Abstract
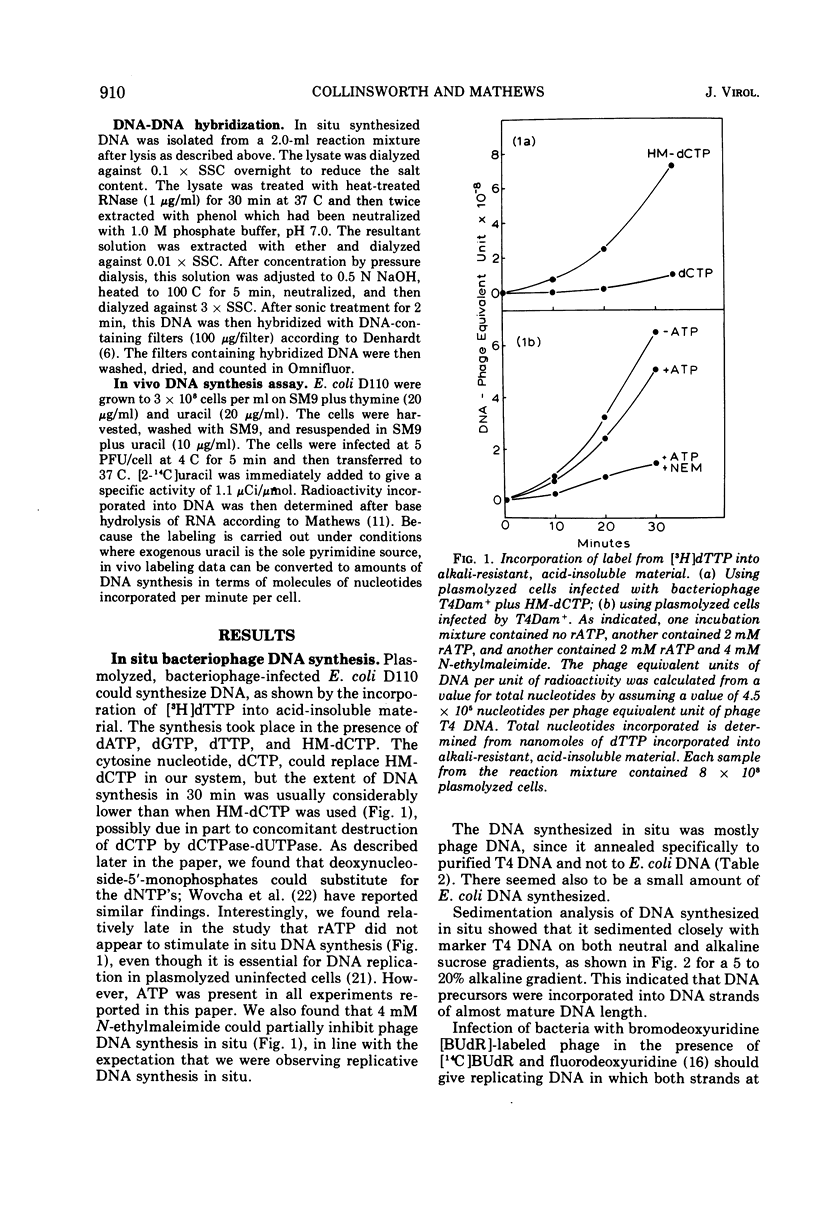
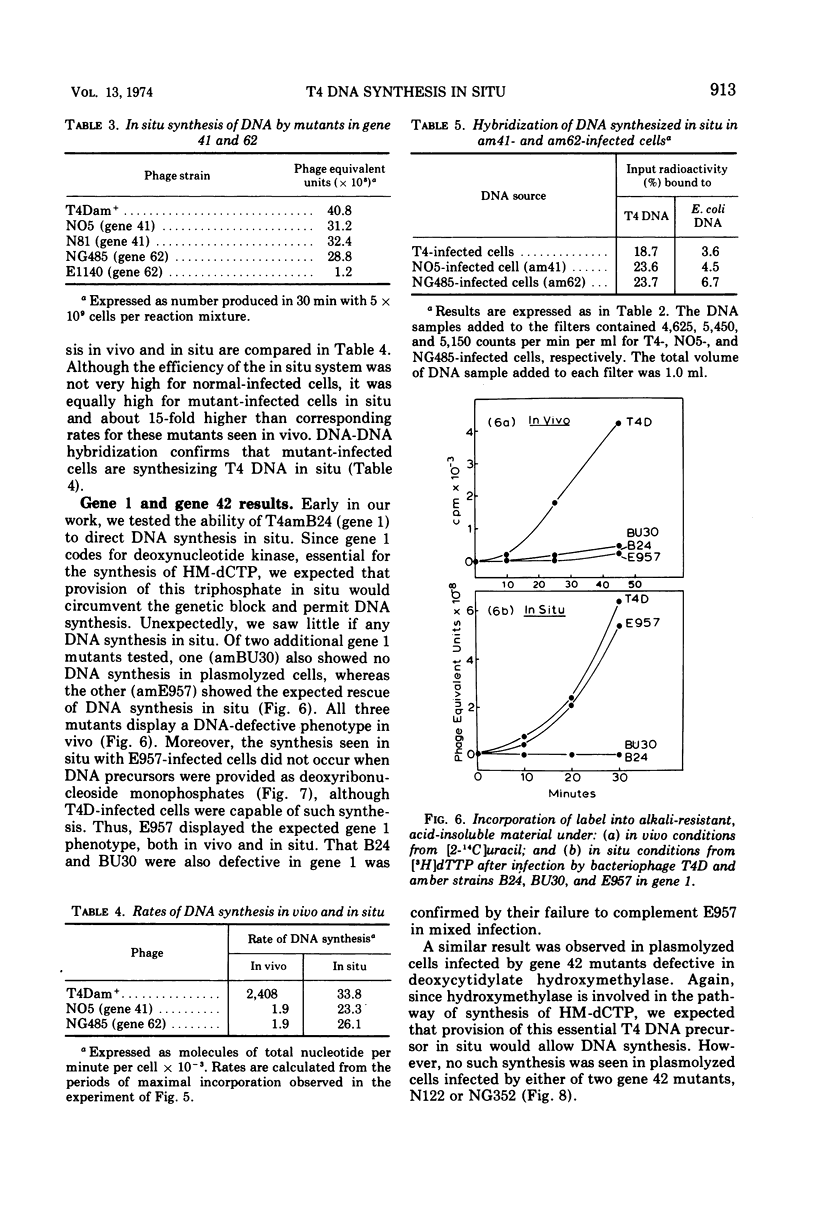
Requirements for bacteriophage T4 DNA synthesis have been investigated in situ by use of plasmolyzed infected cells. When such cells are incubated with dATP, dGTP, dTTP, hydroxymethyldeoxycytidine triphosphate, and rATP, significant semiconservative synthesis of DNA occurs. This DNA hybridizes preferentially to T4 DNA. T4 amber mutants defective in genes 44 and 45, which display a DNA-negative phenotype in vivo, are unable to synthesize DNA in situ. By contrast, T4 amber mutants bearing lesions in genes 41 and 62, which also display a DNA-negative phenotype in vivo, do allow DNA synthesis in situ, the extent of synthesis being 80 to 90% that of the wild-type synthesis under the same conditions. Cells infected with gene 42 mutants (dCMP hydroxymethylase) are unable to synthesize DNA in situ even though exogenous nucleotides are provided. Also one gene 1 mutant (deoxynucleotide kinase) was found to synthesize DNA in situ, but two other gene 1 mutants did not. These results point to possible roles of hydroxymethylase and kinase in DNA metabolism, in addition to provision of essential DNA precursors, as has recently been suggested by Wovcha et al. (1973).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLO L. J., BESSMAN M. J. The enzymology of virus-infected bacteria. IV. Purification and properties of the deoxynucleotide kinase induced by bacteriophage T2. J Biol Chem. 1963 May;238:1777–1787. [PubMed] [Google Scholar]
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. M. Toluene-treated Escherichia coli replicate only that DNA which was about to be replicated in vivo. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2124–2126. doi: 10.1073/pnas.68.9.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Vallée M. The map position of the immunity (imm) gene of bacteriophage T4. Virology. 1973 Feb;51(2):506–508. doi: 10.1016/0042-6822(73)90452-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dicou L., Cozzarelli N. R. Bacteriophage T4-directed DNA synthesis in toluene-treated cells. J Virol. 1973 Dec;12(6):1293–1302. doi: 10.1128/jvi.12.6.1293-1302.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Richter C., Souther A., Bruner R. Synthesis of bacteriophage and host DNA in toluene-treated cells prepared from T4-infected Escherichia coli: role of bacteriophage gene D2a. J Virol. 1973 Dec;12(6):1253–1258. doi: 10.1128/jvi.12.6.1253-1258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. The enzymic synthesis of 5-hydroxymethyldeoxycytidylic acid. Biochim Biophys Acta. 1957 Sep;25(3):667–668. doi: 10.1016/0006-3002(57)90553-x. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutant of bacteriophage T4. I. Ribonucleic acid metabolism. J Biol Chem. 1968 Nov 10;243(21):5610–5615. [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutants of bacteriophage T4. 3. Nucleotide pools. J Biol Chem. 1972 Nov 25;247(22):7430–7438. [PubMed] [Google Scholar]
- Miller R. C., Jr, Taylor D. M., MacKay K., Smith H. W. Replication of T4 DNA in Escherichia coli treated with toluene. J Virol. 1973 Dec;12(6):1195–1203. doi: 10.1128/jvi.12.6.1195-1203.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Mathews C. K. Addition of nucleotides to parental DNA early in infection by bacteriophage T4. J Mol Biol. 1969 Sep 14;44(2):233–248. doi: 10.1016/0022-2836(69)90172-7. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Nucleotide accumulations in Escherichia coli infected with some bacteriophage T4 amber mutants. Virology. 1968 Dec;36(4):527–537. doi: 10.1016/0042-6822(68)90184-0. [DOI] [PubMed] [Google Scholar]
- Werner R. Nature of DNA precursors. Nat New Biol. 1971 Sep 22;233(38):99–103. doi: 10.1038/newbio233099a0. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]
- Wickner R. B., Hurwitz J. DNA replication in Escherichia coli made permeable by treatment with high sucrose. Biochem Biophys Res Commun. 1972 Apr 14;47(1):202–211. doi: 10.1016/s0006-291x(72)80029-9. [DOI] [PubMed] [Google Scholar]
- Wovcha M. G., Tomich P. K., Chiu C. S., Greenberg G. R. Direct participation of dCMP hydroxymethylase in synthesis of bacteriophage T4 DNA. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2196–2200. doi: 10.1073/pnas.70.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]


