Abstract
A soluble protein, FliJ, along with a membrane protein, FlhA, plays a role in the energy coupling mechanism for bacterial flagellar protein export. The water-soluble FliHX-FliI6 ATPase ring complex allows FliJ to efficiently interact with FlhA. However, the FlhA binding site of FliJ remains unknown. Here, we carried out genetic analysis of a region formed by well-conserved residues—Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83—of FliJ. A structural model of the FliI6-FliJ ring complex suggests that they extend out of the FliI6 ring. Glutathione S-transferase (GST)-FliJ inhibited the motility of and flagellar protein export by both wild-type cells and a fliH-fliI flhB(P28T) bypass mutant. Pulldown assays revealed that the reduced export activity of the export apparatus results from the binding of GST-FliJ to FlhA. The F72A and L76A mutations of FliJ significantly reduced the binding affinity of FliJ for FlhA, thereby suppressing the inhibitory effect of GST-FliJ on the protein export. The F72A and L76A mutations were tolerated in the presence of FliH and FliI but considerably reduced motility in their absence. These two mutations affected neither the interaction with FliI nor the FliI ATPase activity. These results suggest that FliJ(F72A) and FliJ(L76A) require the support of FliH and FliI to exert their export function. Therefore, we propose that the well-conserved surface of FliJ is involved in the interaction with FlhA.
INTRODUCTION
For construction of the bacterial flagellum, which is an organelle for motility, most of the flagellar component proteins are transported to the distal end of the flagellar structure by the flagellar type III export apparatus. The export apparatus consists of the six membrane proteins FlhA, FlhB, FliO, FliP, FliQ, and FliR and the three soluble proteins FliH, FliI, and FliJ (1–3). These component proteins share substantial sequence and functional similarities with those of the type III secretion system of animal- and plant-pathogenic Gram-negative bacteria, which directly injects effectors into their host cells for their invasion (4). In addition, FliH, FliI, and FliJ are evolutionally related to major components of F1-ATPase (5–7). Genetic analyses have shown that FliH, FliI, FliJ, and FliO are not essential for flagellar protein export because flagellar assembly occasionally occurs in their absence (8–12).
The six membrane proteins are thought to form a proton-driven export gate at the flagellar base (11). The C-terminal cytoplasmic domains of FlhA (FlhAC) and FlhB (FlhBC) provide the docking sites for FliH, FliI, FliJ, flagellar chaperones, and export substrates (13–17). FliI is a Walker-type ATPase (18) and forms a stable FliH2-FliI complex with FliH (19). The FliH2-FliI complex, along with FliJ, binds to export substrates and chaperone-substrate complexes in the cytoplasm (20–22) and efficiently recruits them to the FlhAC-FlhBC platform of the export gate through specific interactions of the extreme N-terminal region of FliH with a C-ring protein FliN (23–25) and with FlhA (26). The export gate utilizes proton motive force (PMF) across the cytoplasmic membrane as an energy source and drives protein translocation into the central channel of the growing flagellar structure (11, 12). The interaction of FliJ with FlhA brought about by FliH and FliI turns the export gate into a highly efficient PMF-driven export apparatus (27). Interestingly, it has been shown that FliJ increases the binding affinity of FlhAC for the FliT-FliD chaperone-substrate complex, suggesting that FliJ modulates the binding properties of FlhAC or provides an additional binding site for the FliT-FliD complex in close proximity to the export gate (16). These observations suggest that FliJ and FlhA play an important role in the energy coupling mechanism for flagellar protein export.
FliJ is a small protein consisting of 147 amino acids. The C-terminal half of FliJ is sufficient for its interaction with FliH, and the in-frame deletion of residues 101 to 110 shows the most severe effect on the FliJ-FliH interaction, suggesting that this deleted region forms important part of the FliH binding site (28). In-frame deletion of residues 13 to 24 of FliJ significantly weakens the interaction with FliI, suggesting that the N-terminal region of FliJ contains a binding site for FliI (27). Residues 20 to 50 and 60 to 100 of FliJ are involved in the interaction with the flagellar substrate-specific chaperones FlgN and FliT, respectively (29) (see Fig. S1A in the supplemental material). In-frame deletion of residues 13 to 24 in FliJ reduces its binding affinity for FlhA. However, extragenic suppressor mutations in FliH or FliI significantly improve the interaction of FliJ(Δ13-24) with FlhA. Because these suppressor mutations do not affect the FliH-FlhA or FliI-FlhA interaction at all, residues 13 to 24 in FliJ may not be directly involved in the interaction with FlhA (27). Therefore, the FlhA binding site of FliJ remains obscure.
The crystal structure of FliJ adopts an antiparallel coiled coil structure composed of two long α-helices: α1 and α2. A well-conserved, surface-exposed region of FliJ is between the C-terminal region of α1 (residues 38 to 49) and the N-terminal region of α1 (residues 72 to 83) and is formed by Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83, which are highly conserved among FliJ orthologues (see Fig. S1B and C in the supplemental material). The crystal structure of FliJ is considerably similar to that of the two-stranded α-helical coiled coil part of the γ subunit of F1-ATPase. FliJ promotes FliI ring formation by binding to the center of the ring in a way similar to the antiparallel α-helical coiled coil formed by the N- and C-terminal regions of the γ subunit penetrating into the central cavity of the α3β3 ring (7). Interestingly, structure-based sequence alignment of FliJ and the γ subunit has revealed that the well-conserved region of FliJ corresponds to the γ subunit surface involved in the interaction with the ε subunit of the bacterial F1-ATPase (the δ subunit in the bovine mitochondrial F1-ATPase) (7). This raises the possibility that this conserved surface of FliJ is involved in the interaction with its binding partner(s).
In order to identify the FlhA binding site of FliJ, we performed genetic analysis of eight highly conserved, surface-exposed residues—Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83—of FliJ (see Fig. S1B and C in the supplemental material) (7). We show that the F72A and L76A mutations significantly reduce the binding affinity of FliJ for FlhA. We also show that the F72A and L76A mutations are tolerated in the presence of FliH and FliI but considerably reduce the export activity of a ΔfliH-fliI flhB(P28T) bypass mutant, which can form some flagella even in the absence of FliH and FliI.
MATERIALS AND METHODS
Bacterial strains, plasmids, DNA manipulations, and media.
The bacterial strains and plasmids used in the present study are listed in Table 1. DNA manipulations, site-directed mutagenesis, and DNA sequencing were carried out as described previously (34, 35). L broth (LB) and soft tryptone agar plates were prepared as described previously (13, 36). Ampicillin and chloramphenicol were added to LB at final concentrations of 50 and 30 mg/ml, respectively.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli BL21(DE3)pLysS | For overproduction of proteins | Novagen |
| Salmonella enterica | ||
| SJW1103 | Wild type for motility and chemotaxis | 30 |
| SJW1368 | ΔcheW-flhD | 45 |
| MKM40 | ΔfliJ | 25 |
| MMB017 | flhB(P28T) | 11 |
| MMHI001 | ΔfliH-fliI | 32 |
| MMHI0117 | ΔfliH-fliI flhB(P28T) | 11 |
| MMHIJ0117 | ΔfliH-fliI-fliJ flhB(P28T) | 27 |
| Plasmids | ||
| pET15b | Expression vector | Novagen |
| pGEX6p-1 | Expression vector | GE Healthcare |
| pTrc99A | Expression vector | GE Healthcare |
| pTrc99AFF4 | Expression vector | 31 |
| pMM104 | pET19b/His-FlhAc | 13 |
| pMM404 | pTrc99AFF4/FliJ | 10 |
| pMMIJ001 | pET15b/His-FliJ | 33 |
| pMMJ1001 | pGEX6p-1/GST-FliJ | 25 |
| pTIJ007 | pET15b/His-FliJ(F72A) | This study |
| pTIJ008 | pET15b/His-FliJ(L76A) | This study |
| pTIJ105 | pTrc99AFF4/FliJ(F72A) | This study |
| pTIJ106 | pTrc99AFF4/FliJ(L76A) | This study |
| pTIJ201 | pGEX6p-1/GST-FliJ(Q38A) | This study |
| pTIJ202 | pGEX6p-1/GST-FliJ(L42A) | This study |
| pTIJ203 | pGEX6p-1/GST-FliJ(Y45A) | This study |
| pTIJ204 | pGEX6p-1/GST-FliJ(Y49A) | This study |
| pTIJ205 | pGEX6p-1/GST-FliJ(F72A) | This study |
| pTIJ206 | pGEX6p-1/GST-FliJ(L76A) | This study |
| pTIJ207 | pGEX6p-1/GST-FliJ(A79S) | This study |
| pTIJ208 | pGEX6p-1/GST-FliJ(H83A) | This study |
Secretion assays.
Salmonella enterica cells were grown with shaking in 5 ml of LB at 30°C until the cell density had reached an optical density at 600 nm (OD600) of ∼1.2. To test the effects of the ΔpH component of PMF across the cytoplasmic membrane on flagellar protein export, the cells were washed twice with motility buffer (10 mM potassium phosphate, 0.1 mM EDTA, 10 mM sodium lactate) and resuspended in 5 ml of the motility buffer at pH 6.0 or 7.5, followed by a 1-h incubation at 30°C with shaking. Cultures were centrifuged to obtain cell pellets and culture supernatants. Cell pellets were resuspended in an sodium dodecyl sulfate (SDS)-loading buffer, normalized to a cell density to give a constant amount of cells. The proteins in the culture supernatants were precipitated by 10% trichloroacetic acid and suspended in a Tris-SDS loading buffer. The samples were heated at 95°C for 5 min. After SDS-PAGE, Coomassie brilliant blue (CBB) staining or immunoblotting with polyclonal anti-FlgD antibody was carried out as described before (36). Detection was performed with ECL Western blotting detection reagents (GE Healthcare).
Motility assays.
Fresh colonies were inoculated onto soft tryptone agar plates and incubated at 30°C.
Purification of GST and GST-FliJ.
The soluble fractions prepared from SJW1368 (ΔcheW-flhD) expressing glutathione S-transferase (GST) or GST-FliJ were loaded onto a glutathione-Sepharose 4B column (GE Healthcare). After a washing step with phosphate-buffered saline (PBS; 8 g of NaCl, 0.2 g of KCl, 3.63 g of Na2HPO4·12H2O, and 0.24 g of KH2PO4 [pH 7.4] per liter), proteins were eluted with 50 mM Tris-HCl (pH 8.0) and 10 mM reduced glutathione. Fractions containing GST or GST-FliJ were pooled and stored at 4°C.
Purification of His-FlhAC.
His-FlhAC was purified by Ni-NTA affinity chromatography as described previously (13).
Pulldown assays by GST affinity chromatography.
The soluble fractions were prepared from the ΔfliH-fliI flhB(P28T) bypass mutant transformed with pGEX-6p-1 (GST) or pGEX-6p-1-based plasmids encoding various forms of GST-FliJ and then loaded onto a glutathione-Sepharose 4B column whose bed volume was 1 ml. Purified His-FlhAC were mixed with purified GST, GST-FliJ, or various point mutant variants of GST-FliJ. These mixtures were dialyzed overnight against PBS at 4°C with two changes of PBS and then loaded onto the glutathione column (bed volume, 1 ml). After the column was washed with 10 ml of PBS, the proteins were eluted with 50 mM Tris-HCl (pH 8.0) and 10 mM reduced glutathione. The eluted fractions were analyzed by both CBB staining and immunoblotting with polyclonal anti-FlhAC antibody.
To analyze the FliJ-FliI interaction by pulldown assays, the soluble fractions prepared from SJW1364 (ΔflhDC-cheW) expressing GST, GST-FliJ, GST-FliJ(F72A), or GST-FliJ(L76A) were mixed with those from the ΔflhDC-cheW mutant transformed with pMM1702 (His-FliI) and loaded onto the glutathione column (bed volume, 1 ml). After the column was washed with PBS, the proteins were eluted. The eluted fractions were analyzed by CBB staining and immunoblotting with polyclonal anti-FliI antibody.
Purification of FliI and FliJ and ATPase activity measurement of FliI.
Details of the expression and purification of FliI and FliJ have been described previously (7, 33).
The ATPase activity of FliI was measured at 37°C with an enzyme-coupled ATP-regenerating system using a spectrophotometer V-630BIO (Jasco) as described previously (37). The ATPase reaction mixture contained 50 mM Tris-HCl (pH 8.0), 100 mM KCl, 4 mM Na-ATP, 4 mM MgCl2, 2 mM phosphoenolpyruvate, 50 mg of pyruvate kinase/ml, and 50 mg of lactate dehydrogenase/ml. NADH was added to the reaction mixture when its density had reached an OD340 of 1.0 to 1.2. ATP hydrolysis by FliI was started by addition of purified FliI alone or a mixture of purified FliI and FliJ at a molar ratio of 6:1 to the reaction mixture. The ATP activity was calculated from the rate of oxidation of NADH monitored at 340 nm.
Model building of the FliI6-FliJ ring complex.
The structural model of the FliI6-FliJ complex was built by fitting the crystal structure of FliI (PDB ID, 2DPY) and FliJ (PDB ID, 3AJW) onto the α3β3γ structure of bovine mitochondrial F1-ATPase (PDB ID, 1BMF). Fitting of FliI to the α3β3 ring was previously described (6). FliJ was divided into three segments: the upper segment (residues 31 to 87), the middle segment (residues 17 to 30 and residues 88 to 101), and the lower segment (residues 1 to 16 and residues 102 to 136). Each segment was separately fitted onto the corresponding region of the γ subunit with a program LSQKAB in the CCP4 package (38). The three segments were then manually connected with a graphic program Coot (39).
RESULTS
Inhibitory effect of GST-FliJ on flagellar protein export.
FliJ is known to interact with many flagellar proteins involved in the export process (13, 23, 28, 29). It has been shown that FliJ exerts a strong inhibitory effect on flagellar protein export by a Salmonella wild-type strain, but it remained unknown which components are titrated away by overexpression of FliJ (13). To find the binding partner(s), we carried out pulldown assays by GST affinity chromatography (Fig. 1). When wild-type FliJ was added in trans, the motility of a ΔfliJ mutant was restored. However, GST-FliJ failed to recover the motility of the ΔfliJ mutant (Fig. 1A, left panel). Consistently, only a very small amount of the hook-capping protein FlgD was observed in the culture supernatants (Fig. 1A, right panel, lane 8). We obtained the same results with other flagellar proteins, such as FlgE (the hook protein), FliK (the hook-length control protein), FlgK (the first hook-filament junction protein), FlgL (the second hook-filament junction protein), and FliC (flagellin). These results indicate that the GST tag considerably abolishes the export function of FliJ. When GST-FliJ was expressed in wild-type cells, the motility was significantly reduced compared to that of the wild-type overexpressing GST alone (Fig. 1B, upper panel). In agreement with this, the overexpression of GST-FliJ significantly reduced the levels of FlgD, FlgE, FliK, FlgK, and FliC secreted by wild-type cells (data not shown). GST-FliJ also inhibited the motility of the ΔfliH-fliI flhB(P28T) bypass mutant (Fig. 1B, lower panel). These results indicate that GST-FliJ exerts the strong inhibitory effect even in the absence of FliH and FliI.
Fig 1.
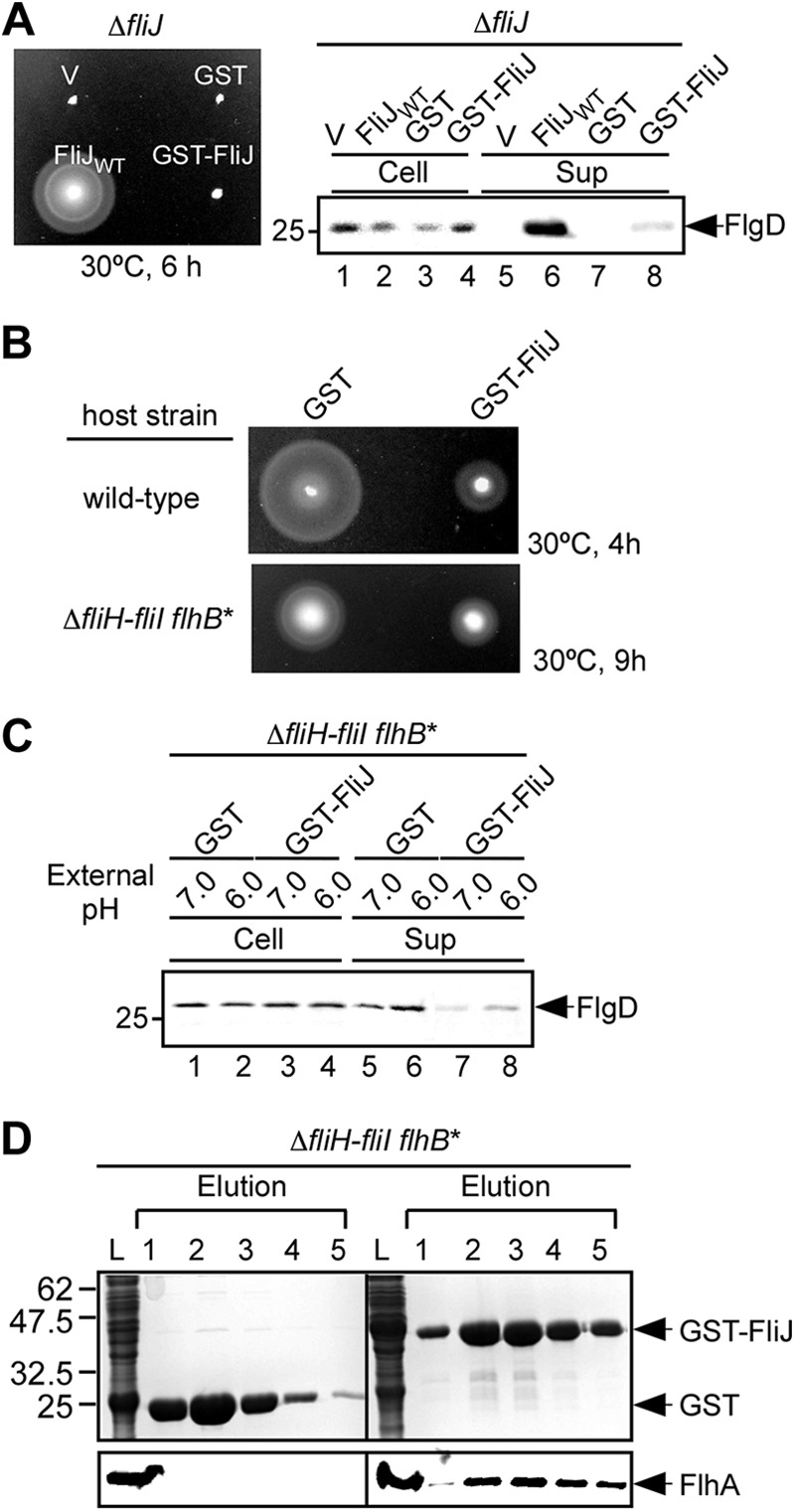
Interaction of GST-FliJ with FlhA. (A) Motility assays of MKM40 (ΔfliJ) transformed with pTrc99AFF4 (V), pGEX6p-1 (GST), pMM404 (wild-type FliJ, indicated as FliJWT), or pMMJ1001 (GST-FliJ) in soft agar plates. The plates were incubated at 30°C for 6 h. (B) Dominant-negative effect of GST-FliJ on motility of wild-type cells (upper panel) and the ΔfliH-fliI flhB(P28T) bypass mutant (lower panel). The motility of SJW1103 (wild type) and MMHI0017 (ΔfliH-fliI, flhB*) transformed with pGEX-6p-1 (GST) or pMMJ1001 (GST-FliJ) in soft agar plates was evaluated. (C) Effect of external pH on FlgD secretion by the ΔfliH-fliI flhB(P28T) bypass mutant. Immunoblotting was performed with the polyclonal anti-FlgD antibody of whole-cell and culture supernatant fractions prepared from the ΔfliH-fliI flhB(P28T) bypass mutant overexpressing GST or GST-FliJ grown at external pH values of 6.0 and 7.0. (D) Pulldown assays by GST affinity chromatography. The soluble fractions (indicated as L) from the ΔfliH-fliI flhB(P28T) bypass mutant overproducing GST or GST-FliJ were loaded onto a GST column. After being washed with 10 ml of PBS, the proteins were eluted with a buffer containing 10 mM reduced glutathione. The eluted factions containing GST or GST-FliJ were analyzed by CBB staining (upper panels), while the eluted FlhA protein was done by immunoblotting with polyclonal anti-FlhAC antibody (lower panels).
It has been shown that an increase in the ΔpH component of PMF across the cytoplasmic membrane stimulates the export activity of the export gate in the ΔfliH-fliI flhB(P28T) bypass mutant (27). Therefore, we analyzed the ΔpH dependence of protein export by the ΔfliH-fliI flhB(P28T) bypass mutant overexpressing GST-FliJ (Fig. 1C). A downward shift of external pH from 7.0 to 6.0, which increased the ΔpH component (data not shown), increased the levels of FlgD secreted by the bypass mutant overexpressing GST alone (Fig. 1C, lanes 5 and 6), in agreement with a previous report (27). GST-FliJ significantly reduced the FlgD secretion levels at both external pH values of 6.0 and 7.0 but did not affect the ΔpH dependence of the protein export (Fig. 1C, lanes 7 and 8). These results suggest that GST-FliJ may compete against FliJ expressed from the chromosome in the interaction with a component of the export gate, thereby causing the reduction in the PMF-driven export activity of the gate.
Because GST-FliJ binds to FlhA (27), we investigated whether the strong inhibitory effect of GST-FliJ on the protein export results from the binding of GST-FliJ to FlhA. Whole-cell lysates were prepared from the ΔfliH-fliI flhB(P28T) bypass mutant overexpressing GST alone or GST-FliJ, followed by GST affinity chromatography (Fig. 2D). FlhA coeluted with GST-FliJ from a GST column but not with GST alone. Because the overexpression of GST-FliJ significantly reduced the secretion levels of flagellar proteins, we suggest that the reduced PMF-driven export activity of the export gate may be a consequence of the binding of GST-FliJ to FlhA.
Fig 2.

Molecular model of the FliI6-FliJ ring complex. (A) Cα backbone trace of the FliI6-FliJ complex. The FliI subunits are colored blue, violet, green, yellow, lilac, and red, and FliJ is colored cyan. (B) Model viewed from the top of panel A. (C) The eight conserved residues are labeled and highlighted in red. The FliI subunits are colored gray. (D) Binding region of FliJ for FliI. The subunit shown in violet in panel A is removed for better visualization of FliJ. Residues 13 to 24 of FliJ are shown in magenta. The region including these residues interacts with FliI.
Structural model of the FliI6-FliJ ring complex.
The FliI ring structure is observed at the flagellar base by electron cryotomography (40). FliI and FliJ show extensive structural similarity to the α/β subunits and the γ subunit of F1-ATPase, respectively (6, 7). FliI assembles into a homohexamer to fully exert its ATPase activity (32, 41). The crystal structure of an ADP-bound form of FliI has revealed that ADP binds to the P-loop of FliI in a way similar to that found in the α/β subunits, suggesting that FliI and F1-ATPase share a similar catalytic pathway for ATP hydrolysis (6). FliJ binds to the C-terminal region of the C1 α-helix of FliI, which corresponds to the region of the β subunit where the γ subunit interacts in the F1-ATPase structure, and forms a FliI6-FliJ ring complex with FliI (7). The electron cryomicroscopic image analysis of the FliI6-FliJ ring complex has shown that FliJ is located at the center of the FliI hexameric ring (7). These observations strongly suggest that the structure of the FliI6-FliJ complex closely resembles the F1-ATPase structure. Eight highly conserved, surface-exposed residues of FliJ—Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83—have been identified (see Fig. S1B and C in the supplemental material). Interestingly, Tyr45, Tyr49, and Phe72 are also conserved between FliJ and the γ subunit (7). To investigate whether these conserved residues are exposed to solvent on the surface of the FliI6 ring, a structural model of the FliI6-FliJ ring complex was built using the F1-ATPase structure as a template (Fig. 2). In this model, FliJ penetrates into the central hole of the FliI hexameric ring in a way similar to the α-helical coiled coil region of the γ subunit in the α3β3 ring in F1-ATPase (Fig. 2A and B). Residues 31 to 91 of FliJ stick out of the FliI ring, and these conserved residues are located in this region (Fig. 2C).
Effect of FliJ mutations on the interaction with FlhA.
The structural model of the FliI6-FliJ ring complex predicts that a well-conserved surface formed by Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83 extends out of the FliI hexameric ring (Fig. 2C). To test whether the conserved surface of FliJ provides a binding site for FlhA, we replaced each conserved residue with alanine except for Ala79, which was replaced with serine, and first tested whether these mutations affect the dominant-negative effect of GST-FliJ on motility of the wild type (see Fig. S2 in the supplemental material) and the ΔfliH-fliI flhB(P28T) bypass mutant (Fig. 3). These mutations did not affect the protein stability of GST-FliJ (Fig. 3A and see Fig. S2A in the supplemental material). When GST-FliJ(Q38A), GST-FliJ(L42A), GST-FliJ(Y45A), GST-FliJ(Y49A), GST-FliJ(A79S), and GST-FliH(H83A) were added in trans, they still showed an inhibitory effect on the motility of the wild type (Fig. S2B) and the ΔfliH-fliI flhB(P28T) bypass mutant (Fig. 3B). Interestingly, the inhibitory effect of GST-FliJ(L42A), GST-FliJ(Y45A), GST-FliJ(Y49A), and GST-FliJ(A79S) on the motility of the bypass mutant was much stronger than that of GST-FliJ (Fig. 3B). In contrast, the F72A and L76A mutations suppressed the inhibitory effect of GST-FliJ on the motility (Fig. 3A and see Fig. S2A in the supplemental material).
Fig 3.
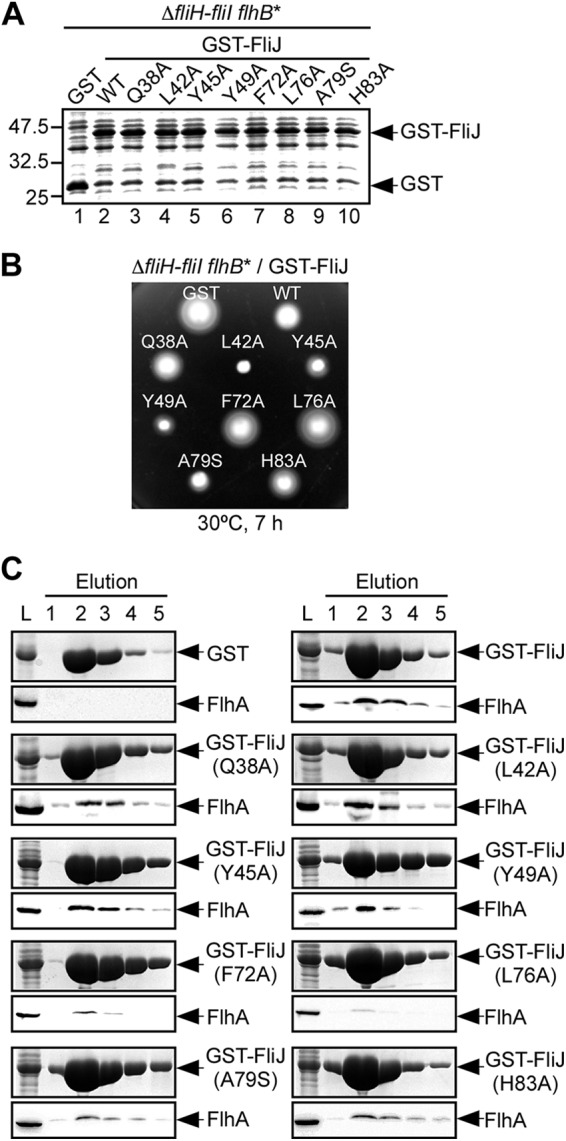
Effect of FliJ mutations on the FliJ-FlhA interaction. (A) Expression levels of various point mutant variants of GST-FliJ. Whole-cell lysates were prepared from the ΔfliH-fliI flhB(P28T) bypass mutant (ΔfliH-fliI flhB*) transformed with pGEX-6p-1-based plasmids encoding various forms of GST-FliJ and subjected to SDS-PAGE, followed by CBB staining. Lane 1, GST; lane 2, GST-FliJ (indicated as wild type [WT]); lane 3, GST-FliJ(Q38A) (indicated as Q38A); lane 4, GST-FliJ(L42A) (indicated as L42A); lane 5, GST-FliJ(Y45A) (indicated as Y45A); lane 6, GST-FliJ(Y49A) (indicated as Y49A); lane 7, GST-FliJ(F72A) (indicated as F72A); lane 8, GST-FliJ(L76A) (indicated as L76A); lane 9, GST-FliJ(A79S) (indicated as A79S); lane 10, GST-FliJ(H83A) (indicated as H83A). (B) Dominant-negative effect of GST-FliJ point mutant variants on motility of the ΔfliH-fliI flhB(P28T) bypass mutant (ΔfliH-fliI flhB*). The motility of the same transformants in soft agar was evaluated. The plates were incubated at 30°C for 7 h. (C) Pulldown assays by GST affinity chromatography. The soluble fractions (L) prepared from the above strains were loaded onto a GST column. After extensive washing with 10 ml of PBS, the proteins were eluted with a buffer containing 10 mM reduced glutathione. The eluted fractions were analyzed by CBB staining (upper panel) and immunoblotting with polyclonal anti-FlhA antibody (lower panel).
To test whether these FliJ mutations affect the interaction with FlhA, we prepared whole-cell lysates from the ΔfliH-fliI flhB(P28T) bypass mutant overexpressing point mutant variants of GST-FliJ and then performed pulldown assays by GST affinity chromatography (Fig. 3C). The F72A and L76A mutations weakened the interaction of GST-FliJ with FlhA significantly, while the others did not.
FlhAC consists of a compactly folded core domain (FlhAC38K) formed by four subdomains—D1, D2, D3, and D4—and a flexible linker region (FlhAL), which connects FlhAC38K to the N-terminal integral membrane domain (FlhATM) with eight predicted transmembrane helices (15, 34, 42). It has been shown that FliJ binds to FlhAL (16, 27). To confirm that the F72A and L76A mutations reduce the binding affinity of FliJ for FlhAL, we analyzed the FliJ-FlhAC interaction in vitro (see Fig. S3 in the supplemental material). His-FlhAC coeluted with GST-FliJ from a GST column (lanes 3 and 4) but not with GST alone (lanes 1 and 2), in agreement with a previous report (16). The eluted amounts of His-FlhAC copurified with GST-FliJ(Q38A) (data not shown), GST-FliJ(L42A) (data not shown), GST-FliJ(Y45A) (lanes 5 and 6), GST-FliJ(Y49A) (lanes 7 and 8), GST-FliJ(A79S) (data not shown), and GST-FliJ(H83A) were essentially the same as that with GST-FliJ, indicating that these mutations do not affect the FliJ-FlhAC interaction at all. In contrast, the F72A and L76A mutations reduced the binding affinity of GST-FliJ for His-FlhAC (lanes 8 to 12). These results suggest that Phe72 and Leu76 are involved in the interaction with FlhAC.
Effect of the F72A and L76A mutations on the export function of FliJ.
We found that the FliJ(F72A) and FliJ(L76A) mutations significantly affected the interaction of FliJ with FlhA (Fig. 3C and see Fig. S3 in the supplemental material). To investigate whether these two mutations cause loss of function, we analyzed the motility of a ΔfliJ mutant expressing each of the FliJ point mutant variants in soft agar (Fig. 4A). Immunoblotting with polyclonal anti-FliJ antibody revealed that these mutant FliJ proteins were as stable as wild-type FliJ (Fig. 4A, right panel). When FliJ(F72A) and FliJ(L76A) were expressed from the plasmid, both of them fully restored the motility of the ΔfliJ mutant (Fig. 4A, left panel). Consistently, the amount of FlgD secreted by these two fliJ mutants was detected at the wild-type level (Fig. 4A, right panel, lanes 7 and 8). These results indicate that the F72A and L76A mutations are tolerated.
Fig 4.

Effect of the F72A and L76A mutations on the export function of FliJ. (A) Motility (left panel) and secretion assays (right panel) of a ΔfliJ mutant harboring pTrc99AFF4 (V), pMM404 (pTrc99AFF4/wild-type FliJ, indicated as FliJWT), pTIJ105 [pTrc99AFF4/FliJ(F72A), indicated as F72A], or pTIJ106 [pTrc99AFF4/FliJ(F76A), indicated as F76A]. The plates were incubated at 30°C for 4.5 h. Immunoblotting with polyclonal anti-FlgD or anti-FliJ antibody of whole-cell (Cell) and culture supernatant (Sup) fractions prepared from the above transformants. (B) Motility (left panel) of and FlgD secretion (right panel) by a ΔfliH-fliI-fliJ flhB(P28T) mutant transformed with the above plasmids. The plates were incubated at 30°C for 8 h. The level of FlgD secretion was analyzed by immunoblotting with the polyclonal anti-FlgD antibody. The expression level of FliJ was detected by immunoblotting with polyclonal anti-FliJ antibody.
FliJ requires the support of FliH and FliI to efficiently and properly interact with FlhA (27), raising the possibility that FliH and FliI allow FliJ(F72A) and FliJ(L76A) to exert their export function. Therefore, we tested whether these two mutant FliJ proteins retain their function in the ΔfliH-fliI flhB(P28T) bypass mutant background (Fig. 4B). When wild-type FliJ was added in trans, the motility of the ΔfliH-fliI-fliJ flhB(P28T) mutant was enhanced significantly (Fig. 4B, left panel), in agreement with a previous report (27). In contrast, the motility of the ΔfliH-fliI-fliJ flhB(P28T) mutant strain expressing FliJ(F72A) or FliJ(L76A) was much poorer than that of the strain harboring the wild-type FliJ plasmid. Consistently, the secretion level of FlgD by these mutants was significantly reduced (Fig. 4B, right panel, lanes 7 and 8). These results indicate that the mutated residues are critical for the function of FliJ in the absence of FliH and FliI.
Effect of the F72A and L76A mutations on the interaction with FliI.
We found that FliJ(F72A) and FliJ(L76A) were functional in the presence of FliH and FliI but not in their absence (Fig. 4). The structural model predicts that the F72A and L76A mutations may not affect the interaction of FliJ with FliI (Fig. 2). To confirm this, we analyzed the effect of the F72A and L76A mutations on the FliJ-FliI interaction by pulldown assays (Fig. 5A). FliI coeluted with GST-FliJ(F72A) and GST-FliJ(L76A) from a GST column, indicating that these mutations do not weaken the FliJ-FliI interaction significantly.
Fig 5.
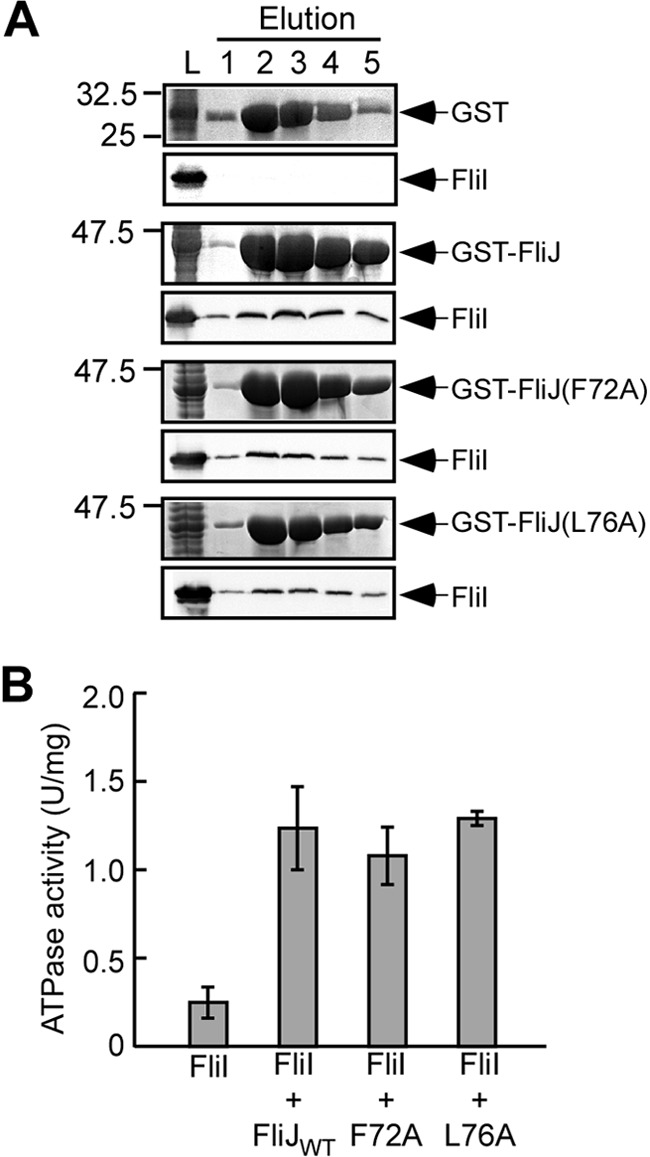
Effect of the F72A and L76A mutations on the interaction with FliI ATPase. (A) Pulldown assays by GST affinity chromatography. A mixture of the soluble fractions (L) prepared from a ΔflhDC-cheW mutant expressing GST (first row), GST-FliJ (second row), GST-FliJ(F72A) (third row), or GST-FliJ(L76A) (fourth row) with those from the ΔflhDC-cheW mutant producing His-FliI were loaded onto a GST column. The eluted fractions were analyzed by both CBB staining (upper panels) and immunoblotting with polyclonal anti-FliI antibody (lower panels). (B) Effect of FliJ substitutions on the ATPase activity of FliI. The ATPase activity of FliI alone and the mixture of FliI with wild-type FliJ (FliJWT), FliJ(F72A) (F72A), or FliJ(L76A) (L76A) at the molar ratio of 6 FliI to 1 FliJ was measured at 37°C with an enzyme-coupled ATP-regenerating system. At least three independent experiments were carried out for each mixture. Vertical bars indicate the standard deviations.
FliJ promotes FliI hexameric ring formation by binding to the center of the ring, thereby facilitating the FliI ATPase activity (7, 29). To investigate whether the F72A and L76A mutations affect the ATPase activity, we measured the FliI ATPase activity in a mixture of FliI and mutant FliJ with a molar ratio of 6:1 using an enzyme-coupled ATP-regenerating system (37). This molar ratio is used because FliI ring formation is significantly enhanced (7). FliI alone displayed an ATPase activity of 0.26 ± 0.03 U mg−1 FliI protein (Fig. 5B). The FliI/FliJ mixture with a molar ratio of 6:1 displayed an ATPase activity of 1.24 ± 0.23 U mg−1 FliI protein. The ATPase activities of the FliI/FliJ(F72A) and FliI/FliJ(L75A) mixtures were nearly the same as that of FliI/FliJ, indicating that these FliJ point mutant retained the ability to enhance the FliI ATPase activity. These results suggest that FliJ(F72A) and FliJ(L75A) retain the ability to facilitate FliI hexamer formation.
DISCUSSION
The crystal structure of FliJ shows extensive similarity to the antiparallel α-helical coiled coil formed by the N- and C-terminal regions of the γ subunit. A well-conserved, surface-exposed region of FliJ is formed by Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83 (Fig. S1B and C). Among these eight conserved residues, Tyr45, Tyr49, and Phe72 correspond to the residues of the γ subunit being involved in the interaction with the e-subunit in the bacterial F1-ATPase (7). However, it remained unknown which components of the flagellar export apparatus bind to this conserved surface of FliJ. Here, we analyzed this conserved surface of FliJ by mutational analysis combined with pulldown assays by GST affinity chromatography. GST-FliJ, which was nonfunctional (Fig. 1A), exerted a strong dominant-negative effect on motility of and flagellar protein export by both the wild type and the ΔfliH-fliI flhB(P28T) bypass mutant (Fig. 1B). An increase in the ΔpH component of PMF across the cytoplasmic membrane still stimulated the level of FlgD secretion by the ΔfliH-fliI flhB(P28T) bypass mutant overexpressing GST-FliJ (Fig. 1C), indicating that GST-FliJ competes against FliJ in the interaction with component(s) of the export gate and hence reduces the PMF-driven export activity of the gate. The negative dominant effect of GST-FliJ on flagellar protein export was substantially relieved by the F72A and L76A mutations but not by the remaining six mutations (Fig. 3B and see Fig. S2B in the supplemental material). The F72A and L76A mutations significantly reduced the binding affinity of FliJ for FlhA, while the others did not show any effect (Fig. 3C and see Fig. S3 in the supplemental material). These results suggest that the reduced PMF-driven export activity of the export gate is a consequence of the binding of GST-FliJ to FlhA and that Phe72 and Leu76 of FliJ strongly contribute to the interaction with FlhA. Because it has been reported that the FliJ(1-73) and FliJ(74-147) fragments do not bind to FlhA (28), we propose that a well-conserved surface formed by Gln38, Leu42, Tyr45, Tyr49, Phe72, Leu76, Ala79, and His83 of FliJ provides a binding site for FlhA.
In-frame deletion of residues 13 to 24 of FliJ, which results in loss-of-function (10), not only abolishes the interaction with FlhA but also weakens the interaction with FliI (27). The in-frame deletion is located at the interface between FliJ and the C1 α-helix of FliI in the FliI6-FliJ complex (Fig. 4D), explaining why the deletion weakens the interaction between FliJ and FliI. Extragenic suppressor mutations in FliH significantly recovers the interaction between FliJ(Δ13-24) and FlhA. These suppressor mutations do not affect the FliH-FlhA or FliI-FlhA interaction at all, suggesting that the pseudorevertants with the second-site mutations are compensating for the FliJ(Δ13-24) mutation by altering the binding interface between FliJ and FlhA (27). The FliI ring structure has been visualized in situ by electron cryomicroscopy (40). Because this in-frame deletion is buried inside of the FliI ring structure, we assume that this in-frame deletion may cause some misalignment of the conserved surface of FliJ relative to FlhA, significantly reducing its binding affinity for FlhA.
FliJ requires the support of FliH and FliI to properly and efficiently interact with FlhA (27). Here, we showed that the F72A and L76A mutations do not affect the export function in the presence of FliH and FliI (Fig. 4A). In contrast, these two mutations resulted in loss of function in the ΔfliH-fliI flhB(P28T) bypass mutant background (Fig. 4B). These results indicate that FliH and FliI overcome the reduced export function of FliJ(F72A) and FliJ(L76A). The F72A and L76A mutations affect neither the interaction with FliI nor the stimulation of the FliI ATPase activity (Fig. 5). Therefore, these results suggest that FliH and FliI stabilize the weak interaction of FliJ(F72A) or FliJ(L76A) with FlhA, allowing these two FliJ mutant to work at the wild-type level.
FliJ binds to FlhAL connecting FlhAC38K to FlhATM (16, 27). Mapping of the conserved residues on the γ subunit of the bovine mitochondrial structure showed that six of the eight conserved residues (Leu42, Tyr45, Tyr49, Phe72, Leu76, and Ala79) are located on the surface interacting with the δ subunit (the ε subunit in the bacterial F1-ATPase). Interestingly, the γ subunit residues corresponding to Phe72 and Leu76 of FliJ are also in close proximity to an α-helix of the ε subunit, which is unique to the bovine mitochondrial F1-ATPase. This raises the possibility that that Phe72 and Lue76 of FliJ interact with FlhAL in a manner similar to the γ subunit interacting with the ε subunit. The ε subunit of the bovine mitochondrial F1-ATPase is a small protein composed of about 50 amino acid residues with a helix-loop-helix structure (43). FlhAL (residues 328 to 362) is unfolded in the crystal structure of the Salmonella FlhAC; its N-terminal 19 residues (residues 328 to 346) are invisible, and the remaining 15 residues (residues 347 to 362) are in an extended conformation stabilized by the interaction with a neighboring molecule in the crystal packing (42). FlhAL in the FlhAC crystals of Helicobacter pylori (44) and Bacillus subtilis (16), however, adopts an α-helical structure. Therefore, it is possible that the FlhAL of the Salmonella FlhAC is folded into an α-helix when it interacts with FliJ.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Kihara-Macnab for continuous support and encouragement.
T.I. was a research fellow of the Japan Society for the Promotion of Science. This study was supported in part by Grants-in-Aid for Scientific Research (21227006 to K.N. and 22570161 and 2312516 to T.M.), Targeted Proteins Research Program (to K.I.), and a Grant-in-Aid for Scientific Research on Innovative Areas “Spying Minority in Biological Phenomena” (23115008 to K.I. and T.M.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print 16 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01711-12.
REFERENCES
- 1. Macnab RM. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77–100 [DOI] [PubMed] [Google Scholar]
- 2. Minamino T, Imada K, Namba K. 2008. Mechanisms of type III protein export for bacterial flagellar assembly. Mol. Biosyst. 4:1105–1115 [DOI] [PubMed] [Google Scholar]
- 3. Minamino T, Namba K. 2004. Self-assembly and type III protein export of the bacterial flagellum. J. Mol. Microbiol. Biotechnol. 7:5–17 [DOI] [PubMed] [Google Scholar]
- 4. Cornelis GR. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811–825 [DOI] [PubMed] [Google Scholar]
- 5. Pallen MJ, Bailey CM, Beatson SA. 2006. Evolutionary links between FliH/YscL-like proteins from bacterial type III secretion systems and second-stalk components of the FoF1 and vacuolar ATPases. Protein Sci. 15:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imada K, Minamino T, Tahara A, Namba K. 2007. Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proc. Natl. Acad. Sci. U. S. A. 104:485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ibuki T, Imada K, Minamino T, Kato T, Miyata T, Namba K. 2011. Common architecture between the flagellar protein export apparatus and F- and V-ATPases. Nat. Struct. Mol. Biol. 18:277–282 [DOI] [PubMed] [Google Scholar]
- 8. Barker CS, Meshcheryakova IV, Kostyukova AS, Samatey FA. 2010. FliO regulation of FliP in the formation of the Salmonella enterica flagellum. PLoS Genet. 6:e1001143 doi:10.1371/journal.pgen.1001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. González-Pedrajo B, Fraser GM, Minamino T, Macnab RM. 2002. Molecular dissection of Salmonella FliH, a regulator of the ATPase FliI and the type III flagellar protein export pathway. Mol. Microbiol. 45:967–982 [DOI] [PubMed] [Google Scholar]
- 10. Minamino T, Chu R, Yamaguchi S, Macnab RM. 2000. Role of FliJ in flagellar protein export in Salmonella. J. Bacteriol. 182:4207–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minamino T, Namba K. 2008. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature 451:485–488 [DOI] [PubMed] [Google Scholar]
- 12. Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. 2008. Energy source of flagellar type III secretion. Nature 451:489–492 [DOI] [PubMed] [Google Scholar]
- 13. Minamino T, Macnab RM. 2000. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 35:1052–1064 [DOI] [PubMed] [Google Scholar]
- 14. Minamino T, Gonzalez-Pedrajo B, Kihara M, Namba K, Macnab RM. 2003. The ATPase FliI can interact with the type III flagellar protein export apparatus in the absence of its regulator, FliH. J. Bacteriol. 185:3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minamino T, Shimada M, Okabe M, Saijo-Hamano Y, Imada K, Kihara M, Namba K. 2010. Role of the C-terminal cytoplasmic domain of FlhA in bacterial flagellar type III protein export. J. Bacteriol. 192:1929–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bange G, Kümmerer N, Engel C, Bozkurt G, Wild K, Sinning I. 2010. FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system. Proc. Natl. Acad. Sci. U. S. A. 107:11295–11300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minamino T, Kinoshita M, Hara N, Takeuchi S, Hida A, Koya S, Glenwright H, Imada K, Aldridge PD, Namba K. 2012. Interaction of a bacterial flagellar chaperone FlgN with FlhA is required for efficient export of its cognate substrates. Mol. Microbiol. 83:775–788 [DOI] [PubMed] [Google Scholar]
- 18. Fan F, Macnab RM. 1996. Enzymatic characterization of FliI: an ATPase involved in flagellar assembly in Salmonella typhimurium. J. Biol. Chem. 271:31981–31988 [DOI] [PubMed] [Google Scholar]
- 19. Minamino T, Macnab RM. 2000. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol. Microbiol. 37:1494–1503 [DOI] [PubMed] [Google Scholar]
- 20. Thomas J, Stafford GP, Hughes C. 2004. Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export. Proc. Natl. Acad. Sci. U. S. A. 101:3945–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imada K, Minamino T, Kinoshita M, Furukawa Y, Namba K. 2010. Structural insight into the regulatory mechanisms of interactions of the flagellar type III chaperone FliT with its binding partners. Proc. Natl. Acad. Sci. U. S. A. 107:8812–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Minamino T, Kinoshita M, Imada K, Namba K. 2012. Interaction between FliI ATPase and a flagellar chaperone FliT during bacterial flagellar protein export. Mol. Microbiol. 83:168–178 [DOI] [PubMed] [Google Scholar]
- 23. González-Pedrajo B, Minamino T, Kihara M, Namba K. 2006. Interactions between C ring proteins and export apparatus components: a possible mechanism for facilitating type III protein export. Mol. Microbiol. 60:984–998 [DOI] [PubMed] [Google Scholar]
- 24. McMurry JL, Murphy JW, Gonzalez-Pedrajo B. 2006. The FliN-FliH interaction mediates localization of flagellar export ATPase FliI to the C ring complex. Biochemistry 45:11790–11798 [DOI] [PubMed] [Google Scholar]
- 25. Minamino T, Yoshimura SDJ, Morimoto YV, González-Pedrajo B, Kami-ike N, Namba K. 2009. Roles of the extreme N-terminal region of FliH for efficient localization of the FliH-FliI complex to the bacterial flagellar type III export apparatus. Mol. Microbiol. 74:1471–1483 [DOI] [PubMed] [Google Scholar]
- 26. Hara N, Morimoto YV, Kawamoto A, Namba K, Minamino T. 2012. Interaction of the extreme N-terminal region of FliH with FlhA is required for efficient bacterial flagellar protein export. J. Bacteriol. 194:5353–5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minamino T, Morimoto YV, Hara N, Namba K. 2011. An energy transduction mechanism used in bacterial type III protein export. Nat. Commun. 2:475 doi:10.1038/ncomms1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fraser GM, González-Pedrajo B, Tame JRH, Macnab RM. 2003. Interactions of FliJ with the Salmonella type III flagellar export apparatus. J. Bacteriol. 185:5546–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans LDB, Stafford GP, Ahmed S, Fraser GM, Hughes C. 2006. An escort mechanism for cycling of export chaperones during flagellum assembly. Proc. Natl. Acad. Sci. U. S. A. 103:17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi S, Fujita H, Sugata K, Taira T, Iino T. 1984. Genetic analysis of H2, the structural gene for phase-2 flagellin in Salmonella. J. Gen. Microbiol. 130:255–265 [DOI] [PubMed] [Google Scholar]
- 31. Ohnishi K, Fan F, Schoenhals GJ, Kihara M, Macnab RM. 1997. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J. Bacteriol. 179:6092–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minamino T, Kazetani K, Tahara A, Suzuki H, Furukawa Y, Kihara M, Namba K. 2006. Oligomerization of the bacterial flagellar ATPase FliI is controlled by its extreme N-terminal region. J. Mol. Biol. 360:510–519 [DOI] [PubMed] [Google Scholar]
- 33. Ibuki T, Shimada M, Minamino T, Namba K, Imada K. 2009. Crystallization and preliminary X-ray analysis of FliJ, a cytoplasmic component of the flagellar type III protein-export apparatus from Salmonella sp. Acta Crystallogr. Sect. F Struct. Biol. Crystallogr. Commun. 65:47–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saijo-Hamano Y, Minamino T, Macnab RM, Namba K. 2004. Structural and functional analysis of the C-terminal cytoplasmic domain of FlhA, an integral membrane component of the type III flagellar protein export apparatus in Salmonella. J. Mol. Biol. 343:457–466 [DOI] [PubMed] [Google Scholar]
- 35. Hara N, Namba K, Minamino T. 2011. Genetic characterization of conserved charged residues in the bacterial flagellar type III export protein FlhA. PLoS One 6:e22417 doi:10.1371/journal.pone.0022417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minamino T, Macnab RM. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 181:1388–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vogel G, Steinhart R. 1976. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry 15:208–216 [DOI] [PubMed] [Google Scholar]
- 38. Collaborative Computational Project Number 4 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50:760–763 [DOI] [PubMed] [Google Scholar]
- 39. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- 40. Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Müller A, Dobro MJ, Jensen GJ. 2011. Structural diversity of bacterial flagellar motors. EMBO J. 30:2972–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Claret L, Susannah CR, Higgins M, Hughes C. 2003. Oligomerisation and activation of the FliI ATPase central to bacterial flagellum assembly. Mol. Microbiol. 48:1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saijo-Hamano Y, Imada K, Minamino T, Kihara M, Shimada M, Kitao A, Namba K. 2010. Structure of the cytoplasmic domain of FlhA and implication for flagellar type III protein export. Mol. Microbiol. 76:260–268 [DOI] [PubMed] [Google Scholar]
- 43. Gibbons C, Montgomery MG, Leslle AGW, Walker JE. 2000. The structure of the central stalk in bovine F1-ATPase at 2.4 Å resolution. Nat. Struct. Biol. 7:1055–1061 [DOI] [PubMed] [Google Scholar]
- 44. Moore SA, Jia Y. 2010. Structure of the cytoplasmic domain of the flagellar secretion apparatus component FlhA from Helicobacter pylori. J. Biol. Chem. 285:21060–21069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohnishi K, Ohto Y, Aizawa S, Macnab RM, Iino T. 1994. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J. Bacteriol. 176:2272–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


