTS
Distribution of Rickettsia and OLO in B. tabaci species China 1.
OLO was detected in six out of the eight China 1 field populations collected in south China in 2009, and 52.2% (105/201) of the adult whiteflies examined were positive (Table 1). OLO was found in whiteflies collected on three host plants (sweet potato, soybean, and eggplant) in five provinces. Rickettsia was detected in only 1.8% (4/201) of adult China 1 whiteflies, belonging to two whitefly populations collected on sweet potato and soybean in Sichuan (Table 1). Digestion of OLO 16S rRNA amplicons by HphI produced one bright band and one blurred band with sizes of 566 and 101 bp, respectively, a pattern that is consistent with that of the predicted RFLP for OLO (see Fig. S1 in the supplemental material). Digestion of the 16S rRNA amplicons of Rickettsia by HphI consistently produced four bands, the largest of which (411 bp) was bright, while the others (128 bp, 99 bp, and 27 bp) were blurred (see Fig. S1 in the supplemental material).
Phylogenetic analysis and taxonomic position of OLO.
A 1,426-bp sequence of the OLO 16S rRNA gene was obtained using the combination of universal primers with the OLO primers OLO-F and OLO-R. This 16S rRNA sequence showed the highest sequence similarity (98.8%, on average) to sequences from the grain aphid Sitobion miscanthi L-type symbionts (SMLS) by a BLAST search of the sequences in GenBank. The next BLAST-hit sequence was from one causative agent of scrub typhus, Orientia chuto (GenBank accession no. HM852447), which had a sequence similarity of 95%. A 91.3% sequence similarity was obtained between the 16S rRNA genes of OLO and the Rickettsia symbiont of B. tabaci (GenBank accession no. DQ077707). The A+T content of the OLO 16S rRNA gene was 48.5%.
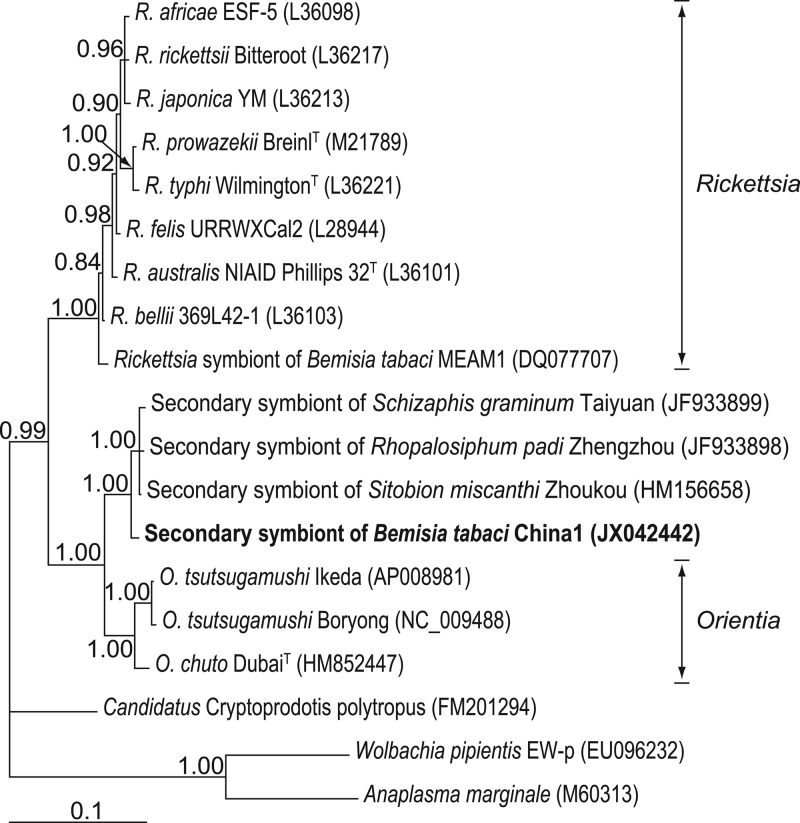
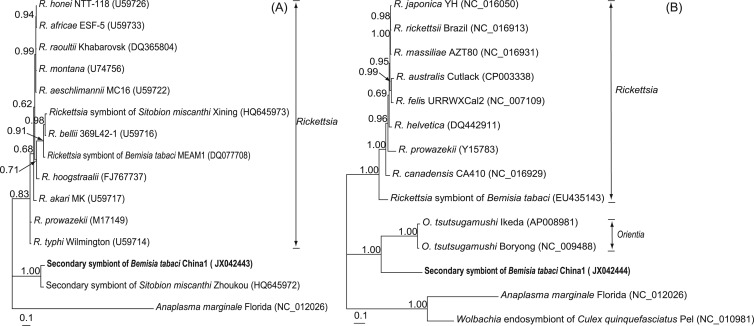
Phylogenetic analysis of the 16S rRNA sequence of OLO using Bayesian methods placed this bacterium near the Orientia spp. in the Rickettsiales order. Phylogenetic analyses consistently gave very strong support (1.00 support values) for a single clade corresponding to OLO in the family Rickettsiaceae together with symbionts of Sitobion miscanthi. This clade was distinct from Rickettsia, which is the known secondary symbiont nearest OLO in B. tabaci (Fig. 1; see Fig. S2 in the supplemental material). Additional phylogenetic analyses based on the gltA gene and groEL gene confirmed the results of the 16S rRNA gene analyses (Fig. 2).
Fig 1.
Phylogenetic position of the OLO identified from Bemisia tabaci putative species China 1 based on bacterial 16S rRNA gene sequences (1,063 sites). The tree was constructed using a TIM3+G substitution model for Bayesian analysis. Bayesian posterior probabilities (>0.50) are shown at the nodes. The names and sequence GenBank accession numbers (in parentheses) are shown. The sequence obtained in this study is shown in bold.
Fig 2.
Phylogenetic position of the OLO identified from Bemisia tabaci putative species China 1. (A) A Bayesian tree based on bacterial gltA gene sequences (495 sites); (B) a Bayesian tree based on bacterial groEL gene sequences (1,519 sites). The Bayesian trees were constructed using an HKY+G substitution model for the gltA gene and a GTR+G substitution model for the groEL gene. Bayesian posterior probabilities (>0.50) are shown at the nodes. The names and sequence GenBank accession numbers (in parentheses) are shown. Sequences obtained in this study are shown in bold.
In situ hybridization of OLO in B. tabaci.
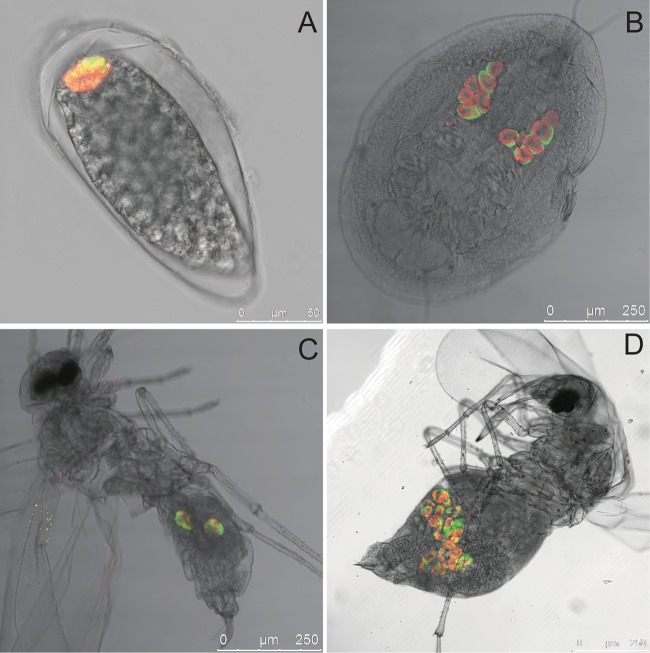
Localization of “Ca. Portiera aleyrodidarum” and OLO was studied in oocytes, nymphs, and adults of B. tabaci using fluorescently modified specific probes targeting the 16 rRNA genes of these bacteria. “Ca. Portiera aleyrodidarum” was detected exclusively inside the bacteriocytes at all developmental stages (Fig. 3A to D). OLO was strictly located in the bacteriocytes among the abundant “Ca. Portiera aleyrodidarum” organisms and was never detected in any other host organs (Fig. 3A to D). The signals of the bacteriocytes in the female adult whitefly were relatively stronger than those in the male whitefly (Fig. 3C and D). The presence of OLO at the anterior pole of the oocytes indicated vertical transmission of these symbionts (Fig. 3A). The specificity of the detected signals was confirmed by the no-probe control, competitive control, and OLO-free whitefly control (see Fig. S3 in the supplemental material).
Fig 3.
Whole-mount FISH of B. tabaci oocytes, nymphs, and adults using a “Ca. Portiera aleyrodidarum”-specific probe (red) and an OLO-specific probe (green). (A to D) Overlay of “Ca. Portiera aleyrodidarum” and OLO channels of oocytes, nymphs, male adults, and female adults, respectively, on a bright-field channel.
Population dynamics of “Ca. Portiera aleyrodidarum” and OLO in whitefly.
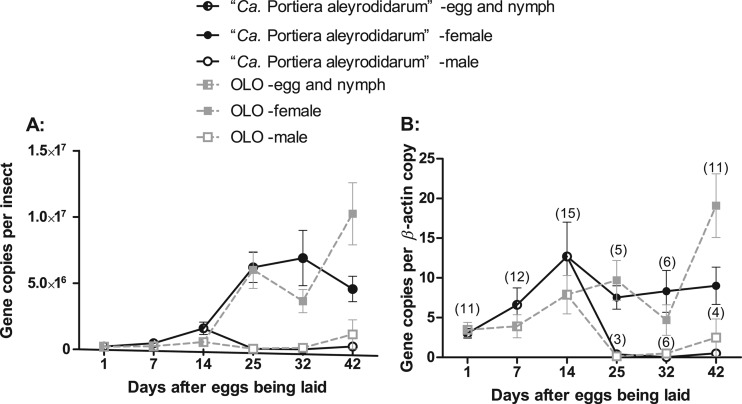
The data in Fig. 4A indicate that when expressed as the number of 16S rRNA gene copies per insect, the densities for both “Ca. Portiera aleyrodidarum” and OLO remained relatively constant during the immature stages of the hosts (i.e., <25 days) but then substantially increased in females as the hosts reached adulthood (25 days and after). The data in Fig. 4B indicate that when expressed as the number of 16S rRNA gene copies per β-actin gene copy, the densities for both “Ca. Portiera aleyrodidarum” and OLO did not vary greatly as the hosts developed. Because the sex of whiteflies is indistinguishable in the egg and nymph stages, only data for adult whiteflies were analyzed using a three-factor ANOVA. The results of ANOVA indicate that, of the three factors considered, host sex was the most influential, and there were complex two-factor and three-factor interactions (see Table S1 in the supplemental material). Although each of these multiple-factor interactions was not significant alone, their combined effects could affect the significance between the mean values when the means were subsequently compared by the LSD test.
Fig 4.
Infection dynamics of “Ca. Portiera aleyrodidarum” and OLO in the developmental course of the host whitefly. (A) “Ca. Portiera aleyrodidarum” and OLO titers in terms of the number of 16S rRNA gene copies per insect; (B) “Ca. Portiera aleyrodidarum” and OLO titers in terms of the number of 16S rRNA gene copies per β-actin gene copy. Whitefly sexes are indistinguishable before the adult phase (earlier than day 25 after hatching). Means and standard errors of the means are shown. Sample sizes are shown in parentheses above the bars.
The densities of both “Ca. Portiera aleyrodidarum” and OLO in female whiteflies were significantly higher than those in males when expressed as either the number of 16S rRNA gene copies per insect or the number of 16S rRNA gene copies per β-actin gene copy (Fig. 4; see Table S1 in the supplemental material). Of the various multiple-factor interactions, the time-symbiont interaction had the largest effects (see Table S1 in the supplemental material). The LSD tests indicate that while at days 25 and 32 there were no significant differences in density between the two bacteria, at day 42 the density of OLO was significantly higher than that of “Ca. Portiera aleyrodidarum” when expressed as either the number of 16S rRNA gene copies per insect (P = 0.028) or the number of 16S rRNA gene copies per β-actin gene (P = 0.024).
DISCUSSION
Recently, an Orientia-like organism (OLO) was detected by primers designed for Rickettsia 16S rRNA genes from two native species (Asia II 7 and China 1) of the whitefly B. tabaci species complex in China (15). The current study was initiated to further characterize that symbiont and determine its phylogenetic status. On the basis of the nearly complete 16S rRNA gene, partial gltA gene, and groEL gene, Bayesian phylogenetic analyses demonstrated that this OLO belongs to the Rickettsiales order within the Alphaproteobacteria subdivision of the Proteobacteria (Fig. 1 and 2). 16S rRNA genes from OLO and SMLS, which are highly similar (98.8%, on average), formed a distinct and robust monophyletic clade in the Rickettsiaceae family and as a sister group of Rickettsia and Orientia. The base composition of 16S rRNA genes from representatives of the Alphaproteobacteria and arthropod symbionts is shown in Table S2 in the supplemental material. Primary symbionts of various insects exhibit high A+T contents (over 51%), while free-living bacteria have much lower percentages of A+T contents (from 43.2% to 45.2%) (28). The A+T content of the OLO 16S rRNA gene (48.5%) places it in the group of secondary symbionts, whose A+T contents vary between those of primary symbionts and those of free-living bacteria.
We followed the guidelines of Tindall et al. (29) to characterize the phylogenetic affiliation of OLO and further clarify its taxonomic status. When identifying bacteria by 16S rRNA gene analysis, a <97% similarity of a sequence to all others is acceptable for classifying a microorganism to a new species, and a new genus name should be given when the similarity between bacterial isolates is ≤95% (29), though additional data are needed as complementary evidence. The 16S rRNA gene of OLO exhibits 94.98% sequence similarity with that of the previously formally described organism O. chuto, and thus, OLO may be considered a new genus.
In this study, OLO was detected in six out of the eight B. tabaci China 1 field populations collected in China. What seems to be the same bacterium has also been found from whiteflies in India. Recently, Singh et al. (30) reported that of the 14 whitefly populations belonging to the Asia I and Asia II genetic groups of B. tabaci from different locations in north India, OLO was present in 7. Apart from whiteflies, bacteria belonging to the same genus as OLO have so far been reported only in genera of the family Aphididae (19, 31).
The presence of OLO in oocytes, as demonstrated by whole-mount FISH, suggests vertical transovarial transmission of that bacterium (Fig. 3A). In addition, OLO was found to be strictly localized in the bacteriocytes together with “Ca. Portiera aleyrodidarum” at all B. tabaci developmental stages. Its distribution was similar to that of “Ca. Hamiltonella defensa” and Arsenophonus (32). This phenomenon is possibly related to the particular mechanism of intact migration of the bacteriocyte into the ovaries in the whitefly, through which OLO could escape host immune responses (33). Because OLO is strictly limited to the whitefly bacteriocytes and is unlikely to go into the hemolymph and stylet, OLO is probably not horizontally transmitted.
When the population dynamics and density of “Ca. Portiera aleyrodidarum” and OLO during host development were monitored using quantitative PCR, “Ca. Portiera aleyrodidarum” proliferation was found to be correlated with the reproductively active stage of the whitefly (Fig. 4A). This coincidence of the symbiont with its host is similar to that of the primary symbiont Buchnera with its aphid hosts and probably reflects the biological role of providing missing essential amino acids for the insect hosts (1, 20). The density of OLO exhibited a pattern of dynamics similar to that of “Ca. Portiera aleyrodidarum”; however, OLO's density outstripped that of “Ca. Portiera aleyrodidarum” in the late developmental stage of the adults (Fig. 4). Because both “Ca. Portiera aleyrodidarum” and OLO are confined in the same bacteriocytes (Fig. 3), interspecies interactions may be expected.
In this study, “Ca. Portiera aleyrodidarum” and OLO were detected in larger quantities in adult female whiteflies than in adult males. FISH analyses revealed that the number of bacteriocytes in the female whiteflies was greater than that in the males (Fig. 3C and D). The densities of both “Ca. Portiera aleyrodidarum” and OLO were higher in the females than in the males (Fig. 4; Table S1 in the supplemental material). This difference between female and male individuals could be explained, at least in part, by the biology of whiteflies. Bemisia tabaci has haplodiploid sex determination: fertilized (diploid) eggs develop into females, and unfertilized (haploid) eggs develop into males (34). Therefore, the higher density in female whiteflies may help the maintenance and transmission of symbionts.
After its first report from MEAM1 whiteflies in 2006, Rickettsia has so far been reported from three other B. tabaci species, MED, Asia II 3 (formerly known as ZHJ1), and Indian Ocean (formerly known as MS) (10, 15, 35, 36). In this study, although the sample size was small, we also found Rickettsia in wild populations of B. tabaci China 1 (Table 1) and thus added another species of the B. tabaci complex to the host range of Rickettsia.
Proposal of “Candidatus Hemipteriphilus asiaticus” gen. nov., sp. nov.
Though the function of OLO is largely unknown, the data collected permit the assignment of OLO as a new genus. The arguments for this proposal include the facts that (i) the highest sequence similarity between OLO and its closely related bacteria is 94.98%, lower than the 95% cutoff value for separating different genera; (ii) the sequences of the OLO 16S rRNA gene (though partial) from field-collected whiteflies showed nearly 100% similarity with each other, suggesting that OLO has its own well-defined identity; (iii) Orientia spp. are mainly found in Leptotrombidium spp. (mites) (37), whereas OLO and its relatives, SMLS (the Sitobion miscanthi L-type symbiont), are endosymbionts of Hemipteran insects; and (iv) Orientia spp. are harbored in the salivary glands of mites and are transmitted to humans during larval feeding (38), whereas OLO and SMLS have not been detected in the salivary glands of their host insects.
Based on its distinct phylogeny and biological traits, we propose to name the newly discovered bacterium associated with B. tabaci “Candidatus Hemipteriphilus asiaticus.” The genus name Hemipteriphilus (He.mi.pte′ri.phi.lus, N.L. neut. plur. n.) is derived from the systematic name of the organism's host order, Hemiptera, N.L. masc. n., and philus (from Gr. philos, friendly to), meaning the friend of Hemiptera. The species name refers to the place, Asia, where this bacterium was found (a.si′a.ti.cus. L. masc. adj. asiaticus, Asiatic, belonging to Asia). “Ca. Hemipteriphilus asiaticus” gen. nov., sp. nov. belongs to the phylum Proteobacteria of bacteria, to the class Alphaproteobacteria, to the order Rickettsiales, and to the family Rickettsiaceae (39). “Ca. Hemipteriphilus asiaticus” is a Gram-negative and transovarialy transmitted intracellular symbiont of arthropods. The genus is assigned mainly on the basis of the 16S rRNA gene sequence (GenBank accession no. JX042442), the gltA gene sequence (GenBank accession no. JX042443), and the groEL gene sequence (GenBank accession no. JX042444).
The bacterium found in the population of the whitefly B. tabaci putative species China 1 from Hangzhou, Zhejiang Province, China, is proposed as the type strain. The G+C content of the 1,426-bp OLO 16S rRNA gene is 51.5%. The level of similarity based on the 16S rRNA gene sequence between “Ca. Hemipteriphilus asiaticus” and the aphid symbiont SMLS (98.8% on average) places them within the genus “Candidatus Hemipteriphilus.” So far, “Candidatus Hemipteriphilus” has been detected only from Asian populations of Hemiptera (15, 19, 30, 31).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China (2009CB119203) and the National Natural Science Foundation of China (31101437).
We thank Hans G. Trüper, Rheinische Friedrich-Wilhelms-Universität, Germany, for advice on naming the newly found bacterium and Qi-Yi Tang for help with the statistical analysis. We also thank Gen-Hong Yan, Hua Zhao, Jia Wang, and Jian Hu for assistance in collecting the whitefly samples; Fabrice Vavre, Helene Delatte, Jun-Min Li, Qiong Rao, Wen-Wu Zhou, and Yun-Lin Su for providing valuable suggestions; and Yun-Qin Li for technical assistance with confocal microscopy.
Footnotes
Published ahead of print 9 November 2012
Supplemental material for this article may be found at 10.1128/AEM.03030-12.
REFERENCES
- 1. Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59:155–189 [DOI] [PubMed] [Google Scholar]
- 2. Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55:247–266 [DOI] [PubMed] [Google Scholar]
- 3. Haine ER. 2008. Symbiont-mediated protection. Proc. Biol. Sci. 275:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelstädter J, Hurst GDD. 2009. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst. 40:127–149 [Google Scholar]
- 5. De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. 2011. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56:1–19 [DOI] [PubMed] [Google Scholar]
- 6. Hu JA, De Barro P, Zhao H, Wang J, Nardi F, Liu SS. 2011. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS One 6:e16061 doi:10.1371/journal.pone.0016061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu SS, Colvin J, De Barro PJ. 2012. Species concepts as applied to the whitefly Bemisia tabaci systematics: how many species are there? J. Integr. Agric. 11:176–186 [Google Scholar]
- 8. Oliveira MRV, Henneberry TJ, Anderson P. 2001. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 20:709–723 [Google Scholar]
- 9. Sloan DB, Moran NA. 2012. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol. Lett. 8:986–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, Kontsedalov S, Gershon M, Gal S, Katzir N, Zchori-Fein E. 2006. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 72:3646–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weeks AR, Velten R, Stouthamer R. 2003. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. Biol. Sci. 270:1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everett KDE, Thao ML, Horn M, Dyszynski GE, Baumann P. 2005. Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int. J. Syst. Evol. Microbiol. 55:1581–1587 [DOI] [PubMed] [Google Scholar]
- 13. Zchori-Fein E, Brown JK. 2002. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 95:711–718 [Google Scholar]
- 14. Nirgianaki A, Banks GK, Frohlich DR, Veneti Z, Braig HR, Miller TA, Bedford ID, Markham PG, Savakis C, Bourtzis K. 2003. Wolbachia infections of the whitefly Bemisia tabaci. Curr. Microbiol. 47:93–101 [DOI] [PubMed] [Google Scholar]
- 15. Bing XL, Ruan YM, Rao Q, Wang XW, Liu SS. 1 June 2012, posting date Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci. doi:10.1111/j.1744-7917.2012.01522.x [DOI] [PubMed] [Google Scholar]
- 16. Liu SS, De Barro PJ, Xu J, Luan JB, Zang LS, Ruan YM, Wan FH. 2007. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 318:1769–1772 [DOI] [PubMed] [Google Scholar]
- 17. Frohlich DR, Torres-Jerez I, Bedford ID, Markham PG, Brown JK. 1999. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 8:1683–1691 [DOI] [PubMed] [Google Scholar]
- 18. Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li T, Xiao J-H, Xu Z-H, Murphy RW, Huang D-W. 2011. Cellular tropism, population dynamics, host range and taxonomic status of an aphid secondary symbiont, SMLS (Sitobion miscanthi L type symbiont). PLoS One 6:e21944 doi:10.1371/journal.pone.0021944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. 2005. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl. Environ. Microbiol. 71:4069–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120 [DOI] [PubMed] [Google Scholar]
- 23. Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 24. Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25:1253–1256 [DOI] [PubMed] [Google Scholar]
- 25. Caspi-Fluger A, Inbar M, Mozes-Daube N, Mouton L, Hunter MS, Zchori-Fein E. 2011. Rickettsia ‘in’ and ‘out’: two different localization patterns of a bacterial symbiont in the same insect species. PLoS One 6:e21096 doi:10.1371/journal.pone.0021096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinisterra XH, McKenzie C, Hunter WB, Powell CA, Shatters RG. 2005. Differential transcriptional activity of plant-pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J. Gen. Virol. 86:1525–1532 [DOI] [PubMed] [Google Scholar]
- 27. Tang Q-Y, Zhang C-X. 1 June 2012, posting date Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. doi:10.1111/j.1744-7917.2012.01519.x [DOI] [PubMed] [Google Scholar]
- 28. Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4:e337 doi:10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tindall BJ, Rosselló-Móra R, Busse H-J, Ludwig W, Kämpfer P. 2010. Notes on the characterization of prokaryote strains for taxonomic purposes. Int. J. Syst. Evol. Microbiol. 60:249–266 [DOI] [PubMed] [Google Scholar]
- 30. Singh ST, Priya NG, Kumar J, Rana VS, Ellango R, Joshi A, Priyadarshini G, Asokan R, Rajagopal R. 2012. Diversity and phylogenetic analysis of endosymbiotic bacteria from field caught Bemisia tabaci from different locations of north India based on 16S rDNA library screening. Infect. Genet. Evol. 12:411–419 [DOI] [PubMed] [Google Scholar]
- 31. Li T, Xiao J-H, Xu Z-H, Murphy RW, Huang D-W. 2011. A possibly new Rickettsia-like genus symbiont is found in Chinese wheat pest aphid, Sitobion miscanthi (Hemiptera: Aphididae). J. Invertebr. Pathol. 106:418–421 [DOI] [PubMed] [Google Scholar]
- 32. Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, Fleury F, Zchori-Fein E. 2008. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 22:2591–2599 [DOI] [PubMed] [Google Scholar]
- 33. Szklarzewicz T, Moskal A. 2001. Ultrastructure, distribution, and transmission of endosymbionts in the whitefly Aleurochiton aceris Modeer (Insecta, Hemiptera, Aleyrodinea). Protoplasma 218:45–53 [DOI] [PubMed] [Google Scholar]
- 34. Byrne DN, Bellows TS. 1991. Whitefly biology. Annu. Rev. Entomol. 36:431–457 [Google Scholar]
- 35. Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, Ghanim M. 2007. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull. Entomol. Res. 97:407–413 [DOI] [PubMed] [Google Scholar]
- 36. Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, Chiel E, Gottlieb Y, Ghanim M, Zchori-Fein E, Fleury F. 2010. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol. Ecol. 19:4365–4378 [DOI] [PubMed] [Google Scholar]
- 37. Kelly DJ, Fuerst PA, Ching W-M, Richards AL. 2009. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin. Infect. Dis. 48:S203–S230 [DOI] [PubMed] [Google Scholar]
- 38. Cho N-H, Kim H-R, Lee J-H, Kim S-Y, Kim J, Cha S, Kim S-Y, Darby AC, Fuxelius H-H, Yin J, Kim JH, Kim J, Lee SJ, Koh Y-S, Jang W-J, Park K-H, Andersson SGE, Choi M-S, Kim I-S. 2007. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc. Natl. Acad. Sci. U. S. A. 104:7981–7986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dumler JS, Walker DH. 2005. Order II. Rickettsiales Gieszczykiewicz 1939, 25 AL emend. Dumler, Barbet, Bekker, Dasch, Palmer, Ray, Rikihisa and Rurangirwa 2001, 2156, p 96–160 In Brenner DJ, Krieg NR, Staley JT. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer, New York, NY [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.