Abstract
Mucosal tissues are the primary route of transmission for most respiratory and sexually transmitted diseases, including human immunodeficiency virus (HIV). There is epidemiological evidence that genital mucosal inflammation leads to enhanced HIV type 1 (HIV-1) transmission. The objective of this study was to assess the influence of periodontal inflammation on oral HIV transmission using a nonhuman primate model of teeth ligature-induced periodontitis. Simian immunodeficiency virus (SIV) was nontraumatically applied to the gingiva after moderate gingivitis was identified through clinical and immunologic analyses (presence of inflammatory cytokines). Overall oral SIV infection rates were similar in the gingivitis-induced and control groups (5 infections following 12 SIV administrations for each), although more macaques were infected with multiple viral variants in the gingivitis group. SIV infection also affected the levels of antiviral and inflammatory cytokines in the gingival crevicular fluid, and a synergistic effect was observed, with alpha interferon and interferon-inducible protein 10 undergoing significant elevations following SIV infection in macaques with gingivitis compared to controls. These increases in antiviral and inflammatory immune modulators in the SIV-infected gingivitis macaques could also be observed in blood plasma, although the effects at both compartments were generally restricted to the acute phase of the infection. In conclusion, while moderate gingivitis was not associated with increased susceptibility to oral SIV infection, it resulted in elevated levels of cytokines in the oral mucosa and plasma of the SIV-infected macaques. These findings suggest a synergy between mucosal inflammation and SIV infection, creating an immune milieu that impacts the early stages of the SIV infection with potential implications for long-term pathogenesis.
INTRODUCTION
The current human immunodeficiency virus (HIV) epidemic is spread primarily during transmission across a mucosal membrane, including the vaginal, rectal, penile, and oral mucosa (reviewed in reference 1). Epidemiologic data indicate that the risk of genital HIV transmission in adults is substantially higher than the risk of oral transmission (2–4). In general, infection with HIV via the oral route can occur during mother-to-child transmission due to virus in breast milk or oral-genital transmission due to virus in semen; the rate of oral-genital transmission of HIV is difficult to ascertain, although a few studies have documented that infection can occur during receptive oral intercourse (5). Overall, these epidemiological studies indicate that while oral transmission of HIV can occur, it only occurs under certain circumstances, and understanding the environmental events or genetic factors that influence oral transmission of HIV would be useful in preventing acquisition of the virus via this route (6). The model of simian immunodeficiency virus (SIV) infection using rhesus macaques has been utilized to investigate the earliest events following oral transmission (7–13). In this model, there is experimental evidence that orally applied SIV enters the body through the oral or esophageal mucosa, although entry via the tonsils is also likely, as SIV can be detected in these tissues at 1 to 2 days postinfection (10, 13).
There is epidemiological evidence that coinfections or inflammation of the genital mucosal tissue lead to enhanced HIV type 1 (HIV-1) transmission (14–17). Genital coinfections, such as genital herpes, gonorrhea, syphilis, and chlamydia, as well as yeast and bacterial vaginal infections, or host responses to these pathogens, create an inflammatory environment that favors HIV-1 infection and dissemination (18). Periodontal disease in the form of gingivitis represents a form of mucosal inflammation that is found in a significant proportion of the world population (19, 20), and there is a well-established and reproducible nonhuman primate model of ligature-induced gingivitis (21–23). The study described here addresses the question of whether the induction of a common moderate inflammatory state of the gingival mucosa impacts oral SIV transmission and the induction of immune modulators in the rhesus macaque model.
MATERIALS AND METHODS
Animals.
Animal care and treatment were in accordance with Texas Biomedical Research Institute IACUC policies and approved protocols. Sixteen male Indian rhesus macaques, free of infection with simian retrovirus, simian T cell leukemia virus, SIV, and Macacine herpesvirus 1 (formerly herpes B virus), were divided into two equal groups of similar average weight and age. Gingivitis was induced in one group, whereas the second group acted as a control.
Induction of gingivitis.
The gingivitis phase was initiated at the beginning of the study by first switching to a soft diet, consisting of commercial chow biscuits soaked in warm water for 10 min and drained and without providing any mechanical oral hygiene (24). Ligatures were tied on the first and second molar and second premolar teeth (teeth five, six, and seven) in all quadrants of the oral cavity using 3-0 silk sutures that were packed subgingivally; silk ligatures and soft food were maintained for the duration of the study. Control animals received normal food and no ligatures. A complete periodontal evaluation was performed for all animals at each sampling time point by D. Cappelli. A Maryland probe (William's markings) was used to determine the plaque index (PI) (25), pocket depth (PD), recession, and bleeding upon probing (BOP) at four sites on each tooth: distobuccal, buccal, mesiobuccal, and lingual (premolar and first and second molar) in each quadrant. Clinical attachment level (CAL) values were calculated from the pocket depth and recession measures. A gingival bleeding score, following determination of the pocket depth measure, was obtained (21–23).
Viral challenge.
In a first study, one group of 4 control animals and a second group of 4 gingivitis macaques were challenged by depositing 1 ml (1,833 50% tissue culture infectious doses [TCID50]) of SIVmac251 suspension over the gingiva, at the location of the ligatures, or in equivalent areas in control macaques; this procedure was repeated on two occasions, 48 and 96 h after the first challenge. This viral dose and type of delivery was previously determined to infect approximately 50% of control animals (26). In a second study, two groups of 5 animals each were exposed to virus by placing a strip of Whatman 3-mm paper soaked in virus on the same area of the gum used in the first study and leaving the strip for at least 10 min; the procedure was repeated 48 and 96 h later. Blood samples, oral mucosal punch biopsy specimens, and microbiological samples were also obtained from sites of gingivitis induction or a comparable site for the control macaques.
GCF collection.
Gingival crevicular fluid (GCF) samples were obtained as previously described (22, 27, 28). Briefly, gingival sites were isolated and gently dried using cotton gauze. Absorbent filter strips (Periopaper strips; Oraflow, Inc.) were placed below the gingival margin at mesial sites of premolar and first and second molar teeth in the maxillary and mandibular quadrants on both sides of the oral cavity. The strips were maintained, isolated from saliva, for a period of approximately 15 to 30 s. The filter strips were removed, placed in a vial containing transport buffer, stored on ice, transported to the laboratory, and maintained at −80°C until analysis.
Viral loads.
SIV loads were quantified in plasma by NASBA (nucleic acid sequence-based analysis) assay (Ranajit Pal, Advanced Bioscience Labs, Inc.) with a lower detection limit of 50 genome equivalents per ml of plasma (29).
Cytokines in plasma and GCF.
Plasma and GCF cytokines were determined by a nonhuman primate (NHP)-specific Luminex assay that detected 36 cytokines as described elsewhere (30–32). These cytokines included granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF), growth-regulated oncogene alpha (GRO-α), alpha interferon (IFN-α), IFN-γ, interleukin-1β (IL-1β), IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-18, IL-23, interferon-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage-derived chemokine (MDC), MIP-1α, MIP-1β, perforin, RANTES, sCD40L, sFASL, tumor growth factor alpha (TGF-α), TGF-β, tumor necrosis factor alpha (TNF-α), TNF-β, and vascular endothelial growth factor (VEGF).
For GCF, cytokines were eluted from two filter strips in 50 μl of phosphate-buffered saline containing 0.05% Tween 20 and protease inhibitors and was stored at −80°C.
Quantitative real-time PCR analysis of immune effector genes.
Changes in expression of the IFN-α, CXCL10 (IP-10), IL-6, 2′-5′-oligoadenylate synthetase (OAS), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were assessed by real-time reverse transcription-PCR (RT-PCR) utilizing gene-specific primers/probes as described previously (33). These assays were performed on an ABI 7700 or ABI 7300 (Applied Biosystems, Foster City CA) sequencer, and changes were calculated by the delta delta threshold cycle (ΔΔCT) method (34).
Assessment of plasma viral RNA envelope V1-V2 sequences by single-genome amplification (SGA).
Identification of viral variants in SIV-infected macaques was done by limiting-dilution cloning and sequencing as described previously (35, 36) with minor modifications. Sequences were proofread and double checked with single peaks at every nucleotide position using the software Bioedit (Ibis Therapeutics, Carlsbad, CA). p-distances were calculated by MEGA by dividing the number of nucleotide differences by the total number of nucleotides being compared. Verified sequences were subject to Highlighter analysis through the HIV database online tool to determine the number of viral variants (http://www.hiv.lanl.gov/content/sequence/HIGHLIGHT/highlighter_top.html).
Statistical analysis.
Statistical analyses were performed with GraphPad Prism software. Measurement differences between gingivitis and control groups were determined with the Mann-Whitney test, whereas measurement differences within each group at different time points were done with the Wilcoxon matched pairs test with the confidence interval set at 95% (P < 0.05).
RESULTS
Induction of gingivitis.
In order to study the influence of mucosal inflammation in the acquisition of SIV infection, we induced gingivitis in a group of rhesus macaques with a combination of ligation of the posterior teeth and a diet of softened food, while animals with no ligatures that received standard monkey chow served as controls (21–23). The development of inflammation in both groups was evaluated by detection of cytokines in GCF and clinical periodontal measurements.
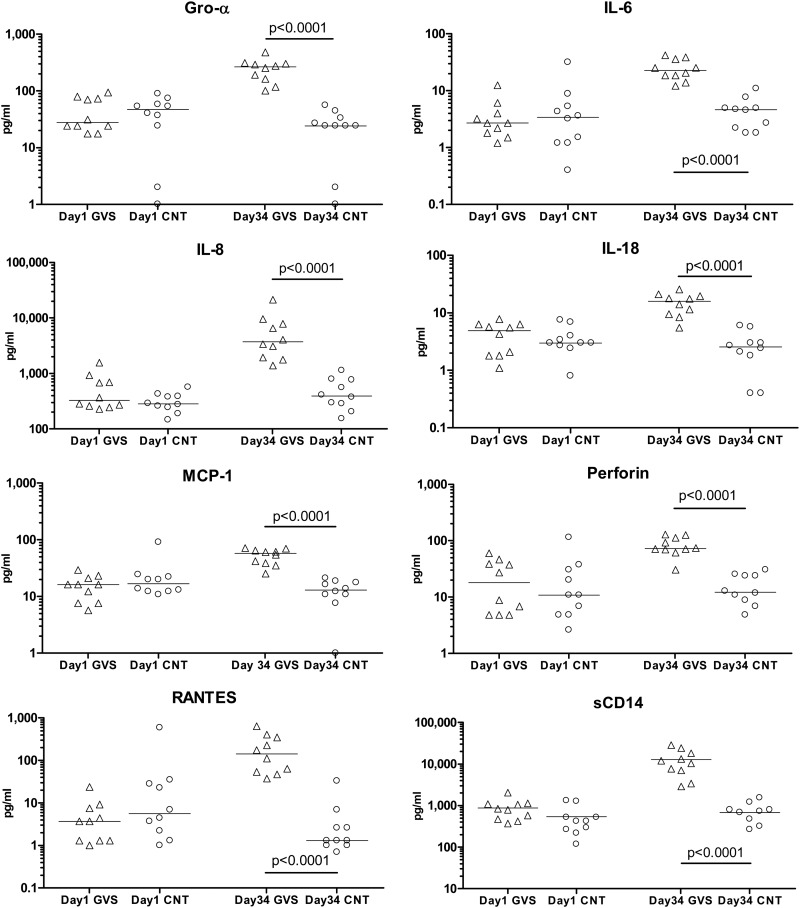
Analysis of cytokines in the GCF, performed for each group at the beginning of the study and after 5 weeks of ligature placement, demonstrated clear inflammation in the experimental group. While differences between groups were not statistically significant at the beginning of the study (day 1), animals with ligated teeth had significantly higher concentrations of the inflammatory markers GRO-α, IL-6, IL-8, IL-18, MCP-1, perforin, RANTES, and sCD14 by the end of the fifth week of treatment (P < 0.0001 for treated versus control macaques) (Fig. 1).
Fig 1.
Mucosal inflammatory cytokines associated with gingivitis. GCF samples were taken from rhesus macaques before (day 1) and 34 days after induction of gingivitis (GVS) by teeth ligation and a diet of softened food; GCF samples were also taken from control animals (CNT) at the same time points. Cytokine concentrations (in pg/ml) were determined in the GCF by an NHP Luminex assay as described in Materials and Methods; differences in median cytokine concentrations were compared between groups with the Mann-Whitney test.
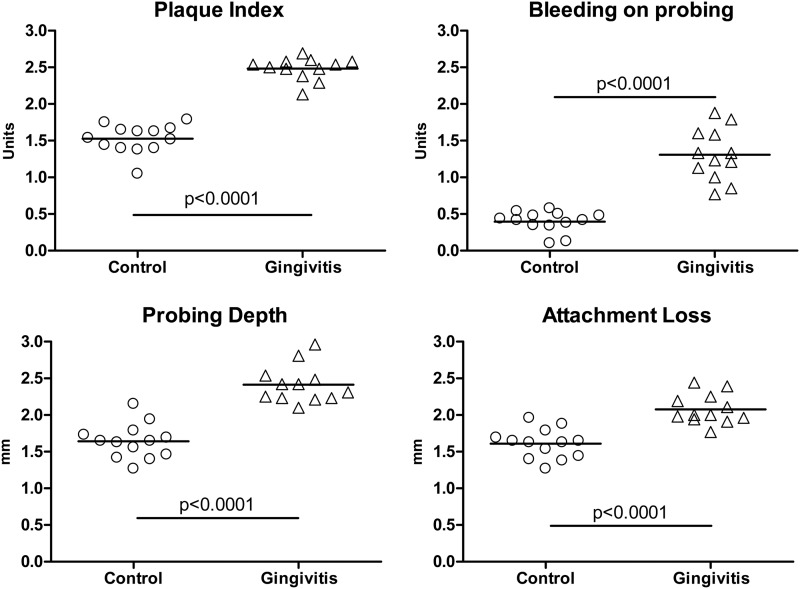
Among the clinical measurements, plaque index (PI) characterizes the extent and quantity of tooth-associated bacterial plaque, whereas probe depth (PD) and clinical attachment loss (CAL) characterize the depth of the gingival crevice/pocket and level of gingival attachment to the tooth, respectively; periodontal breakdown is associated with increased pocketing and clinical attachment loss. The presence and degree of bleeding upon gentle periodontal probing and probing depth measures provide an estimate of the inflammatory changes in the lining of the gingival pocket. After 5 weeks of ligature placement, all of the clinical measurements (PI, PD, and CAL) of treated animals were significantly higher than what was measured in control animals (P < 0.0001 for the four clinical measurements, treated versus control) (Fig. 2), confirming the efficacy of the chosen treatment in the development of gingivitis.
Fig 2.
Clinical manifestation of gingivitis. Untreated controls and monkeys after 5 weeks of teeth ligature and a diet of soft food were evaluated for plaque index, probing depth, bleeding index, and attachment loss as described in Materials and Methods. Median values for both groups were compared by the Mann-Whitney test.
In summary, the combination of soft food and silk ligatures induced a consistent and localized mucosal inflammation in treated animals. In general, the level of inflammation observed in the treated animals corresponded to moderate gingivitis (37).
Susceptibility to oral infection with SIV.
Five weeks after inserting ligatures, and when all animals in the treated group had clear signs of inflammation, animals in both groups were exposed to cell-free SIVmac251 by the oral route. The initial viral dose (1,833 TCID50) and delivery method (needleless syringe applying the virus to the boundary between the teeth and gums) had previously been shown to infect 50% of untreated rhesus macaques (26); in this study, this challenge resulted in three out of four animals in each group becoming infected (Table 1). Since our goal was to evaluate the infectivity of the virus at the site of gingivitis, we designed a second experiment in which 5 macaques (the single uninfected macaque from each group plus four new macaques for each group) again were administered the same amount of virus (1,833 TCID50), but this time the virus was deposited in a piece of Whatman 3-mm paper placed at the junction of teeth and gingiva. The virus-soaked paper was left in place for 15 min in each of the sedated animals. After this exposure, one out of five control animals (20%) and two out of five ligated animals (40%) became infected. Finally, in order to approach a 50% animal infectious dose for untreated animals, we performed a third challenge in which the uninfected macaques in both the gingivitis-induced and control groups were exposed to the Whatman paper soaked with a higher viral inoculum (2,750 TCID50). As a result of this challenge, one more control animal became infected, whereas none of the ligated animals showed the presence of virus. Thus, the final outcome for these three sets of oral SIV challenges was that 5 out of 12 viral exposures in each group resulted in SIV infection (42%), suggesting that oral inflammation did not correlate with increased susceptibility to SIV infection.
Table 1.
Influence of gingivitis on oral SIV acquisition
| Sample set | No. of macaques |
Virus administration | Doseb (TCID50) | SIV infection |
||
|---|---|---|---|---|---|---|
| Control | Gingivitisa | Control | Gingivitis | |||
| 1 | 4 | 4 | Needleless syringe | 1,833 | 3 | 3 |
| 2 | 5 | 5 | Whatman paper | 1,833 | 1 | 2 |
| 3 | 4 | 3 | Whatman paper | 2,750 | 1 | 0 |
Gingivitis was induced by teeth ligature and a diet of softened food for 5 weeks.
The SIVmac251 dose was applied orally every other day for a total of three exposures.
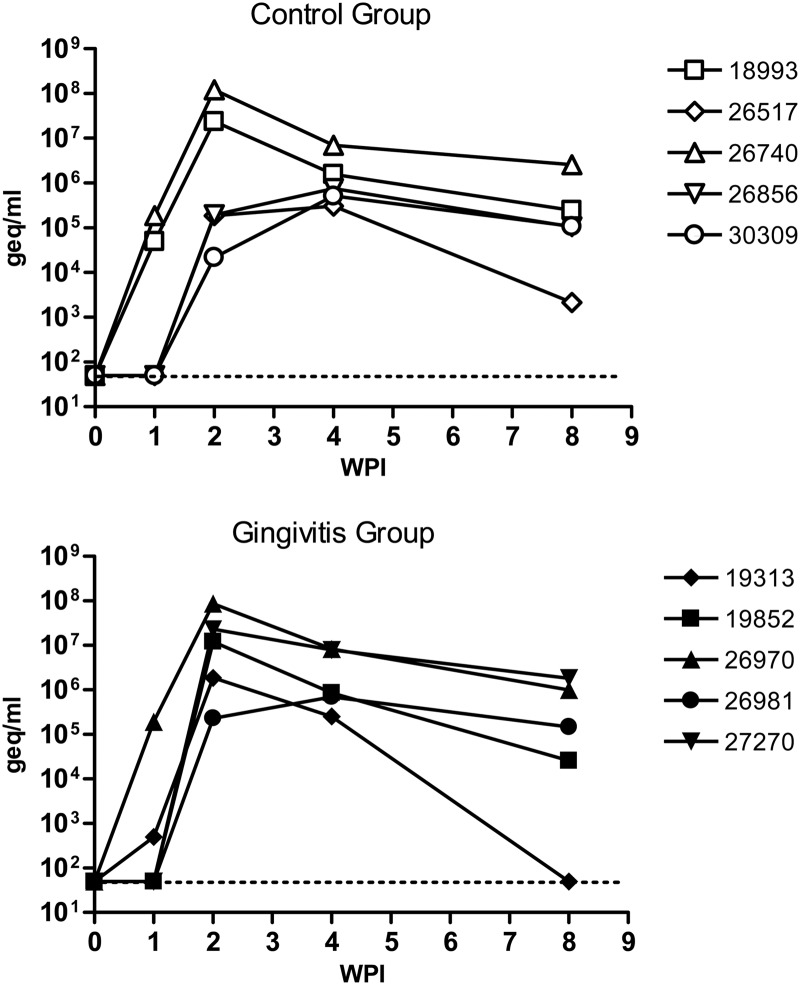
To determine the influence of oral mucosal inflammation in the outcome of systemic viral infection, we also measured viral loads in the plasma of infected animals. The plasma viral loads within each group varied from animal to animal, but the differences between treated and control groups were not statistically significant (Fig. 3).
Fig 3.
Viral loads in rhesus macaques after oral exposure to SIV. Control macaques and animals that developed gingivitis after 5 weeks of tooth ligature were exposed to SIVmac251 by the oral route as described in Materials and Methods. Viral loads were determined by the NASBA method in RNA extracted from plasma of infected macaques and are expressed as genome equivalents (geq)/ml. WPI, weeks postinfection.
We also measured viral diversity and number of founder viruses in infected animals by single-genome amplification (SGA) of the SIVgp120 V1V2 domains early after infection. This analysis demonstrated that macaques with high viral loads in either group (18993 in the control group and 26970, 19852, and 27270 in the gingivitis group) tended to have more viral variants in circulation (see the example provided in Fig. S1 in the supplemental material), and that the group with teeth ligatures had more animals (3 out of 5 infected macaques) (Table 2) with more than one founder virus than the infected control animals (1 out of 5) (Table 2).
Table 2.
Impact of gingivitis on the viral diversity/founder viruses
| Group | Days after first virus administration | Viral RNA characteristic |
|
|---|---|---|---|
| p-distancea (%) | No. of viral variants | ||
| Control | |||
| 18993 | 7 | 0.9 | 4 |
| 26740 | 7 | <0.1 | 1 |
| 26856 | 14 | <0.1 | 1 |
| 26517 | 14 | 0 | 1 |
| 30309 | 14 | 0 | 1 |
| Gingivitis | |||
| 26970 | 7 | 1.4 | 4 |
| 19852 | 14 | 0.2 | 3 |
| 27270 | 14 | 0.1 | 2 |
| 19313 | 14 | <0.1 | 1 |
| 26981 | 14 | <0.1 | 1 |
The p-distance was calculated by dividing the number of nucleotide differences by the total number of nucleotides being compared.
In summary, the presence of ligature-induced gingival inflammation in the treated group did not result in increased susceptibility to infection with SIV or increased systemic viral replication. However, there was a tendency for infection with more than one founder virus in animals with oral inflammation.
Changes in mucosal inflammation markers after SIV infection.
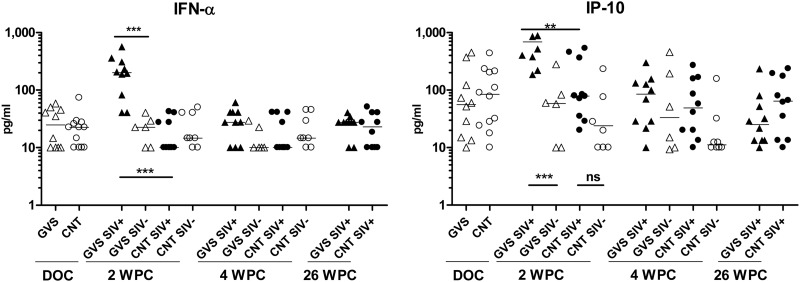
We determined the presence of inflammation markers in the oral mucosa of treated and control animals after exposure to SIV by measuring gingival crevicular fluid (GCF) cytokine concentrations with a Luminex assay. IFN-α is an antiviral cytokine that is produced by numerous cell types (particularly dendritic cells) in response to viral infections, and it also leads to the expression of the chemokine IP-10 (interferon-inducible protein 10, or CXCL10). Assessment of GCF did not identify any difference in the levels of IFN-α or IP-10 prior to SIV infection, indicating that gingivitis induction alone did not affect the expression of these immune modulators (Fig. 4). However, at 2 weeks post-SIV infection, the IFN-α and IP-10 protein levels were both significantly elevated in the GCF of the SIV-infected macaques that had gingivitis compared to the SIV-infected controls (IFN-α, P < 0.0001; IP-10, P = 0.0021). Furthermore, while IFN-α and IP-10 protein levels in the SIV-infected macaques that had gingivitis were significantly elevated compared to those of uninfected macaques with gingivitis, the same was not observed for SIV-infected versus uninfected control animals (Fig. 4). The association between gingivitis and increased levels of IFN-α and IP-10 protein levels within the GCF obtained at the oral mucosa was transient, limited to the 2-week post-SIV challenge time point. By 4 and 26 weeks post-SIV oral exposure (WPC), the levels of IFN-α were similar between the two SIV-infected groups, as well as being similar to the levels observed in macaques that were challenged but remained uninfected.
Fig 4.
Mucosal cytokines induced by SIV infection. GCF samples (two samples per animal) were taken from gingivitis (GVS; triangles) and control (CNT; circles) rhesus macaques on the day of SIV challenge (DOC) and at 2, 4, and 26 weeks post-SIV oral exposure (WPC). SIV-infected macaques are represented by filled symbols, while open symbols are uninfected macaques. Cytokine concentrations (in pg/ml) were determined in the GCF by an NHP Luminex assay as described in Materials and Methods. Differences in median cytokine concentrations were compared between groups with the Mann-Whitney test. ***, extremely significant, P < 0.001; **, very significant, 0.001 < P < 0.01; *, significant, 0.01 < P < 0.05; ns, not significant, P > 0.05.
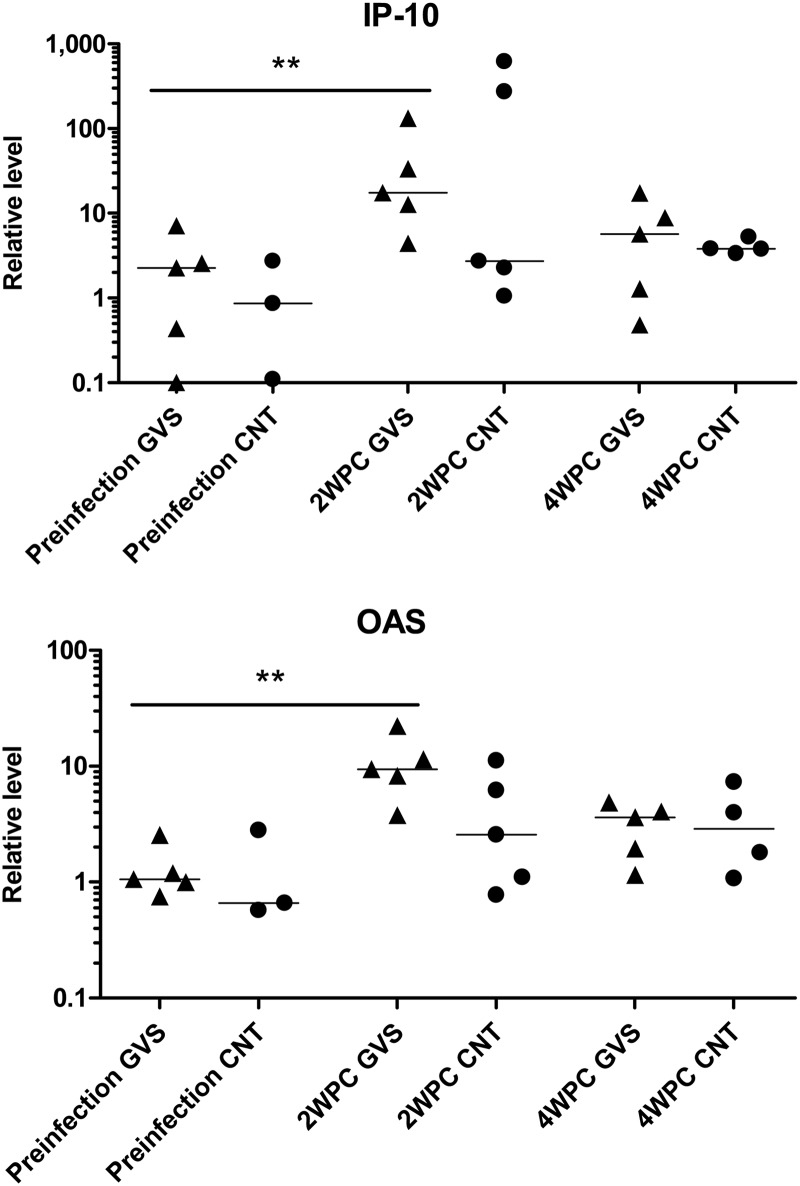
Assessment of punch biopsy specimens obtained from the oral mucosa nearest the ligatures permitted an evaluation of mRNA levels for IFN-α and the IFN-α response genes 2,5-oligoadenylate synthetase (OAS) and IP-10. Surprisingly, the levels of IFN-α mRNA were not elevated in the punch biopsy specimens at 2 WPC when the elevation in IFN-α protein within the GCF was identified (data not shown). However, compared to preinfection levels, mRNA levels for IP-10 and OAS were significantly elevated by 2 WPC only in SIV-infected animals with gingivitis (IP-10 and OAS, P = 0.0079) (Fig. 5). These data indicate that the IFN-α present within the GCF at 2 weeks postinfection is able to influence expression of IFN-inducible genes and proteins in the oral mucosa of SIV+ macaques with gingivitis.
Fig 5.
Inflammatory gene expression levels in oral mucosa. RNA was extracted from oral biopsy specimens obtained from gingivitis (GVS; triangles) and control macaques (CNT; circles) before oral exposure to SIV and at 2 and 4 weeks post-SIV infection (WPC). Levels of mRNA for IP-10 and OAS were determined by real-time RT-PCR using GAPDH levels as a reference. Differences in relative mRNA levels were compared between groups with the Mann-Whitney test. ***, extremely significant, P < 0.001; **, very significant, 0.001 < P < 0.01; *, significant, 0.01 < P < 0.05; ns, not significant, P > 0.05.
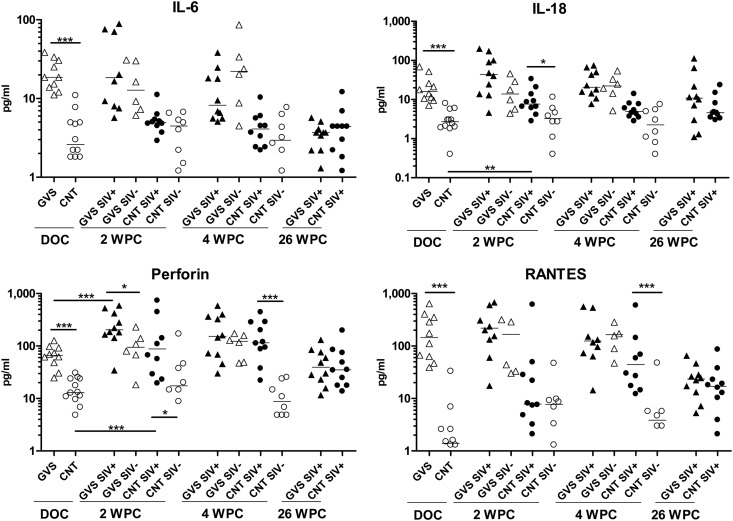
We also evaluated mucosal levels of other proinflammatory cytokines in GCF of control macaques and animals with gingivitis prior to and following SIV infection (Fig. 6). As previously observed (Fig. 1), these immune modulators were elevated in the GCF of macaques that had gingivitis compared to the control macaques prior to SIV infection. At 2 weeks following mucosal viral exposure, SIV-infected animals had an additional increase in many of these inflammatory proteins, which was significant in the control macaques for IL-18 (P = 0.0017) and perforin (P = 0.0205), as well as for the macaques with gingivitis for perforin (P = 0.0312). By 4 WPC, SIV infection in control macaques continued to drive expression of perforin (P = 0.0002) and RANTES (P = 0.0009) to levels comparable to the ones seen for macaques with gingivitis. Interestingly, at the 26-week time point the levels of immune modulators were similar in the SIV-infected gingivitis and control groups, having levels that were generally intermediate between the starting levels of the control macaques and those with gingivitis.
Fig 6.
Mucosal inflammatory cytokines associated with SIV infection. GCF samples (two samples per animal) were taken from gingivitis (GVS; triangles) and control (CNT; circles) rhesus macaques on the day of SIV challenge (DOC) and at 2, 4, and 26 weeks post-SIV oral exposure (WPC). SIV-infected macaques are represented by filled symbols, while open symbols are uninfected macaques. Cytokine concentrations (in pg/ml) were determined in the GCF by an NHP Luminex assay as described in Materials and Methods. Differences in median cytokine concentrations were compared between groups with the Mann-Whitney test. ***, extremely significant, P < 0.001; **, very significant, 0.001 < P < 0.01; *, significant, 0.01 < P < 0.05; ns, not significant, P > 0.05.
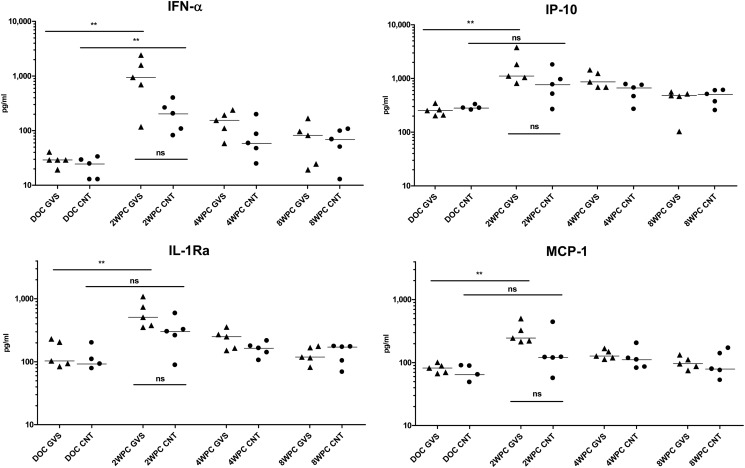
To evaluate whether changes in immune modulators were also manifested within the systemic circulation following SIV infection, the protein levels in plasma were once again evaluated utilizing the Luminex assay. Representative plasma cytokine analysis determined that, in contrast to the GCF, there was no difference in the day of challenge (DOC) levels of the plasma cytokines between the two groups of macaques (Fig. 7). After SIV infection, animals with gingivitis experienced a significant increase for circulating levels of IFN-α, IP-10, IL-1Ra, and MCP-1 (P = 0.0079); however, only IFN-α was significantly increased in SIV-infected control animals (P = 0.0079). When absolute values for these four cytokines were compared at 2 WPC between SIV-infected gingivitis and control animals, there was a tendency for higher values in macaques with gingivitis, but the differences did not reach statistical significance (P = 0.0565). By 4 WPC, cytokine values decreased and were similar between the two groups (Fig. 7). Overall, these data indicate an elevation of select immune modulators during acute infection which reached significance in macaques that had oral mucosal inflammation.
Fig 7.
Systemic inflammatory cytokines associated with SIV infection. Plasma samples from gingivitis (GVS; triangles) and control (CNT; circles) macaques were obtained before oral exposure to SIV (DOC) and at 2, 4, and 8 weeks post-SIV infection (WPC). Cytokine concentrations (in pg/ml) were determined by an NHP Luminex assay as described in Materials and Methods. Differences in median cytokine concentrations were compared between groups with the Mann-Whitney test. ***, extremely significant, P < 0.001; **, very significant, 0.001 < P < 0.01; *, significant, 0.01 < P < 0.05; ns, not significant, P > 0.05.
DISCUSSION
The American Dental Association (ADA) defines periodontal (gum) disease as an infection of the tissues surrounding and supporting the teeth. Periodontal diseases are classified according to the severity of the disease. The two major stages of this inflammatory process are gingivitis and periodontitis. Gingivitis is a reversible form of periodontal disease that reflects an inflammatory process with sequelae that include visible changes to the gingival tissue (e.g., reddening, swelling, and bleeding). Gingivitis may lead to a more serious, destructive form of this inflammatory disease, called periodontitis. The distinguishing characteristic in the progression from gingivitis to periodontitis is the loss of connective tissue and bone support for the tooth. Experimental periodontitis in nonhuman primates, elicited by ligature placement, is accompanied by changes in the subgingival microbial ecology with bacterial species similar to those present in human disease (21, 23, 38). This chronic oral infection elicits elevated levels of local inflammatory, innate, and acquired immune mediators and, in more advances cases of periodontitis, contributes to and/or triggers systemic inflammatory responses (39, 40). In our study, we did not reach the levels of clinical signs, systemic inflammation, or serious local infection seen in advanced periodontitis, but we achieved a stage of periodontal disease consistent with moderate gingivitis. This type of gingivitis is similar to the level of periodontal disease identified in a significant sector of the population of the United States and other countries, reaching as high as 82% of adolescents and more than 50% of adults (19, 20), which makes our study very relevant for addressing a public health concern. Under our experimental conditions, we would have been able to assess an increase in susceptibility to infection from 50 to 90% (one-sided Fisher exact test). While there are inherent limitations in any macaque study due to the numbers of animals that can be evaluated in each group, the identical infection outcome between treated and control animals (5 of 12 viral administrations resulting in a successful infection) provides strong evidence that moderate gingivitis does not increase susceptibility to SIV infection.
Infection with HIV by the oral route is less efficient than through the rectal or vaginal mucosa (6, 41), and the same has been observed for adult macaques experimentally exposed to SIV or simian/human immunodeficiency virus (SHIV) (42). It has been suggested that the adult oropharyngeal stratified epithelium has two lines of defense against HIV: (i) a mechanical barrier of stratified epithelia with tight junctions that prevent penetration of virions into the deeper layers of the epithelium, and (ii) antiviral innate proteins that inactivate those virions that penetrate into the first layers of epithelium (43, 44); in addition to this, human and macaque saliva possess innate and adaptive immune factors that are capable of inhibiting HIV and SIV infection (45, 46). However, the epidemiological evidence that coinfections or inflammation of the genital mucosa increases susceptibility to HIV infection (14–17) raises the possibility that inflammation of the oral mucosa also could increase susceptibility to HIV infection by exposure through the oral route. Several hypotheses have been proposed to explain this increased genital mucosal susceptibility, including that ulceration and inflammation caused by coinfections can lead to the production of proinflammatory cytokines and chemokines which result in the recruitment of immune cells to the mucosal tissue, such as activated CD4+ T cells, where these cells provide a potential target for HIV-1 infection. Our studies suggest, however, that the level of inflammation seen in moderate ligature-induced gingivitis does not increase susceptibility to infection in rhesus macaques orally exposed to SIV. These results seem to contradict a previous study by Chenine et al. that showed increased susceptibility to SHIV in a macaque model of localized buccal inflammation (42). However, there are clear differences between both studies, including the viruses (SIV versus SHIV) and method of delivery of the virus (42). More importantly, Chenine et al. created a fast chemical irritation of the cheek mucosa (injections of 10% acetic acid solution) that developed evident epithelial lesions in 4 days, while we used ligation of the teeth to induce a moderate gum inflammation after 5 weeks of treatment; considering the importance of the buccal epithelial layer discussed previously, the open oral wounds induced by Chenine et al. may explain that increased susceptibility to infection (42).
The main cytokines that we detected at higher levels in GCF from animals with gingivitis before infection were the classic proinflammatory cytokines IL-6, IL-8, IL-18, MCP-1, and sCD14; we also identified high levels of RANTES as the only CCR5 ligand incrementally induced by the ligatures (Fig. 1) that could inhibit binding of HIV/SIV to the coreceptor. However, the clear spike in the virus-induced cytokines IFN-α and IP-10 seen in the GCF of the same animals with gingivitis after SIV infection (Fig. 4 and 5) suggest the presence of increased viral replication at the site of inflammation. Studies from Keele et al. and others have determined that the number of infecting variants can be inferred through the HIV or SIV Env diversity at early time points (36, 47, 48). While it is likely that our studies underestimate Env variability, since we only analyzed the V1V2 region of Env, we did determine that a majority of the SIV-infected macaques with gingivitis had multiple founder viruses (Table 2), suggesting that there was an increased number of target cells in the macaques in which an infection was established. However, this increased presence of potential target cells did not make the animals more susceptible to SIV infection. These differential findings suggest that there is a balance occurring at sites of inflamed mucosa in which immune cells and immune modulators are present that inhibit as well as potentially increase viral transmission.
As mentioned before, the moderate degree of local mucosal inflammation seen in animals with ligatures in our study contrasts with the systemic inflammation and cellular activation reported for other monkey studies with long-term, ligature-induced advanced periodontitis (39, 40). However, the higher levels of systemic inflammatory mediators seen for macaques with gingivitis after SIV infection (Fig. 7) suggests that this localized inflammation is conducive to heightened systemic effects. Long-term studies in SIV-infected macaques with gingivitis may provide answers about whether these transient systemic inflammations and higher numbers of viral founders result in advanced pathogenesis or disease progression.
In conclusion, induction of moderate gingivitis in rhesus macaques was not associated with any increase in oral SIV transmission. This finding provides evidence that mucosal inflammation need not result in an increase in SIV and, by analogy, HIV transmission. The key factors that dictate the impact of any inflammatory state on transmission likely include the degree of breakage of the epithelium permitting viral access to SIV/HIV target cells and the types of cells that infiltrate to the site, including the levels of viral target cells as well as immune cells able to identify and eliminate infected cells. Finally, the elevated levels of cytokines in the oral mucosa and plasma of the SIV-infected macaques with gingivitis indicates a synergy between mucosal inflammation and SIV infection that creates an immune milieu that has the potential to impact the early stages of the SIV infection, with implications for the success of treatments or efficacies of vaccines.
Supplementary Material
ACKNOWLEDGMENTS
We thank Heather Cole and Cassondra Bauer for their excellent work while overseeing the animal experiments. We also recognize the high-quality performance of the veterinary technical personnel of the Southwest National Primate Research Center.
Support for this project comes from NIH grants R01 DE017541 and R24 OD013096. The Southwest National Primate Research Center was funded by the National Center for Research Resources P51 RR013986 and is currently supported by the Office of Research Infrastructure Programs/OD P51 OD011133.
Footnotes
Published ahead of print 21 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02079-12.
REFERENCES
- 1. Tebit DM, Ndembi N, Weinberg A, Quinones-Mateu ME. 2012. Mucosal transmission of human immunodeficiency virus. Curr. HIV Res. 10:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baggaley RF, White RG, Boily MC. 2008. Systematic review of orogenital HIV-1 transmission probabilities. Int. J. Epidemiol. 37:1255–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen MS, Shugars DC, Fiscus SA. 2000. Limits on oral transmission of HIV-1. Lancet 356:272. [DOI] [PubMed] [Google Scholar]
- 4. Scully C, Porter S. 2000. HIV topic update: oro-genital transmission of HIV. Oral Dis. 6:92–98 [DOI] [PubMed] [Google Scholar]
- 5. Perez CL, Hasselrot K, Bratt G, Broliden K, Karlsson AC. 2010. Induction of systemic HIV-1-specific cellular immune responses by oral exposure in the uninfected partner of discordant couples. AIDS 24:969–974 [DOI] [PubMed] [Google Scholar]
- 6. Moutsopoulos NM, Greenwell-Wild T, Wahl SM. 2006. Differential mucosal susceptibility in HIV-1 transmission and infection. Adv. Dent. Res. 19:52–56 [DOI] [PubMed] [Google Scholar]
- 7. Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820–1825 [DOI] [PubMed] [Google Scholar]
- 8. Baba TW, Koch J, Mittler ES, Greene M, Wyand M, Penninck D, Ruprecht RM. 1994. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res. Hum. Retrovir. 10:351–357 [DOI] [PubMed] [Google Scholar]
- 9. Baba TW, Trichel AM, An L, Liska V, Martin LN, Murphey-Corb M, Ruprecht RM. 1996. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272:1486–1489 [DOI] [PubMed] [Google Scholar]
- 10. Milush JM, Kosub D, Marthas M, Schmidt K, Scott F, Wozniakowski A, Brown C, Westmoreland S, Sodora DL. 2004. Rapid dissemination of SIV following oral inoculation. AIDS 18:2371–2380 [PubMed] [Google Scholar]
- 11. Otsyula MG, Miller CJ, Tarantal AF, Marthas ML, Greene TP, Collins JR, van Rompay KK, McChesney MB. 1996. Fetal or neonatal infection with attenuated simian immunodeficiency virus results in protective immunity against oral challenge with pathogenic SIVmac251. Virology 222:275–278 [DOI] [PubMed] [Google Scholar]
- 12. Ruprecht RM, Baba TW, Liska V, Ayehunie S, Andersen J, Montefiori DC, Trichel A, Murphey-Corb M, Martin L, Rizvi TA, Bernacky BJ, Buchl SJ, Keeling M. 1998. Oral SIV, SHIV, and HIV type 1 infection. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S97–S103 [PubMed] [Google Scholar]
- 13. Stahl-Hennig C, Steinman RM, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261–1265 [DOI] [PubMed] [Google Scholar]
- 14. Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, Manigart O, Mulenga J, Keele BF, Shaw GM, Hahn BH, Allen SA, Derdeyn CA, Hunter E. 2009. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 5:e1000274 doi:10.1371/journal.ppat.1000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sagar M, Lavreys L, Baeten JM, Richardson BA, Mandaliya K, Ndinya-Achola JO, Kreiss JK, Overbaugh J. 2004. Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population. AIDS 18:615–619 [DOI] [PubMed] [Google Scholar]
- 16. Schellenberg JJ, Plummer FA. 2012. The microbiological context of HIV resistance: vaginal microbiota and mucosal inflammation at the viral point of entry. Int. J. Inflam. 2012:131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wasserheit JN. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 19:61–77 [PubMed] [Google Scholar]
- 18. Royce RA, Sena A, Cates W, Jr, Cohen MS. 1997. Sexual transmission of HIV. N. Engl. J. Med. 336:1072–1078 [DOI] [PubMed] [Google Scholar]
- 19. Albandar JM, Rams TE. 2002. Global epidemiology of periodontal diseases: an overview. Periodontol. 2000 29:7–10 [DOI] [PubMed] [Google Scholar]
- 20. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. 2010. Accuracy of NHANES periodontal examination protocols. J. Dent. Res. 89:1208–1213 [DOI] [PubMed] [Google Scholar]
- 21. Cappelli D, Holt SC, Singer RE, Pickrum HM, Ebersole JL. 2000. Effects of 0.12% chlorhexidine gluconate on experimental gingivitis in non-human primates: clinical and microbiological alterations. Oral Dis. 6:124–131 [DOI] [PubMed] [Google Scholar]
- 22. Ebersole JL, Cappelli D, Holt SC, Singer RE, Filloon T. 2000. Gingival crevicular fluid inflammatory mediators and bacteriology of gingivitis in nonhuman primates related to susceptibility to periodontitis. Oral Microbiol. Immunol. 15:19–26 [DOI] [PubMed] [Google Scholar]
- 23. Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. 1998. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J. Periodontol. 69:686–697 [DOI] [PubMed] [Google Scholar]
- 24. Kornman KS, Holt SC, Robertson PB. 1981. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J. Periodont. Res. 16:363–371 [DOI] [PubMed] [Google Scholar]
- 25. Loe H. 1967. The gingival index, the plaque index and the retention index systems. J. Periodontol. 38(Suppl.):610–616 [DOI] [PubMed] [Google Scholar]
- 26. Durudas A, Chen HL, Gasper MA, Sundaravaradan V, Milush JM, Silvestri G, Johnson W, Giavedoni LD, Sodora DL. 2011. Differential innate immune responses to low or high dose oral SIV challenge in rhesus macaques. Curr. HIV Res. 9:276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ebersole JL, Taubman MA, Smith DJ, Goodson JM. 1984. Gingival crevicular fluid antibody to oral microorganisms. I. Method of collection and analysis of antibody. J. Periodont. Res. 19:124–132 [DOI] [PubMed] [Google Scholar]
- 28. Reynolds MA, Dawson DR, Novak KF, Ebersole JL, Gunsolley JC, Branch-Mays GL, Holt SC, Mattison JA, Ingram DK, Novak MJ. 2009. Effects of caloric restriction on inflammatory periodontal disease. Nutrition 25:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee EM, Chung HK, Livesay J, Suschak J, Finke L, Hudacik L, Galmin L, Bowen B, Markham P, Cristillo A, Pal R. 2010. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J. Virol. Methods 163:287–294 [DOI] [PubMed] [Google Scholar]
- 30. Giavedoni LD. 2005. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using Luminex technology. J. Immunol. Methods 301:89–101 [DOI] [PubMed] [Google Scholar]
- 31. Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Investig. 119:3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sariol CA, Martinez MI, Rivera F, Rodriguez IV, Pantoja P, Abel K, Arana T, Giavedoni L, Hodara V, White LJ, Anglero YI, Montaner LJ, Kraiselburd EN. 2011. Decreased dengue replication and an increased anti-viral humoral response with the use of combined Toll-like receptor 3 and 7/8 agonists in macaques. PLoS One 6:e19323 doi:10.1371/journal.pone.0019323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. 2009. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J. Virol. 83:12229–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, Miller CJ. 2010. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J. Virol. 84:7083–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ebersole JL, Brunsvold M, Steffensen B, Wood R, Holt SC. 1991. Effects of immunization with Porphyromonas gingivalis and Prevotella intermedia on progression of ligature-induced periodontitis in the nonhuman primate Macaca fascicularis. Infect. Immun. 59:3351–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cappelli D, Steffen MJ, Holt SC, Ebersole JL. 2009. Periodontitis in pregnancy: clinical and serum antibody observations from a baboon model of ligature-induced disease. J. Periodontol. 80:1154–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ebersole JL, Cappelli D, Mathys EC, Steffen MJ, Singer RE, Montgomery M, Mott GE, Novak MJ. 2002. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann. Periodontol. 7:102–111 [DOI] [PubMed] [Google Scholar]
- 40. Ebersole JL, Steffen MJ, Holt SC, Kesavalu L, Chu L, Cappelli D. 2010. Systemic inflammatory responses in progressing periodontitis during pregnancy in a baboon model. Clin. Exp. Immunol. 162:550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powers KA, Poole C, Pettifor AE, Cohen MS. 2008. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect. Dis. 8:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chenine AL, Siddappa NB, Kramer VG, Sciaranghella G, Rasmussen RA, Lee SJ, Santosuosso M, Poznansky MC, Velu V, Amara RR, Souder C, Anderson DC, Villinger F, Else JG, Novembre FJ, Strobert E, O'Neil SP, Secor WE, Ruprecht RM. 2010. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J. Infect. Dis. 201:1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. 2012. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J. Virol. 86:2556–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. 2011. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 409:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moore BE, Flaitz CM, Coppenhaver DH, Nichols M, Kalmaz GD, Bessman JD, Cloyd MW, Lynch DP, Prabhakar BS, Baron S. 1993. HIV recovery from saliva before and after dental treatment: inhibitors may have critical role in viral inactivation. J. Am. Dent. Assoc. 124:67–74 [DOI] [PubMed] [Google Scholar]
- 46. Thomas JS, Lacour N, Kozlowski PA, Nelson S, Bagby GJ, Amedee AM. 2010. Characterization of SIV in the oral cavity and in vitro inhibition of SIV by rhesus macaque saliva. AIDS Res. Hum. Retrovir. 26:901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keele BF, Estes JD. 2011. Barriers to mucosal transmission of immunodeficiency viruses. Blood 118:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salazar-Gonzalez JF, Bailes E, Pham KT, Salazar MG, Guffey MB, Keele BF, Derdeyn CA, Farmer P, Hunter E, Allen S, Manigart O, Mulenga J, Anderson JA, Swanstrom R, Haynes BF, Athreya GS, Korber BT, Sharp PM, Shaw GM, Hahn BH. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.