Abstract
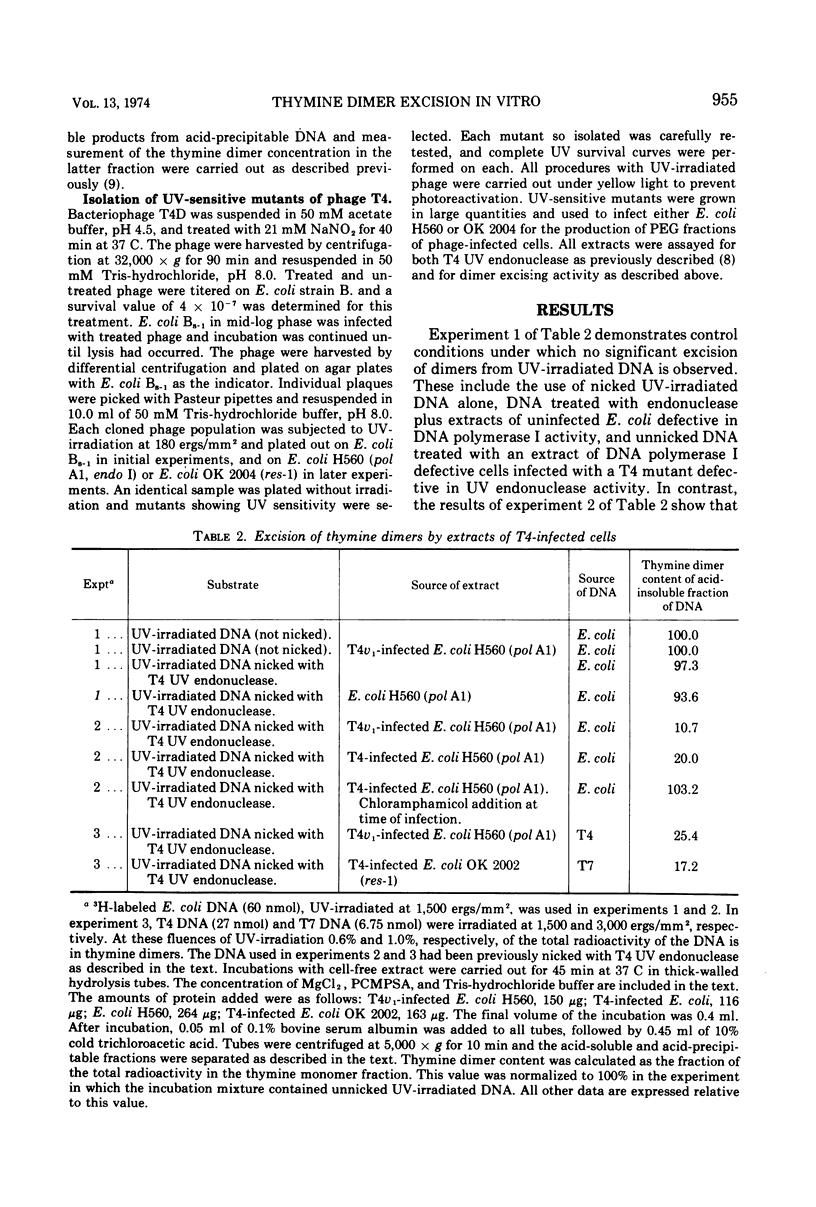
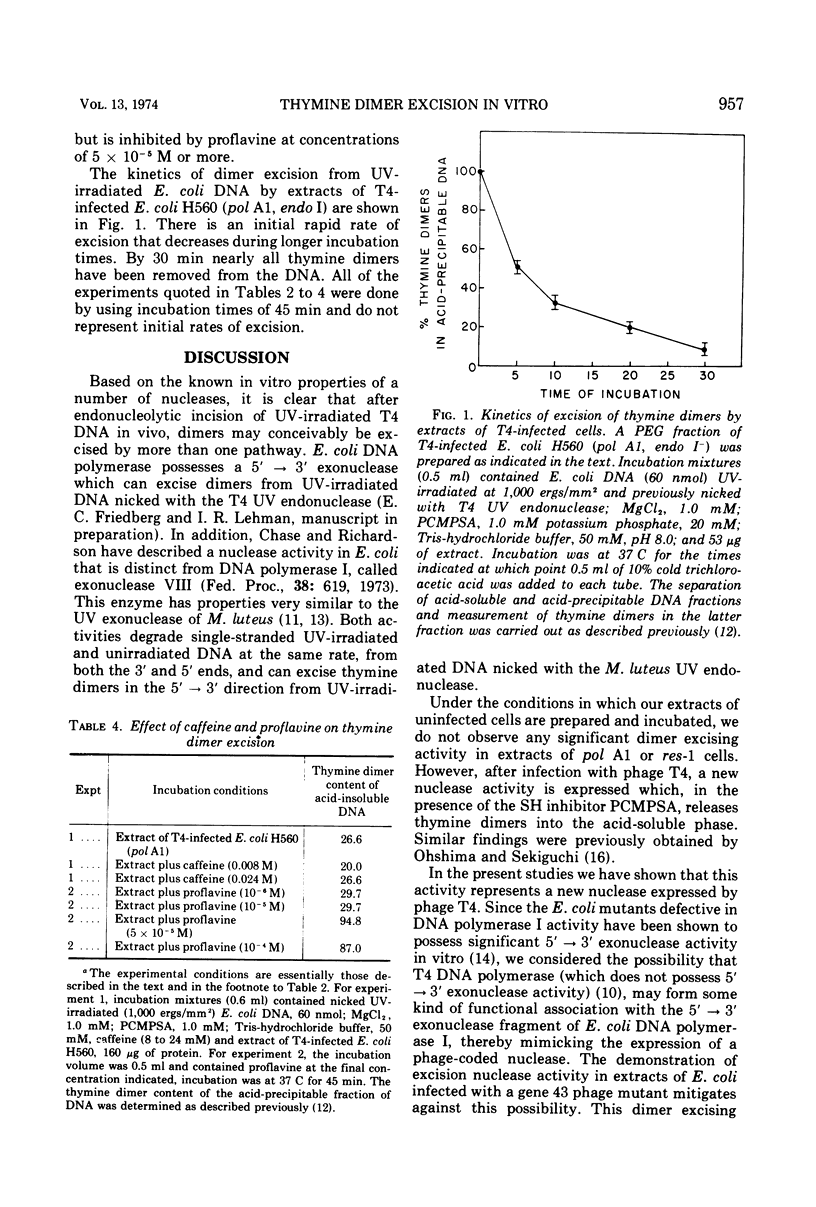
Extracts of DNA polymerase I defective Escherichia coli infected with phage T4 contain an exonuclease activity that removes thymine dimers from UV-irradiated DNA previously nicked with T4 UV endonuclease. This activity is not expressed if cells are infected in the presence of chloramphenicol. The enzyme has a requirement for divalent cation and is not affected by caffeine, but excision is inhibited in the presence of proflavine. The enzyme is present in all phage T4 mutants thus far examined, including 25 UV-sensitive mutants isolated during the course of the experiments, all of which are defective in the v gene. A similar activity can be detected in cells infected with phages T2, T3, and T6, but not in cells infected with phage T7.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldy M. W. Repair and recombination in phage T4. II. Genes affecting UV sensitivity. Cold Spring Harb Symp Quant Biol. 1968;33:333–338. doi: 10.1101/sqb.1968.033.01.038. [DOI] [PubMed] [Google Scholar]
- Brunk C. F. Distribution of dimers in ultraviolet-irradiated DNA. Nat New Biol. 1973 Jan 17;241(107):74–76. doi: 10.1038/newbio241074a0. [DOI] [PubMed] [Google Scholar]
- Frenkel G. D., Richardson C. C. The deoxyribonuclease induced after infection of Escherichia coli by bacteriophage T5. I. Characterization of the enzyme as a 5'-exonuclease. J Biol Chem. 1971 Aug 10;246(15):4839–4847. [PubMed] [Google Scholar]
- Friedberg E. C., Clayton D. A. Electron microscopic studies on substrate specificity of T4 excision repair endonuclease. Nature. 1972 May 12;237(5350):99–100. doi: 10.1038/237099a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Endonucleolytic cleavage of UV-irradiated DNA controlled by the V+ gene in phage T4. Biochem Biophys Res Commun. 1969 Nov 6;37(4):646–651. doi: 10.1016/0006-291x(69)90859-6. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Studies on the substrate specificity of the T 4 excision repair endonuclease. Mutat Res. 1972 Jun;15(2):113–123. doi: 10.1016/0027-5107(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Goldmann K., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. Anal Biochem. 1973 May;53(1):124–131. doi: 10.1016/0003-2697(73)90413-2. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA. 3. Properties of the UV-endonuclease and UV-exonuclease. Biochemistry. 1971 Aug 31;10(18):3315–3324. doi: 10.1021/bi00794a001. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Kaplan J. C., Ono H., Grossman L. Enzymatic repair of deoxyribonucleic acid. IV. Mechanism of photoproduct excision. Biochemistry. 1971 Aug 31;10(18):3325–3334. doi: 10.1021/bi00794a002. [DOI] [PubMed] [Google Scholar]
- OLESON A. E., KOERNER J. F. A DEOXYRIBONUCLEASE INDUCED BY INFECTION WITH BACTERIOPHAGE T2. J Biol Chem. 1964 Sep;239:2935–2943. [PubMed] [Google Scholar]
- Oshima S., Sekiguchi M. Induction of a new enzyme activity to excise pyrimidine dimers in Escherichia coli infected with bacteriophage T4. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1126–1132. doi: 10.1016/0006-291x(72)90951-5. [DOI] [PubMed] [Google Scholar]
- Shafranovskaya N. N., Trifonov E. N., Lazurkin Y. S., Frank-Kamenetskii M. D. Clustering of thymine dimers in ultraviolet irradiated DNA and the long-range transfer of electronic excitation along the molecule. Nat New Biol. 1973 Jan 10;241(106):58–60. doi: 10.1038/newbio241058a0. [DOI] [PubMed] [Google Scholar]
- Short E. C., Jr, Koerner J. F. Separation and characterization of deoxyribonucleases of Escherichia coli B. II. Further purification and properties of an exonuclease induced by infection with bacteriophage T2. J Biol Chem. 1969 Mar 25;244(6):1487–1496. [PubMed] [Google Scholar]
- Warner H. R., Snustad D. P., Koerner J. F., Childs J. D. Identification and genetic characterization of mutants of bacteriophage T4 defective in the ability to induce exonuclease A. J Virol. 1972 Mar;9(3):399–407. doi: 10.1128/jvi.9.3.399-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. Mechanism of repair of DNA in bacteriophage. II. Inability of ultraviolet-sensitive strains of bacteriophage in inducing an enzyme activity to excise pyrimidine dimers. J Mol Biol. 1970 Jan 28;47(2):243–255. doi: 10.1016/0022-2836(70)90343-8. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]


