Abstract
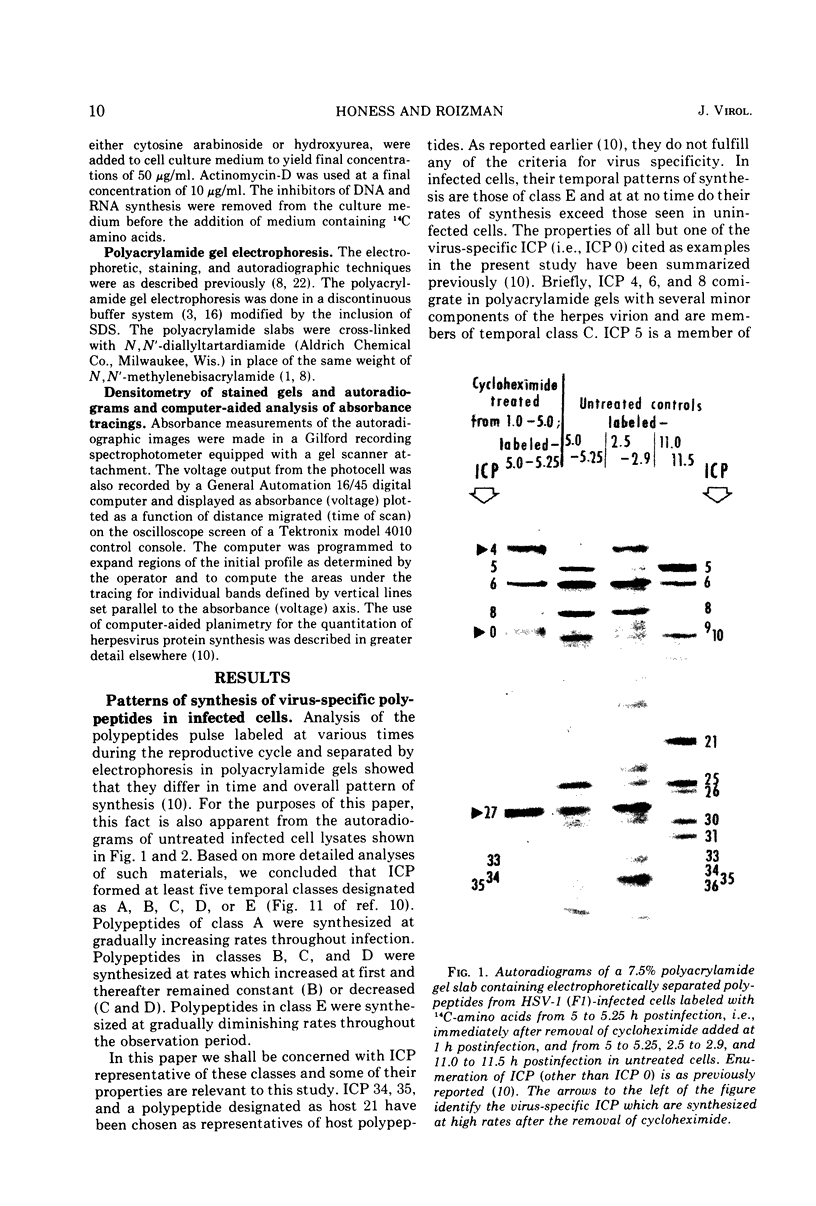
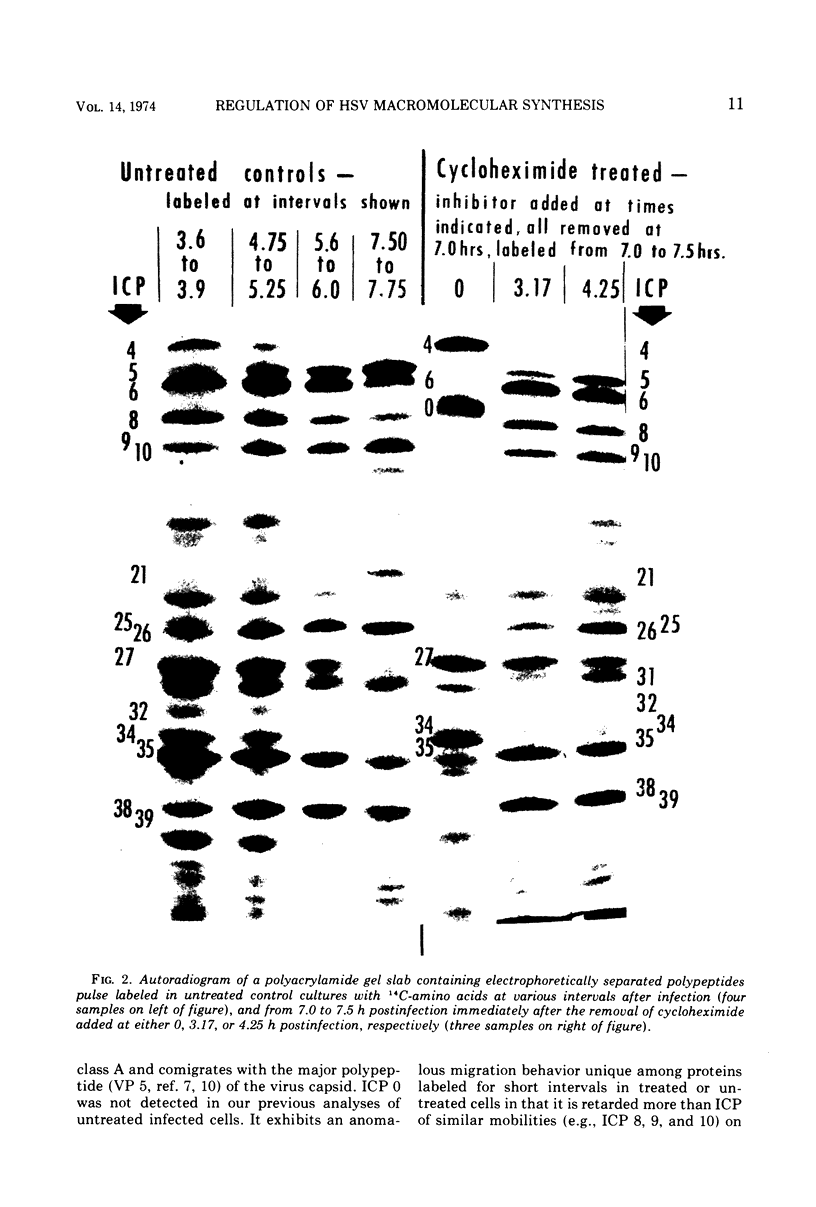
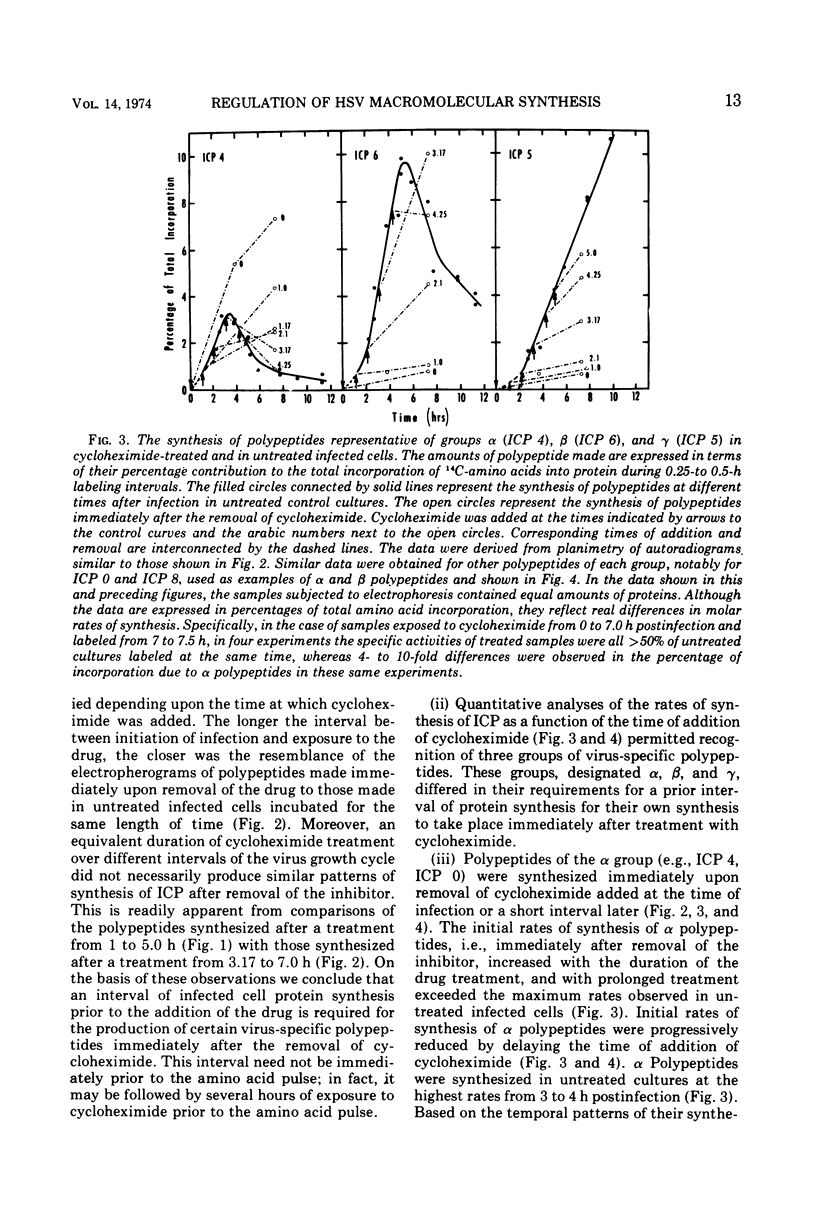
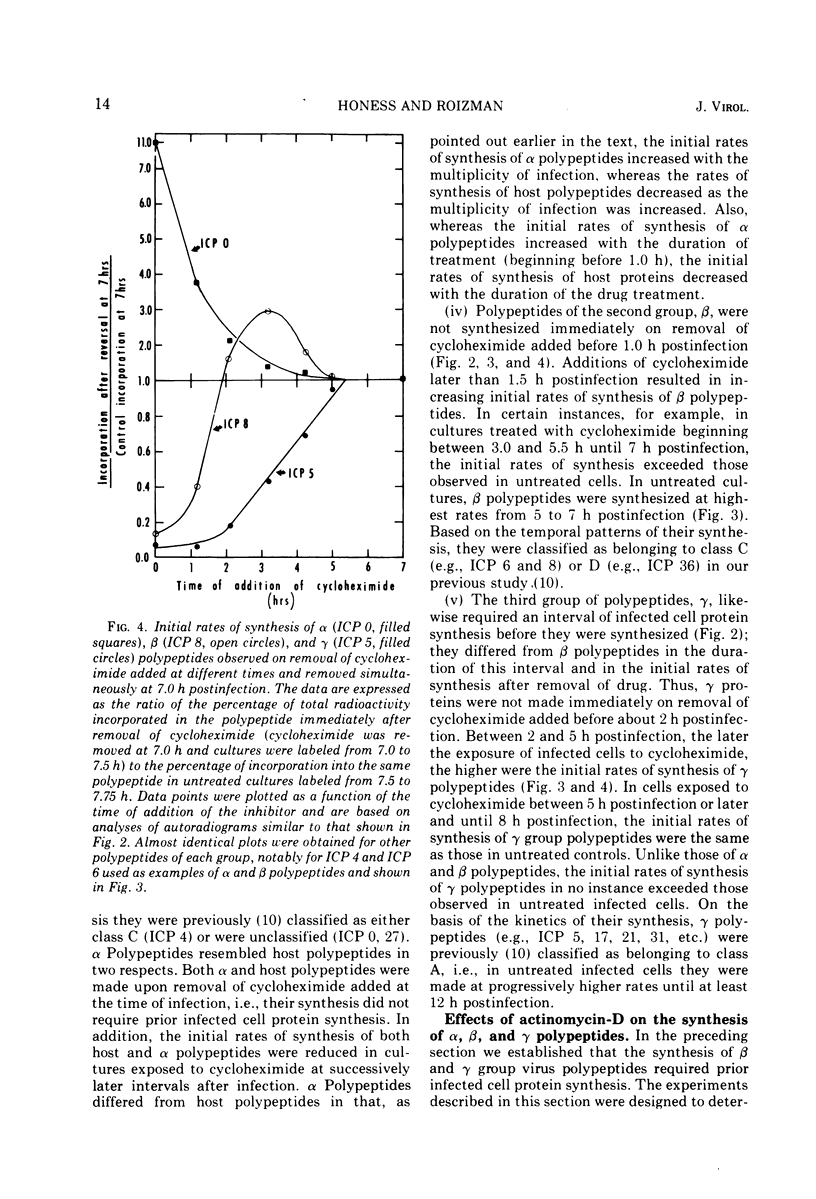
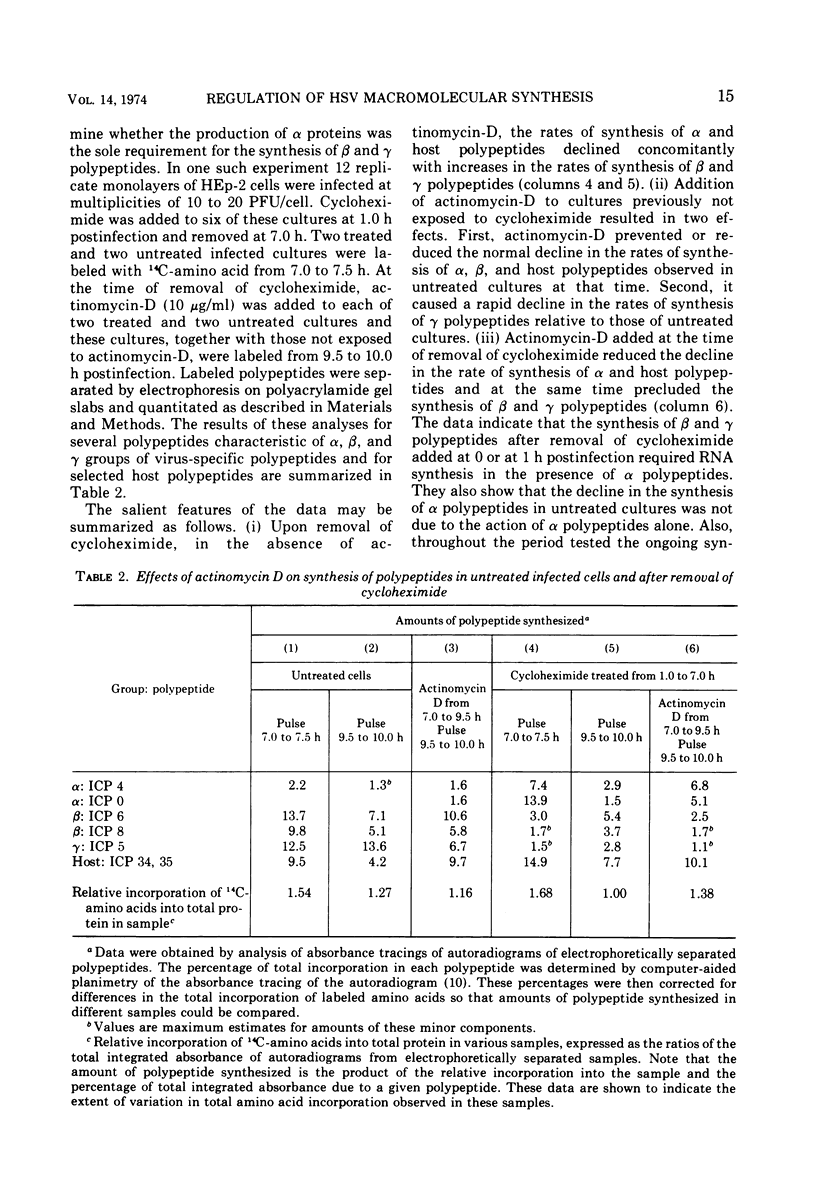
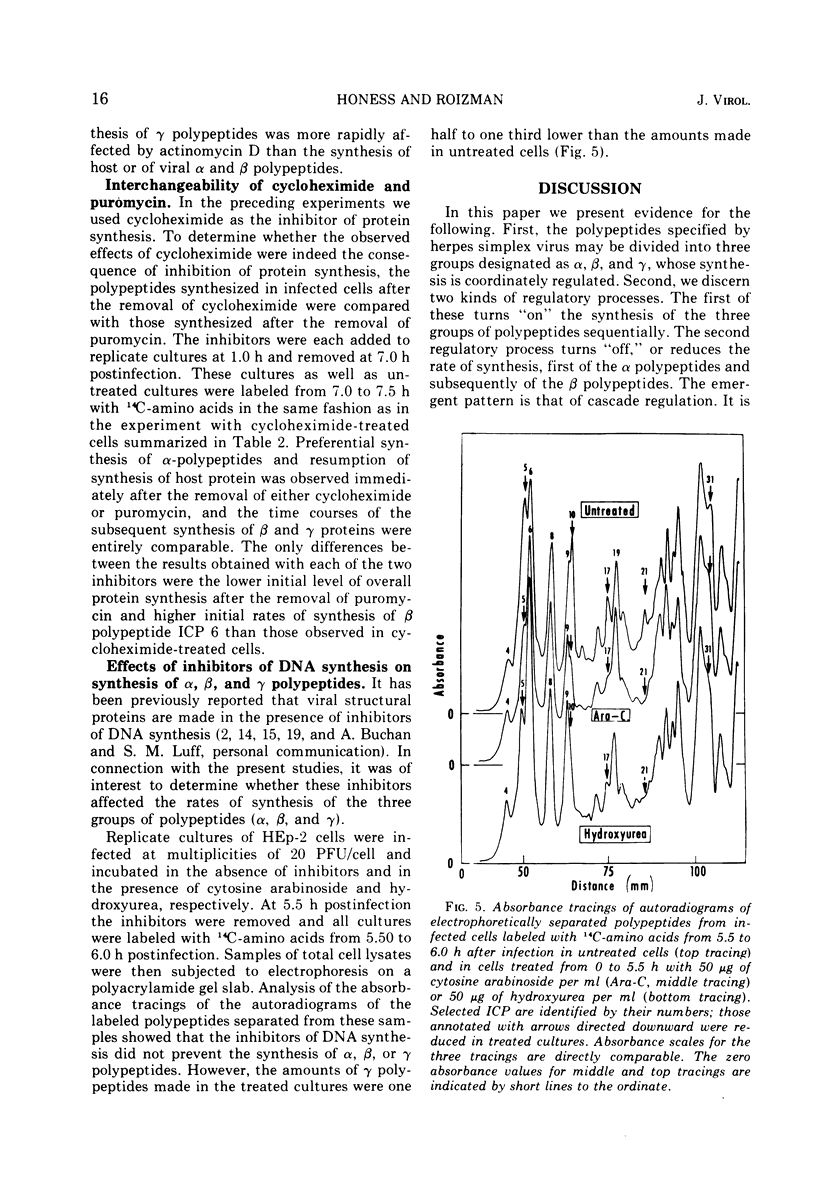
Based on evidence that 50% of herpes simplex 1 DNA is transcribed in HEp-2 cells in the absence of protein synthesis we examined the order and rates of synthesis of viral polypeptides in infected cells after reversal of cycloheximide- or puromycin-mediated inhibition of protein synthesis. These experiments showed that viral polypeptides formed three sequentially synthesized, coordinately regulated groups designated α, β, and γ. Specifically: (i) The α group, containing one minor structural and several nonstructural polypeptides, was synthesized at highest rates from 3 to 4 h postinfection in untreated cells and at diminishing rates thereafter. The β group, also containing minor structural and nonstructural polypeptides, was synthesized at highest rates from 5 to 7 h and at decreasing rates thereafter. The γ group containing major structural polypeptides was synthesized at increasing rates until at least 12 h postinfection. (ii) The synthesis of α polypeptides did not require prior infected cell protein synthesis. In contrast, the synthesis of β polypeptides required both prior α polypeptide synthesis as well as new RNA synthesis, since the addition of actinomycin D immediately after removal of cycloheximide precluded β polypeptide synthesis. The function supplied by the α polypeptides was stable since interruption of protein synthesis after α polypeptide synthesis began and before β polypeptides were made did not prevent the immediate synthesis of β polypeptides once the drug was withdrawn. The requirement of γ polypeptide synthesis for prior synthesis of β polypeptides seemed to be similar to that of β polypeptides for prior synthesis of the α group. (iii) The rates of synthesis of α polypeptides were highest immediately after removal of cycloheximide and declined thereafter concomitant with the initiation of β polypeptide synthesis; this decline in α polypeptide synthesis was less rapid in the presence of actinomycin D which prevented the appearance of β and γ polypeptides. The decrease in rates of synthesis of β polypeptides normally occurring after 7 h postinfection was also less rapid in the presence of actinomycin D than in its absence, whereas ongoing synthesis of γ polypeptides at this time was rapidly reduced by actinomycin D. (iv) Inhibitors of DNA synthesis (cytosine arabinoside or hydroxyurea) did not prevent the synthesis of α, β, or γ polypeptides, but did reduce the amounts of γ polypeptides made.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Shimono H., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. II. Flow of structural viral proteins from cytoplasm to nucleus. Virology. 1969 Jan;37(1):56–61. doi: 10.1016/0042-6822(69)90306-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Editorial: Provisional labels for herpesviruses. J Gen Virol. 1973 Sep;20(3):417–419. doi: 10.1099/0022-1317-20-3-417. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Silverstein S., Cassai E., Roizman B. RNA synthesis in cells infected with herpes simplex virus. VII. Control of transcription and of transcript abundancies of unique and common sequences of herpes simplex virus 1 and 2. J Virol. 1973 Jun;11(6):886–892. doi: 10.1128/jvi.11.6.886-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitt J., Becker Y. The effect of cytosine arabinoside on the replication of herpes simplex virus. Virology. 1967 Jan;31(1):129–134. doi: 10.1016/0042-6822(67)90016-5. [DOI] [PubMed] [Google Scholar]
- Nii S., Rosenkranz H. S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. 3. Effect of hydroxyurea. J Virol. 1968 Oct;2(10):1163–1171. doi: 10.1128/jvi.2.10.1163-1171.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Penman S., Goldstein E., Reichman M., Singer R. The relation of protein synthesis regulation and RNA metabolism. Acta Endocrinol Suppl (Copenh) 1973;180:168–191. doi: 10.1530/acta.0.074s168. [DOI] [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Himeno M., Kaplan A. S. Early functions of the genome of herpesvirus. I. Characterization of the RNA synthesized in cycloheximide-treated, infected cells. Virology. 1971 Dec;46(3):877–889. doi: 10.1016/0042-6822(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Silverstein S., Bachenheimer S. L., Frenkel N., Roizman B. Relationship between post-transcriptional adenylation of herpes virus RNA and messenger RNA abundance. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2101–2104. doi: 10.1073/pnas.70.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. RNA synthesis in cells infected with herpes simplex virus. II. Evidence that a class of viral mRNA is derived from a high molecular weight precursor synthesized in the nucleus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):626–633. doi: 10.1073/pnas.64.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]