Abstract
OBJECTIVE
To assess the possibility of improving nocturnal glycemic control as well as meal glycemic response using closed-loop therapy in children aged <7 years.
RESEARCH DESIGN AND METHODS
This was a randomized controlled crossover trial comparing closed-loop with standard open-loop insulin pump therapy performed in an inpatient clinical research center. Ten subjects aged <7 years with type 1 diabetes for >6 months treated with insulin pump therapy were studied. Closed-loop therapy and standard open-loop therapy were compared from 10:00 p.m. to 12:00 p.m. on 2 consecutive days. The primary outcome was plasma glucose time in range (110–200 mg/dL) during the night (10:00 p.m.–8:00 a.m.). Secondary outcomes included peak postprandial glucose levels, incidence of hypoglycemia, degree of hyperglycemia, and prelunch glucose levels.
RESULTS
A trend toward a higher mean nocturnal time within target range was noted for closed- versus open-loop therapy, although not reaching statistical significance (5.3 vs. 3.2 h, P = 0.12). There was no difference in peak postprandial glucose or number of episodes of hypoglycemia. There was significant improvement in time spent >300 mg/dL overnight with closed-loop therapy (0.18 vs. 1.3 h, P = 0.035) and the total area under the curve of glucose >200 mg/dL (P = 0.049). Closed-loop therapy returned prelunch blood glucose closer to target (189 vs. 273 mg/dL on open loop, P = 0.009).
CONCLUSIONS
Closed-loop insulin delivery decreases the severity of overnight hyperglycemia without increasing the incidence of hypoglycemia. The therapy is better able to reestablish target glucose levels in advance of a subsequent meal. Younger children with type 1 diabetes may reap significant benefits from closed-loop therapy.
In 1993, the landmark Diabetes Control and Complications Trial showed that intensive insulin therapy reduced the rate of long-term microvascular complications from type 1 diabetes (1). However, this improved glycemic control came at the cost of significantly increased rates of hypoglycemia. Since that time, there have been many efforts to improve glycemic control with the goal of developing a completely automated artificial pancreas, i.e., closed-loop insulin therapy. Closed-loop insulin therapy involves the integration of continuous glucose monitoring (CGM) with continuous subcutaneous insulin delivery via a computer-driven mathematical algorithm that calculates insulin dosage (2). In adults and older children, closed-loop therapy has been shown to improve glycemic control while decreasing rates of hypoglycemia (3–7). There has been little research regarding closed-loop insulin therapy in very young children who are particularly vulnerable to frequent episodes of hypoglycemia.
Management of type 1 diabetes in very young children is especially difficult because of unpredictable eating patterns, erratic activity level, and increased susceptibility to severe hypoglycemia (8,9). Furthermore, children diagnosed with type 1 diabetes at a younger age have increased risk of long-term neurocognitive dysfunction, which may be related to episodes of severe hypoglycemia (10,11). A study of CGM in children aged <7 years found that episodes of hypoglycemia occurred on 28% of nights (12). This problem will only become of greater significance, as the incidence of type 1 diabetes is increasing worldwide with the most rapid increase in children aged <5 years (13). One study estimated a doubling of the number of children aged <5 years diagnosed each year with type 1 diabetes by the year 2020 (14). While there have been substantial technological advances with the advent of insulin pumps and CGM, a recent trial of CGM use in young children showed no benefit in glycemic control or decrease in rates of hypoglycemia (15). Novel strategies for treating these young children are desperately needed. Closed-loop therapy has the potential to markedly improve young children’s diabetes care. We performed a randomized crossover inpatient study comparing closed-loop therapy with standard open-loop insulin pump therapy in children aged <7 years with type 1 diabetes.
RESEARCH DESIGN AND METHODS
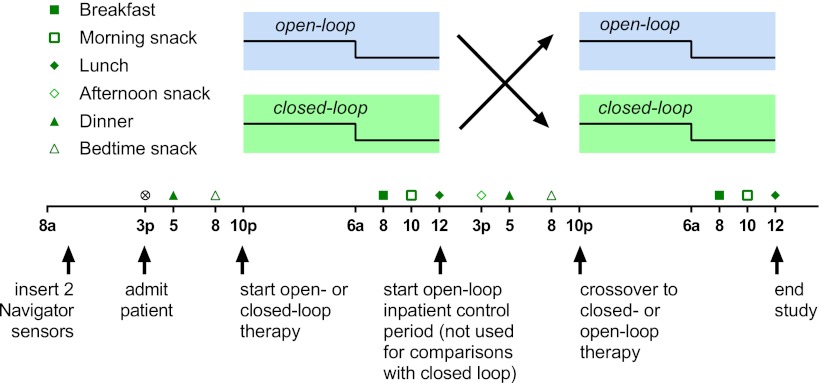
Subjects were recruited from the diabetes clinic at Boston Children’s Hospital between September 2011 and April 2012. Eligibility criteria included type 1 diabetes diagnosed by a pediatric endocrinologist, age <7 years, duration of diabetes >6 months, and insulin pump use >6 weeks. Subjects were excluded for any significant comorbid illnesses except for treated celiac disease. The protocol was approved by the institutional review board at Boston Children's Hospital. Written informed consent was obtained from a parent of each subject. Each subject was admitted to the inpatient clinical research center for a single 45-h admission. This study was a crossover controlled trial comparing standard open-loop therapy with closed-loop therapy from 10:00 p.m. to 12:00 p.m. on each day of the hospitalization (Fig. 1). The order of administration of the two modes of therapy was randomized from a computer-generated list. The individual assignments were sealed in sequentially numbered envelopes, which were opened after enrollment and consent.
Figure 1.
Study design.
Procedures
On the morning of admission, two CGMs (Abbott Freestyle Navigator; Abbott Diabetes Care, Alameda, CA) were placed in the thighs of each subject. An initial blood glucose calibration was performed at 1 h. Subjects then returned for admission at 3:00 p.m., when a repeat calibration was performed. Calibrations were then performed at 10 and 24 h postinsertion as per the manufacturer’s recommendation. Additional calibrations for discrepancy between the sensor and venous glucose were not done. By convention, the sensor in the right leg was used for running the closed-loop algorithm except in one case when sensor data from the right sensor were unavailable at 10:00 p.m., in which case the left-leg sensor was used. The sensor used for control was never switched during the study.
On admission, an intravenous catheter was placed for frequent blood sampling. All subjects were switched to the study OneTouch Ping insulin pump (Animas Corporation, West Chester, PA), which was preprogrammed with their home settings. A new infusion set was placed. All subjects received insulin aspart for the duration of the study. Meals and snacks were served on the following schedule: dinner, 5:00 p.m.; bedtime snack, 8:00 p.m.; breakfast, 8:00 a.m.; morning snack, 10:00 a.m.; lunch, 12:00 p.m.; and afternoon snack, 3:00 p.m. This represents a typical meal schedule for children with diabetes in this age-group. Parents chose the subjects’ meals off a standard menu, and identical meals and snacks were served on both days of the study. All meals and snacks were pre- and postweighed to assess intake.
At 5:00 p.m. and 8:00 p.m. on both days, an insulin bolus was given for dinner and bedtime snack based on the subject’s blood glucose level and planned carbohydrate intake, using the subject’s home carbohydrate ratios, correction factors, and blood glucose targets. The insulin-on-board feature was activated on the OneTouch Ping insulin pump as per the subject’s home insulin pump settings. The study period commenced at 10:00 p.m. From 10:00 p.m. to 7:40 a.m., venous blood glucose values were obtained every 20 min. From 7:40 a.m. to 8:40 a.m., venous samples were obtained every 10 min for glucose and insulin levels, followed by every 20 min until 10:00 a.m., and then samples were obtained at 10:30 a.m., 11:00 a.m., and 12:00 p.m. Fasting serum C-peptide levels were drawn at 8:00 a.m. on the open-loop day.
During open-loop therapy, insulin boluses were given at 8:00 a.m. and 10:00 a.m. per the subject’s home insulin routine. For the 10:00 a.m. snack, carbohydrate coverage was given but a correction dose of insulin for hyperglycemia was not given, as the subject had received a bolus of insulin for breakfast 2 h prior. Basal rates were not adjusted overnight during open-loop therapy, but an additional correction dose of insulin could be given for hyperglycemia at the parents’ discretion to mimic their standard home therapy. During closed-loop therapy, insulin basal rates were adjusted every 20 min overnight from 10:00 p.m. to 8:00 a.m., and mini-boluses of insulin (0.05–0.1 units) were given up to every minute from 8:00 a.m. to 12:00 p.m.. Delivery rates were calculated using closed-loop algorithms (described below) but with the dose approved by a physician and administered via manual entry by the physician into the pump. All episodes of hypoglycemia defined as plasma glucose <70 mg/dL were treated with oral carbohydrates (juice or glucose tabs). One subject (subject 4) did not receive the dinner insulin bolus on the open-loop therapy night as the result of an error. A correction dose of insulin was given with the bedtime snack at 8:00 p.m. (See Supplementary Fig. 4 for details.)
Closed-loop control system
Different algorithms were used to effect control during the nighttime (10:00 p.m. to 8:00 a.m.) and daytime (8:00 a.m. to 12:00 p.m.) closed-loop periods. During the nighttime, control was effected with a proportional-integral component in series with a proportional-derivative component. The proportional-integral component recommended stepwise increases in the basal rate whenever sensor glucose was above target (150 mg/dL, 10:00 p.m.–6:00 a.m.; 120 mg/dL, 6:00 a.m.–8:00 a.m.) and not returning to target at a desired rate: [(sensor glucose − target)/TI], where TI is 120 min. This was modified by the proportional-derivative component to be accompanied by a bolus (bolus = basal change in units per minutes × TD, where TD is 30 min). Recommendations were made every 20 min with the aid of a spreadsheet (Microsoft Excel).
At 8:00 a.m., control was transferred to an algorithm using a proportional-integral component in parallel with a proportional-derivative component. The proportional-integral component increased the anticipated basal rate whenever sensor glucose was above target (120 mg/dL) and not returning to target at a desired rate: [(sensor glucose − target)/TI], where TI = 60 min. The anticipated basal rate was then summed with the proportional-derivative component, which recommended insulin delivery be adjusted in proportion to the sensor glucose value expected at a future time point (sensor glucose + TD × rate of change of sensor glucose, where TD = 120 min). The sum of the two components was then modified by insulin feedback as previously described (5,16) but with pharmacokinetic/pharmacodynamic profiles obtained in children (17,18). The modified rate (units/hour) was then converted to a series of 0.05-unit boluses and delivered to the subject on a minute-to-minute interval as needed, with blood glucose assessed every 10–20 min.
During the study (subject 6 and forward), the nighttime control configuration was modified to reduce the desired rate for glucose to return to target (TI increased from 60 to 120 min), reduce the bolus accompanying each change in basal rate (TD reduced from 60 to 30 min), and add insulin feedback. No changes were made in the daytime control algorithm.
Venous glucose was measured on the HemoCue Glucose 201 DM Analyzer (HemoCue, Cypress, CA) and is reported as plasma equivalents. Plasma insulin was measured using a chemiluminescent immunoassay (Beckman Coulter, Fullerton, CA). C-peptide was measured by radioimmunoassay (Siemens, Los Angeles, CA).
Statistics
Data are reported as means ± SEM unless otherwise indicated. Primary outcome was defined as plasma glucose time in range (110–200 mg/dL; HemoCue) during the night (10:00 p.m.–8:00 a.m.). Incidence of hypoglycemia (blood glucose <70 mg/dL as measured by the HemoCue meter) was combined with supplemental carbohydrate given to prevent hypoglycemia with paired difference in open- versus closed-loop control made using McNemar test with continuity correction. Incidences separated by <30 min were treated as a single event. Continuous outcome measures (time in range, mean glucose, etc.) were compared using paired t tests as indicated. Statistical analysis was performed using GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, CA). Statistical significance was taken as P < 0.05; correction for multiple comparisons was not performed.
RESULTS
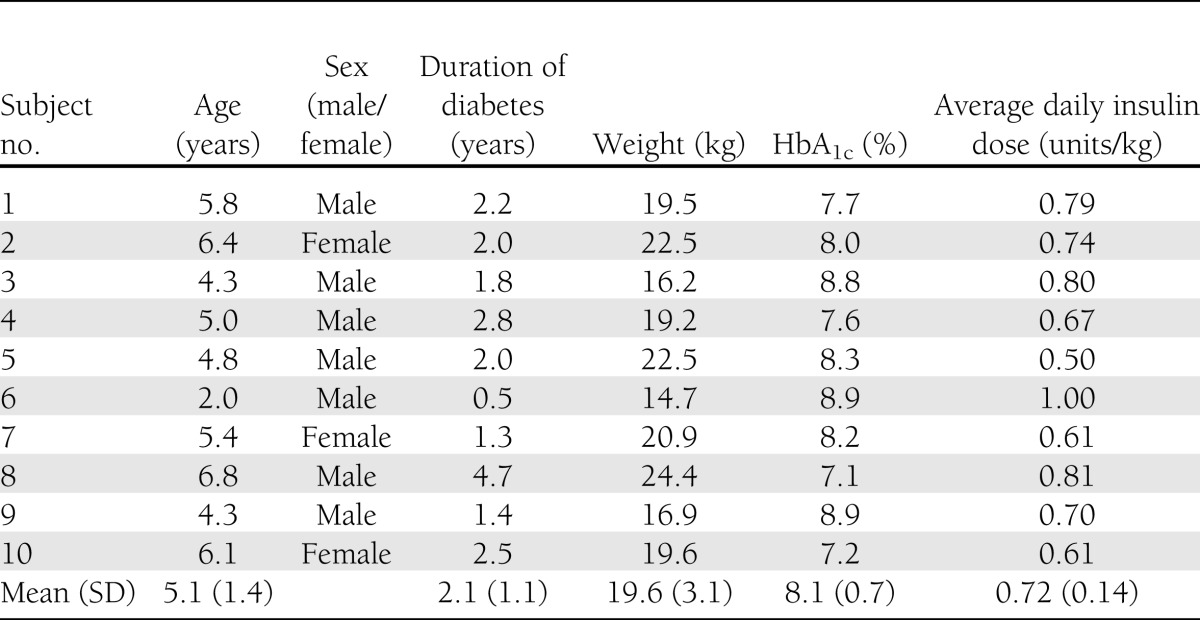
Eleven subjects consented and were randomized. One subject withdrew after randomization but before any study procedures because of scheduling conflicts and is excluded from all analysis. Baseline demographics and clinical characteristics of the remaining 10 subjects are presented in Table 1. Subjects’ ages ranged from 2 to 6 years (mean 5.1) with a mean duration of diabetes of 2.1 years. Seven subjects had A1C values <8.5% (American Diabetes Association target for this age range [19]), and all had A1C values <9%. Mean daily insulin dose was 0.72 units per kg. Fasting C-peptide levels were <0.1 ng/mL in all subjects.
Table 1.
Baseline demographic information
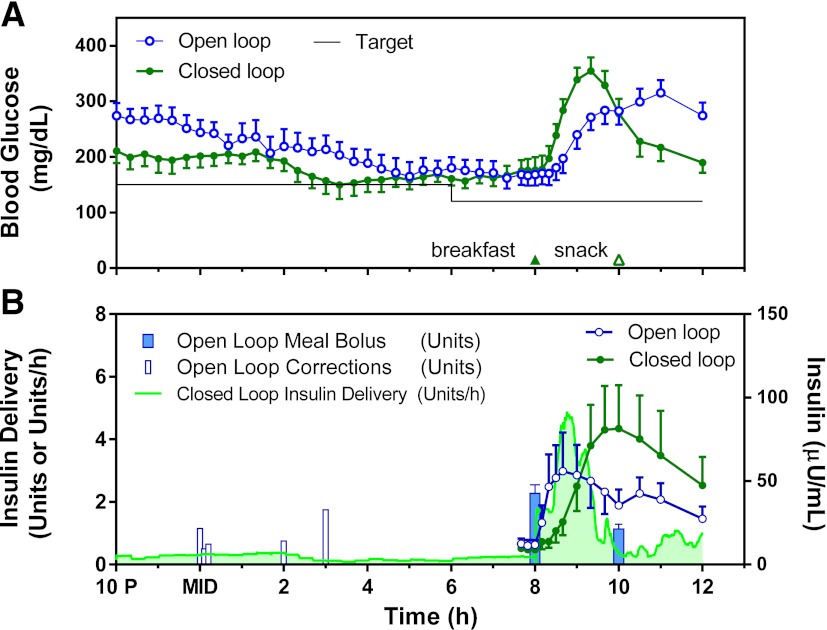
Six subjects were randomized to receive closed-loop therapy on the first night of the admission, with the remaining four receiving closed-loop therapy on the second night. During closed-loop therapy, there was a more rapid rise in postprandial plasma glucose (Fig. 2A) as a result of the delayed delivery of insulin (Fig. 2B). Total daytime insulin delivery was greater during closed-loop therapy (mean 5.9 vs. 4.6 units during open loop; P = 0.03). Five episodes of hypoglycemia occurred between 12:00 p.m. and 5:00 p.m. while the subjects were on their standard open-loop therapy but outside of the study outcome period. Individual profiles for all subjects are available in Supplementary Figs. 1–10.
Figure 2.
Comparison of average (N = 10) open- and closed-loop glucose (A) and insulin delivery (B, left axis) and concentration (B, right axis) curves. MID, midnight.
For the primary nocturnal outcome, a trend toward a higher mean time for plasma glucose within target range (110–200 mg/dL) was noted for closed- versus open-loop therapy (5.3 vs. 3.2 h, respectively), although this difference did not achieve statistical significance (P = 0.12) (Table 2). Closed-loop therapy significantly reduced time spent >300 mg/dL (0.18 vs. 1.3 h, P = 0.035) and the total area under the curve of glucose >200 mg/dL (P = 0.049). During open-loop therapy, five subjects received an additional correction dose of insulin overnight due to persistent hyperglycemia (Fig. 1B). There was no significant difference in the number of episodes of hypoglycemia/supplemental carbohydrate (P = 1.0). There were no significant differences in time spent below the target range or frankly hypoglycemic (Table 2). No subject developed measurable urine ketones at any point in the study.
Table 2.
Outcome measures
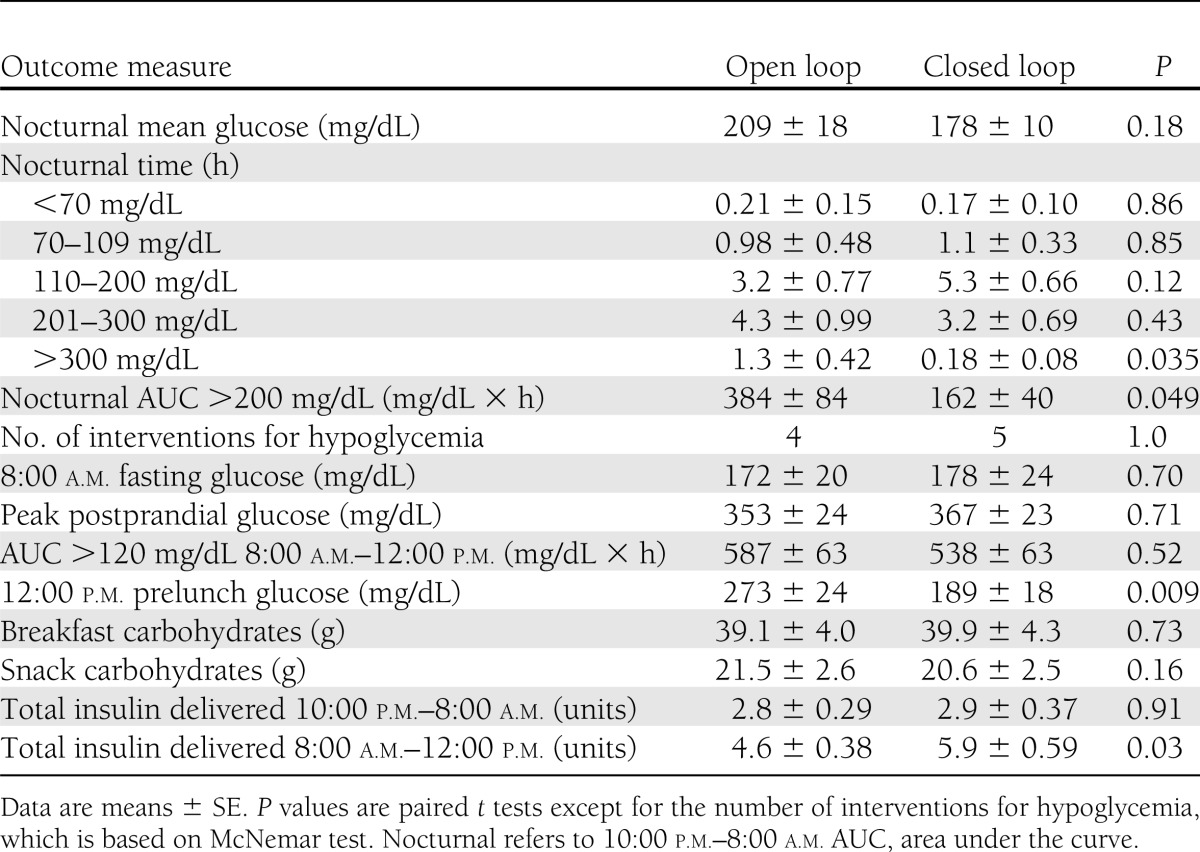
There were no instances of hypoglycemia between 8:00 a.m. and 12:00 p.m. in either group and no differences in the peak postprandial glucose concentration (Table 2). There was significant improvement in the prelunch blood glucose on closed-loop therapy (189 vs. 273 mg/dL on open-loop, P = 0.009). Breakfast and morning snack carbohydrate intake was well matched (Table 2).
CONCLUSIONS
We performed the first study of closed-loop insulin therapy in children aged <7 years. In our study, closed-loop insulin delivery decreased the severity of overnight hyperglycemia without increasing time spent below the glucose target range. Closed-loop therapy was also able to return glucose levels closer to target in advance of consuming a subsequent meal. Having subjects in this age-group (aged <7 years) achieve near-target glucose levels in a timely manner is particularly challenging, as it is not always possible to predict in advance how much the child will eat.
Although our study did not show an improvement in nighttime time in target, 50% of the subjects received additional insulin boluses during the open-loop nighttime. Generally, our protocol sought to mimic the subjects’ home therapy during the open-loop study period; however, we worked closely with the parents in trying to achieve the best possible open-loop control and allowed additional correction insulin boluses to be given overnight in response to the frequent blood glucose measurements. This should have biased our results to the null; yet, a significant decrease in hyperglycemia was still obtained with the closed-loop system. Our study subjects likely represent a highly motivated subgroup of families with type 1 diabetes who are more likely to closely monitor their children’s blood glucose and give additional correction doses as needed. Thus, it is likely that widespread implementation of a similar closed-loop algorithm would yield an even greater benefit to overnight glycemic control, as many patients do not receive supplemental correction doses overnight.
Parents of young children with type 1 diabetes have a significant fear of hypoglycemia, especially at night (20). In a recent randomized controlled trial, use of a CGM did not decrease episodes of hypoglycemia in young children or fear of hypoglycemia in their caregivers (15). Our study was not powered to detect a difference in rate of hypoglycemia. Additional longer-term studies are needed to assess the ability of a closed-loop system to prevent hypoglycemia in young children and to alleviate hypoglycemia fear in their caregivers. A number of subjects did experience episodes of hypoglycemia during closed-loop therapy. There are various factors that account for these episodes. First, our closed-loop system was implemented every 20 min overnight, which significantly hampers the algorithm’s ability to rapidly adjust to changes in glucose values. Implementation on a fully automated minute-to-minute system will improve performance significantly. Second, as this is the first study of closed-loop therapy in this age-group, the initial algorithm parameters were set based on estimates from adult studies. These parameters were initially too aggressive and were adjusted downward during the study. Computer modeling (21–23) of the profiles obtained here may allow for optimization of algorithm performance and should lead to improved outcomes in subsequent clinical studies.
Closed-loop therapy was able to provide similar peak postprandial glucose concentrations and then return the glucose concentration closer to target prior to the next meal. Typically, open-loop therapy has a better meal response than closed-loop therapy, as the insulin is given prior to the subject eating. In closed-loop therapy, insulin is not delivered until the blood glucose begins to rise after eating, leading to a delay in insulin action. It is for this reason that some have suggested using a hybrid approach where a portion of the meal bolus is given prior to eating and the remaining insulin is determined by the closed-loop system. This hybrid approach can improve meal glycemic control (7). However, in the case of young children it is very difficult to predict how much food they are going to eat at an individual meal and thus how much insulin to deliver. Parents often divide the meal bolus, giving a small portion in advance and the remainder postprandially. However, even this approach can prove difficult to implement safely and can result in hypoglycemia when the child eats significantly less than anticipated. A reactive closed-loop insulin algorithm can prevent these episodes from occurring and could serve a great clinical need in these young children. Another possible hybrid closed-loop approach is to give a noncommittal insulin bolus prior to the meal. This bolus could improve the postprandial hyperglycemia, but if the subject decides not to eat at all the controller could still prevent hypoglycemia by reducing future basal delivery. Alternatively, a partial meal bolus could be given postprandially after the parents witness how much the child eats—similar to current practice in young children. Either of these approaches will speed up insulin delivery, allowing for improvement in the meal response and then allowing the hybrid closed-loop system to deliver the remainder of the necessary insulin. Of course, these approaches require input from the patient or parent and thus increase the burden of the system. Finally, other groups have experimented with including glucagon in a closed-loop system to prevent hypoglycemia. The inclusion of glucagon can potentially allow for more aggressive up-front insulin delivery, as the glucagon can counteract the effects of overdelivery of insulin and prevent postprandial hypoglycemia (3,24). While this approach is quite appealing at some level, there are a number of technical challenges to a dual hormonal system such as a substantial increase in the complexity of the system, manufacturing of a dual-chamber pump, and a stable formulation of glucagon, all of which have yet to be resolved.
Our study has a number of limitations. It was performed in an inpatient setting on a limited number of individuals, and the subjects’ activity levels were much lower than their typical daily routine. Yet to be determined is how a closed-loop system will deal with the day-to-day variability in young children’s activity levels and eating patterns. Additionally, our closed-loop arm was implemented manually as opposed to on a fully automated system. This allowed for physician oversight of every dose but limited the frequency of adjustments overnight. Subjects’ insulin management was not optimized prior to entry into the study. However, it is quite difficult to optimize insulin basal rates in young children, and 70% of subjects had A1C levels within the American Diabetes Association target range, indicating that they were relatively well controlled. Finally, our study included a midmorning snack, as is routine for children in this age-group. This snack may have played a role in preventing postbreakfast hypoglycemia due to aggressive insulin delivery. Additional studies will be needed to validate the improved prelunch blood sugars with closed-loop therapy without hypoglycemia in the absence of a midmorning snack.
In conclusion, we performed the first study of closed-loop insulin therapy in children aged <7 years. Closed-loop therapy decreased nocturnal hyperglycemia and improved prelunch blood sugars. The development of a fully closed-loop system is critical for improving the well-being of patients with type 1 diabetes. We believe that young children are especially difficult to manage with standard diabetes therapy because of high risk of nocturnal hypoglycemia, erratic activity, and unpredictable eating patterns and will benefit significantly from closed-loop therapy. Additional research is needed in this underrepresented patient population.
Supplementary Material
Acknowledgments
This work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Institutes of Health Grant UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers).
Abbott Diabetes Care, Animas Corporation, and HemoCue Inc. provided supplies for the study. The companies played no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. No other potential conflicts of interest relevant to this article were reported.
A.D. designed and conducted the study, performed the analysis, and wrote the manuscript. L.C. conducted the study, contributed to the analysis, and revised the manuscript. J.S. contributed to the design and conduct of the study and revised the manuscript. M.S.D.A. contributed to algorithm development and discussion of results. S.E. contributed to the design and conduct of the study and revised the manuscript. G.M.S. designed and conducted the study, performed the analysis, and wrote the manuscript. G.M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in oral form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012, and at The Endocrine Society's 94th Annual Meeting & Expo, Houston, Texas, 23–26 June 2012.
The authors thank the patients and their families for participating in the study. The authors thank the nurses, nutritionists, and entire staff of the Clinical and Translational Studies Unit at Boston Children’s Hospital for their excellent care. The authors also thank Stephanie Smith and Astrid Atakov-Castillo, Joslin Diabetes Center, for help with laboratory processing.
Footnotes
Clinical trial reg. no. NCT01421225, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1079/-/DC1.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol 2011;7:385–395 [DOI] [PubMed] [Google Scholar]
- 3.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 5.Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 7.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 8.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 1997;20:22–25 [DOI] [PubMed] [Google Scholar]
- 9.Wagner VM, Rosenbauer J, Grabert M, Holl RW, German Initiative on Quality Control in Pediatric Diabetology Severe hypoglycemia, metabolic control, and diabetes management in young children with type 1 diabetes using insulin analogs—a follow-up report of a large multicenter database. Eur J Pediatr 2008;167:241–242 [DOI] [PubMed] [Google Scholar]
- 10.Bjørgaas M, Gimse R, Vik T, Sand T. Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr 1997;86:148–153 [DOI] [PubMed] [Google Scholar]
- 11.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 1985;75:921–927 [PubMed] [Google Scholar]
- 12.Gandrud LM, Xing D, Kollman C, et al. The Medtronic Minimed Gold continuous glucose monitoring system: an effective means to discover hypo- and hyperglycemia in children under 7 years of age. Diabetes Technol Ther 2007;9:307–316 [DOI] [PubMed] [Google Scholar]
- 13.DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 14.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 15.Mauras N, Beck R, Xing D, et al. Diabetes Research in Children Network (DirecNet) Study Group A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care 2012;35:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loutseiko M, Voskanyan G, Keenan DB, Steil GM. Closed-loop insulin delivery utilizing pole placement to compensate for delays in subcutaneous insulin delivery. J Diabetes Sci Tech 2011;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swan KL, Dziura JD, Steil GM, et al. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care 2009;32:240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swan KL, Weinzimer SA, Dziura JD, et al. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care 2008;31:44–46 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manag (Lond) 2011;1:627–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanderian SS, Weinzimer S, Voskanyan G, Steil GM. Identification of intraday metabolic profiles during closed-loop glucose control in individuals with type 1 diabetes. J Diabetes Sci Tech 2009;3:1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanderian SS, Weinzimer SA, Steil GM. The identifiable virtual patient model: comparison of simulation and clinical closed-loop study results. J Diabetes Sci Tech 2012;6:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steil GM, Clark B, Kanderian S, Rebrin K. Modeling insulin action for development of a closed-loop artificial pancreas. Diabetes Technol Ther 2005;7:94–108 [DOI] [PubMed] [Google Scholar]
- 24.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.