Abstract
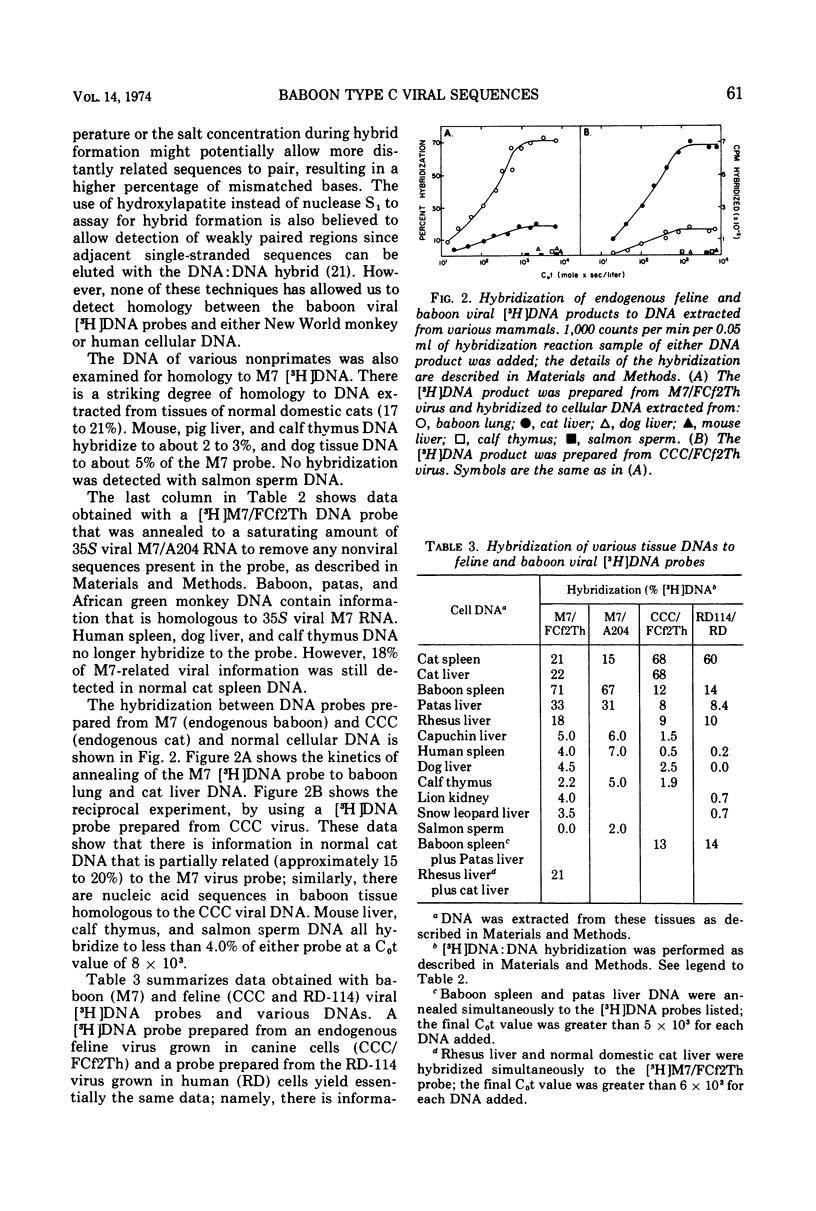
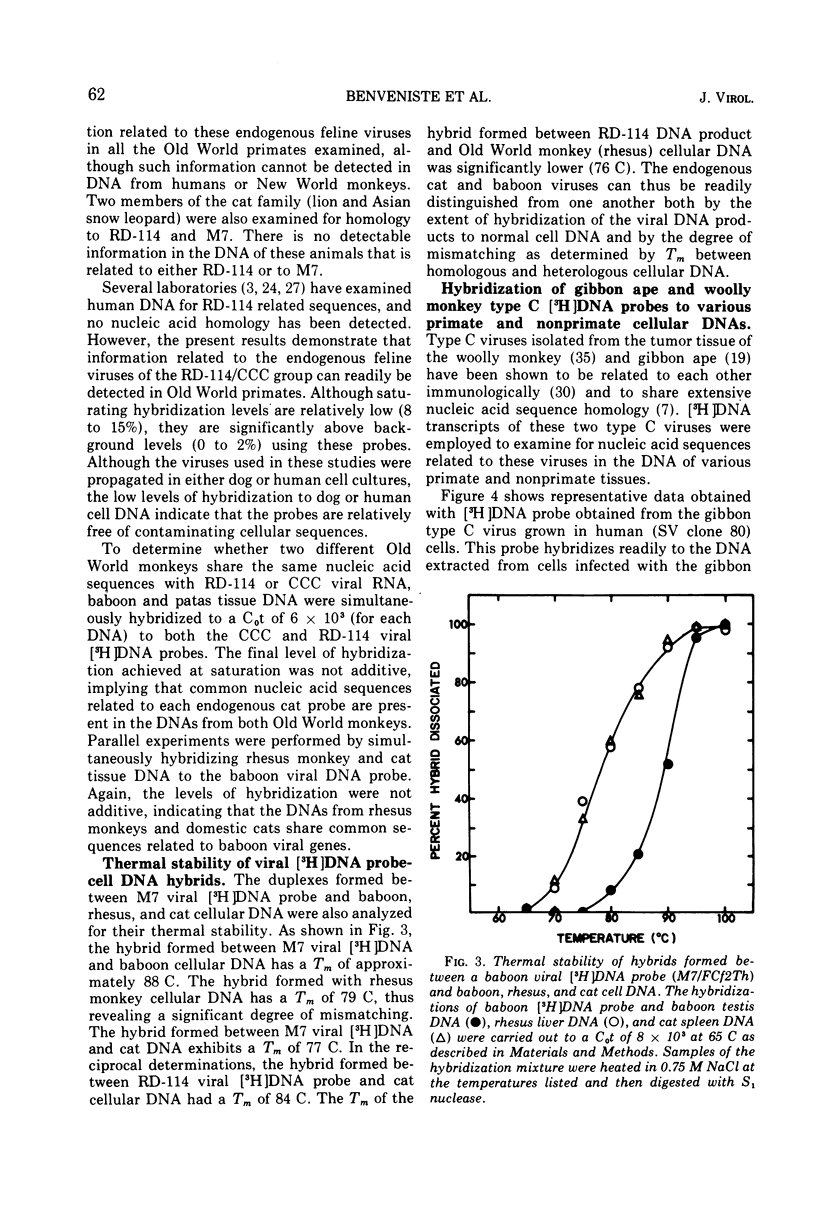
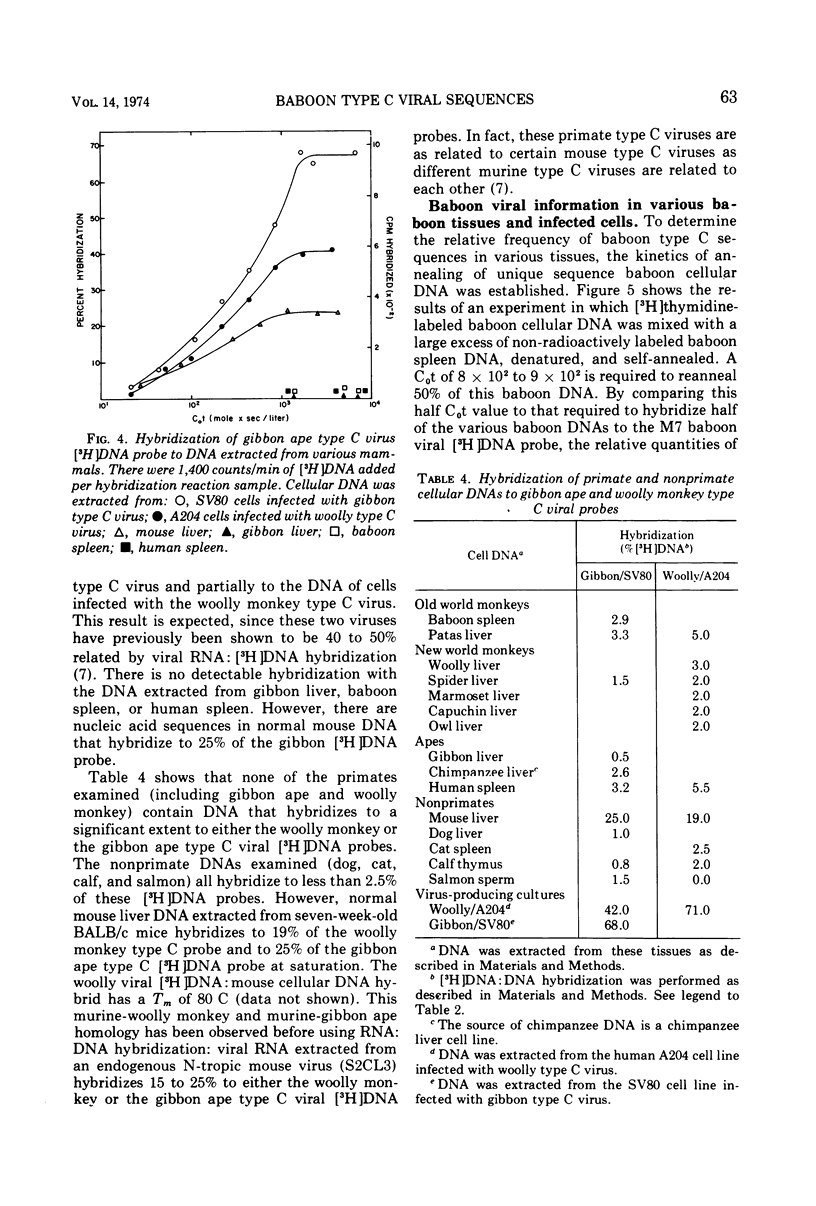
Nucleic acid sequences homologous to a single-stranded [3H]DNA transcript prepared from a baboon type C virus replicating in dog thymus cells can be readily detected in the cellular DNA of several Old World monkeys (baboon, patas, African green, and two species of macaques-rhesus and stumptail). These results demonstrate that primates other than the baboon also contain endogenous type C viral genes. With the hybridization conditions employed (S1 nuclease, 65 C), no homologous sequences were detected in DNA from human or New World monkey tissues. Of various nonprimate tissues examined, only domestic cat cellular DNA was partially homologous to the baboon virus [3H]DNA transcript. In reciprocal experiments, [3H]DNA transcripts of RNAs from endogenous cat viruses (RD-114/CCC group) show a significant partial homology with cellular DNA from Old World primates (baboon, patas, and rhesus monkey). The partial homology between type-C-related information in the DNA of domestic cats and various Old World monkeys suggests the possibility of horizontal transmission between the progenitors of these animals at some point in evolution. No nucleic acid sequences homologous to [3H]DNA transcripts prepared from type C viruses isolated from tumor tissue of a woolly monkey and a gibbon ape could be detected in any primate tissue DNA examined; however, a partial nucleic acid homology was found between woolly monkey and gibbon ape type C viral [3H]DNA and normal mouse cellular DNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Todaro G. J. A distinct class of inducible murine type-C viruses that replicates in the rabbit SIRC cell line. Proc Natl Acad Sci U S A. 1974 Mar;71(3):602–606. doi: 10.1073/pnas.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J., Scolnick E. M., Parks W. P. Partial transcription of murine type C viral genomes in BALB c cell lines. J Virol. 1973 Oct;12(4):711–720. doi: 10.1128/jvi.12.4.711-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Peebles P. T., Nomura S., Haapala D. K. Isolation of RD-114-like oncornavirus from a cat cell line. J Virol. 1973 Jun;11(6):978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Aaronson S. A., Martin M. A. Heterogeneity of murine leukemia virus in vitro DNA; detection of viral DNA in mammalian cells. Science. 1971 Jun 25;172(3990):1353–1355. doi: 10.1126/science.172.3990.1353. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Kohne D. E. Evolution of higher-organism DNA. Q Rev Biophys. 1970 Aug;3(3):327–375. doi: 10.1017/s0033583500004765. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Todaro G. J. Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C virus. Virology. 1973 May;53(1):142–151. doi: 10.1016/0042-6822(73)90473-x. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. Related base sequences in the DNA of simple and complex organisms. VI. The extent of base sequence divergence among the DNAs of various rodents. Biochem Genet. 1970 Jun;4(3):425–446. doi: 10.1007/BF00485758. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of RD114 virus nucleotide sequences in feline cellular DNA. Nat New Biol. 1973 Jul 11;244(132):62–64. doi: 10.1038/newbio244062a0. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Okabe H., Gilden R. V., Hatanaka M. RD 114 virus-specific sequences in feline cellular RNA: detection and characterization. J Virol. 1973 Nov;12(5):984–994. doi: 10.1128/jvi.12.5.984-994.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Murine mammary tumor cell clones with varying degrees of virus expression. Virology. 1973 Sep;55(1):163–173. doi: 10.1016/s0042-6822(73)81018-9. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Noon M. C., Watson C. J., Kawakami T. G. Radioimmunoassay of mammalian type-C polypeptides. IV. Characterization of woolly monkey and gibbon viral antigens. Int J Cancer. 1973 Jul 15;12(1):129–137. doi: 10.1002/ijc.2910120114. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Joysey K. A., Friday A. E. Molecular evolution of myoglobin and the fossil record: a phylogenetic synthesis. Nature. 1973 Dec 14;246(5433):389–395. doi: 10.1038/246389a0. [DOI] [PubMed] [Google Scholar]
- Ruprecht R. M., Goodman N. C., Spiegelman S. Determination of natural host taxonomy of RNA tumor viruses by molecular hybridization: application to RD-114, a candidate human virus. Proc Natl Acad Sci U S A. 1973 May;70(5):1437–1441. doi: 10.1073/pnas.70.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W., Kawakami T., Kohne D., Okabe H., Gilden R., Hatanaka M. Primate and murine type-C viral nucleic acid association kinetics: analysis of model systems and natural tissues. J Virol. 1974 Feb;13(2):363–369. doi: 10.1128/jvi.13.2.363-369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Lieber M. M., Benveniste R. E., Todaro G. J. Endogenous baboon type C virus (M7): biochemical and immunologic characterization. Virology. 1974 Apr;58(2):492–503. doi: 10.1016/0042-6822(74)90083-x. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., WOLMAN S. R., GREEN H. RAPID TRANSFORMATION OF HUMAN FIBROBLASTS WITH LOW GROWTH POTENTIAL INTO ESTABLISHED CELL LINES BY SV40. J Cell Physiol. 1963 Dec;62:257–265. doi: 10.1002/jcp.1030620305. [DOI] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Lieber M. M., Sherr C. J. Characterization of a type C virus released from the porcine cell line PK(15). Virology. 1974 Mar;58(1):65–74. doi: 10.1016/0042-6822(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E., Lieber M. M., Melnick J. L. Type C viruses of baboons: isolation from normal cell cultures. Cell. 1974 May;2(1):55–61. doi: 10.1016/0092-8674(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]


