Abstract
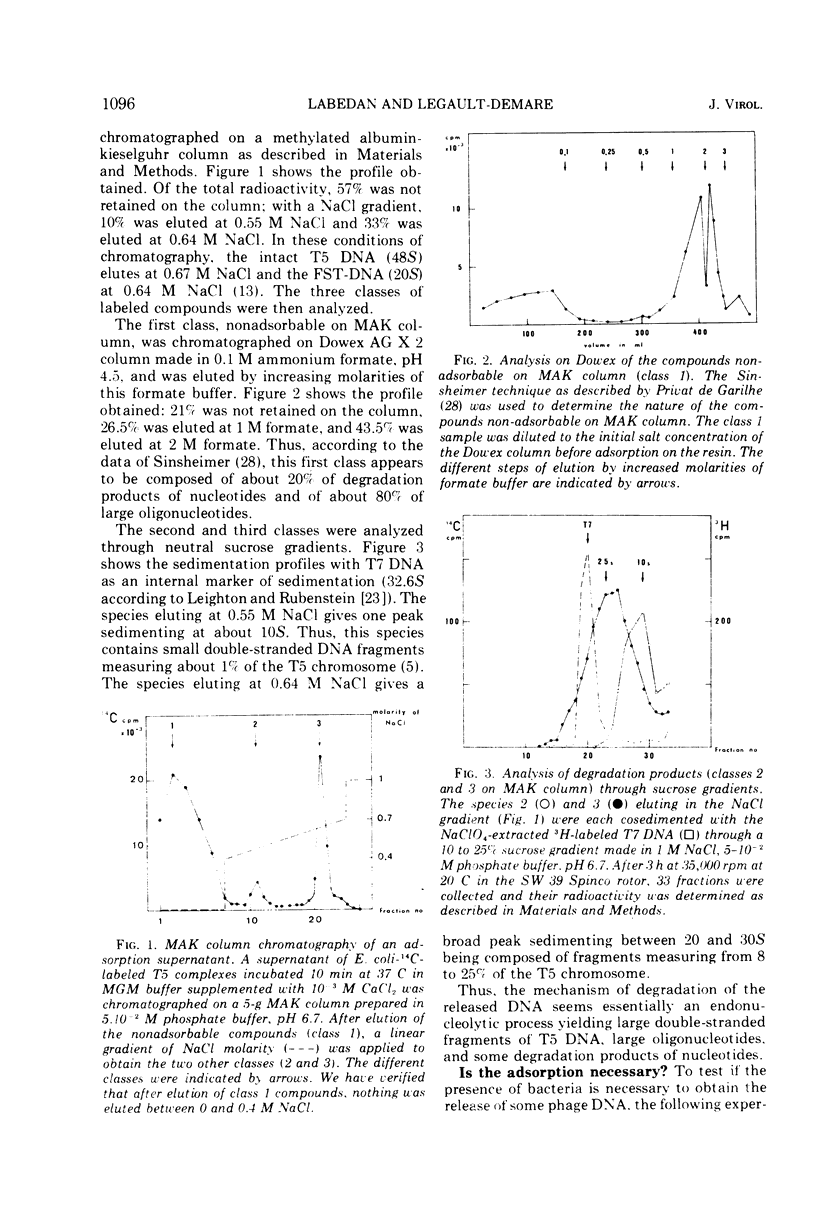
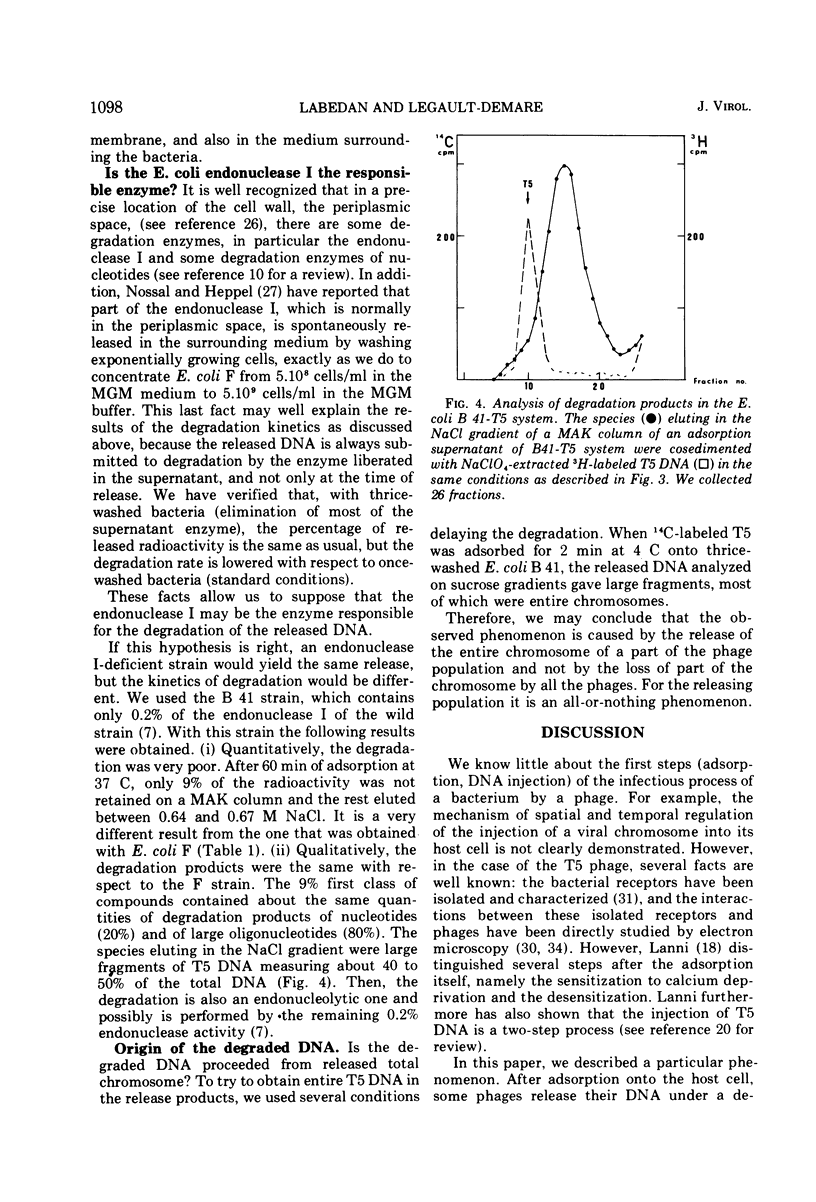
Each T5 stock contains a population of particular phages that, just after adsorption onto the host bacteria. release their entire chromosome outside the bacterial membrane in a place where it is sensitive to bacterial enzymes. This release takes place before the sensitization step to deprivation of calcium and before the transfer of the first-step DNA fragment. Secondarily, this released DNA is degraded by bacterial enzymes, mainly by the endonuclease I; the products of degradation are spontaneously released in the surrounding medium. Thus, in each T5 phage stock it seems that there is a minor population that is deficient for the mechanism of controlled DNA injection into the bacteria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Eigner J. Breakdown and exclusion of superinfecting T-even bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):869–886. doi: 10.1128/jvi.8.6.869-886.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Zimm B. H. Viscosity and sedimentation of the DNA from bacteriophages T2 and T7 and the relation to molecular weight. J Mol Biol. 1965 Jul;12(3):525–536. doi: 10.1016/s0022-2836(65)80310-2. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Fielding P. E., Lunt M. R. The relation between breakdown of superinfecting virus deoxyribonucleic acid and temporal exclusion induced by T4 and T5 bacteriophages. J Gen Virol. 1970 Mar;6(3):333–342. doi: 10.1099/0022-1317-6-3-333. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Richardson C. C. Analysis of the interruptions in bacteriophage T5 DNA. J Mol Biol. 1970 Feb 14;47(3):477–493. doi: 10.1016/0022-2836(70)90316-5. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Infection by bacteriophage T5 and its intracellular growth; a study by complement fixation. J Bacteriol. 1954 Jun;67(6):640–650. doi: 10.1128/jb.67.6.640-650.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. I. Some basic kinetic features. Virology. 1960 Apr;10:501–513. doi: 10.1016/0042-6822(60)90132-x. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. II. Dissociation of calcium-independent and calcium-dependent processes. Virology. 1960 Apr;10:514–529. doi: 10.1016/0042-6822(60)90133-1. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. III. Stages revealed by changes in susceptibility of early complexes to abortive infection. Virology. 1961 Oct;15:127–135. doi: 10.1016/0042-6822(61)90229-x. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., ROUSSOS G. G., PRATT E. A. The deoxyribonucleases of Escherichia coli. II. Purification and properties of a ribonucleic acid-inhibitable endonuclease. J Biol Chem. 1962 Mar;237:819–828. [PubMed] [Google Scholar]
- LURIA S. E., STEINER D. L. The role of calcium in the penetration of bacteriophage T5 into its host. J Bacteriol. 1954 Jun;67(6):635–639. doi: 10.1128/jb.67.6.635-639.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Crochet M., Legault-Demare J., Stevens B. J. Location of the first step transfer fragment and single-strand interruptions in T5stO bacteriophage DNA. J Mol Biol. 1973 Apr 5;75(2):213–234. doi: 10.1016/0022-2836(73)90017-x. [DOI] [PubMed] [Google Scholar]
- Labedan B., Legault-Demare J. Penetration into host cells of naked, partially injected (post-FST) DNA of bacteriophage T5. J Virol. 1973 Aug;12(2):226–229. doi: 10.1128/jvi.12.2.226-229.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. T. DNA transfer from phage T5 to host cells: dependence on intercurrent protein synthesis. Proc Natl Acad Sci U S A. 1965 May;53(5):969–973. doi: 10.1073/pnas.53.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton S. B., Rubenstein I. Calibration of molecular weight scales for DNA. J Mol Biol. 1969 Dec 14;46(2):313–328. doi: 10.1016/0022-2836(69)90424-0. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- WEIDEL W., KELLENBERGER E. The E. coli B-receptor for the phage T5. II. Electron microscopic studies. Biochim Biophys Acta. 1955 May;17(1):1–9. doi: 10.1016/0006-3002(55)90314-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


