Abstract
Autism spectrum disorders (ASD) are neurodevelopmental diseases that affect an alarming number of individuals. The etiological basis of ASD is unclear, and evidence suggests it involves both genetic and environmental factors. There are many reports of cytokine imbalances in ASD. These imbalances could have a pathogenic role, or they may be markers of underlying genetic and environmental influences. Cytokines act primarily as mediators of immunological activity, but they also have significant interactions with the nervous system. They participate in normal neural development and function, and inappropriate activity can have a variety of neurological implications. It is therefore possible that cytokine dysregulation contributes directly to neural dysfunction in ASD. Further, cytokine profiles change dramatically in the face of infection, disease, and toxic exposures. Therefore, imbalances may represent an immune response to environmental contributors to ASD. The following review is presented in two main parts. First, we discuss select cytokines implicated in ASD, including IL-1Β, IL-6, IL-4, IFN-γ, and TGF-Β, and focus on their role in the nervous system. Second, we explore several neurotoxic environmental factors that may be involved in the disorders, and focus on their immunological impacts. This review represents an emerging model that recognizes the importance of both genetic and environmental factors in ASD etiology. We propose that the immune system provides critical clues regarding the nature of the gene by environment interactions that underlie ASD pathophysiology.
Keywords: Autism spectrum disorder, cytokine, toxicant, immunology, environment, neurodevelopment
Introduction
Autism spectrum disorders (ASD) are clinically variable neurodevelopmental disorders that arise in early childhood. ASD is diagnosed in 1 out of every 88 children in the United States, and is characterized by stereotypic behaviors and impaired language and communication (American Psychiatric Association 1994; Lord, Rutter et al. 1994; Prevention 2009; CDC 2012). The biological basis of ASD remains largely elusive. Postmortem investigations have demonstrated abnormal brain growth, including neuronal overgrowth in some regions (Courchesne, Mouton et al. 2011), and undergrowth in others (Schumann and Amaral 2006). Altered ratios of excitatory to inhibitory signaling in the brain may also contribute to ASD (Fatemi, Halt et al. 2002; Rubenstein and Merzenich 2003; Fatemi, Reutiman et al. 2010; Yizhar, Fenno et al. 2011). Inheritance analyses have demonstrated a large genetic component, although a recent twin study suggests that non-genetic environmental factors also make a substantial contribution (Hallmayer, Cleveland et al. 2011; Ronald and Hoekstra 2011). A multitude of genes have been implicated in autism, but only a few cases can be traced to specific rare genetic variants, and many of the implicated genes are also found in typically developing populations (State and Levitt 2011). Collectively, these findings suggest that autism results from complex interactions between multiple susceptibility genes and environmental factors.
A growing body of evidence implicates immunological disturbances in ASD. Several of the genes linked to ASD have critical roles in immune signaling, activation, and regulation (Torres, Maciulis et al. 2002; Campbell, Sutcliffe et al. 2006; Lee, Zachary et al. 2006; Torres, Sweeten et al. 2006; Varga, Pastore et al. 2009; Orlova and Crino 2010). Individuals with autism and their family members (primarily mothers) demonstrate increased autoimmunity, altered cellular immunity, and skewed expression of soluble mediators like cytokines (Vargas, Nascimbene et al. 2005; Braunschweig, Ashwood et al. 2008; Li, Chauhan et al. 2009; Morgan, Chana et al. 2010; Goines, Haapanen et al. 2011; Onore, Careaga et al. 2011). Cytokine abnormalities in ASD are an important clue for researchers as they might result from genetic and environmental factors, and may contribute directly to neurological dysfunction. The following considers the neurological significance of cytokine dysregulation in ASD, and how environmental factors can modulate the cytokine response.
Cytokines: The common language between the immune and nervous system
The immune system and the nervous system interact extensively. It is therefore not surprising that immune dysfunction is often noted in neurological disorders. Immune mediators known as cytokines are key facilitators of cross-systemic communication. Cytokines are proteins that control the nature, duration, and intensity of an immune response. They are highly pleiotropic, and can act in an autocrine, paracrine, and/or endocrine fashions. Immune cells, including dendritic cells, macrophages, neutrophils, T cells, and B cells, are the primary source of cytokines; though many additional cell types, including neurons, produce and respond to them. Cytokines share structural similarities and signaling pathways with neurotrophins and neurologically relevant growth factors. In many ways, cytokines represent a common language between the immune system and the nervous system.
Cytokines influence both the development and function of the nervous system. Their significance varies based on the timing, duration, and intensity of the neuro-immune interaction. For example, cytokines impact the developing brain differently than the adult brain; and may be beneficial at one concentration while harmful at another. Cytokines are involved in normal aspects of neurodevelopment, including progenitor cell differentiation, cellular localization/migration within the nervous system, and synaptic network formation (Deverman and Patterson 2009). During infection and illness, cytokines mediate neurological changes associated with fever and sickness behavior by signaling directly to the hypothalamus (Dantzer 2001; Skurlova, Stofkova et al. 2006). Emerging evidence also implicates cytokines in higher order neurological functions, including cognition and memory (McAfoose and Baune 2009; Derecki, Cardani et al. 2010). Imbalanced cytokine production, signaling, and/or regulation can therefore have a wide range of neurological consequences.
Cytokines in ASD
Aberrant expression of cytokines and their signaling intermediaries is often noted in ASD (Table 1). This is observed in the brain (Vargas, Nascimbene et al. 2005; Grigorenko, Han et al. 2008; Voineagu, Wang et al. 2011; Ziats and Rennert 2011) peripheral blood (Molloy, Morrow et al. 2006; Ashwood, Krakowiak et al. 2011) and the gastrointestinal tract (DeFelice, Ruchelli et al. 2003; Ashwood, Anthony et al. 2004). Cytokine imbalances during development and/or throughout life could impact neural activity and mediate behavioral aspects of the disorder. The following considers the significance of several cytokines linked to ASD.
Table 1. Cytokines in autism spectrum disorders.
A variety of independent clinical studies have linked cytokines to ASD. This table presents detailed findings for each individual cytokine. Often multiple cytokines were associated with ASD in a single study, which is noted in parentheses.
| Cytokine | Findings in autism | Reference |
|---|---|---|
| IL-1Β | Elevated plasma levels in children with ASD, correlated with regressive onset. (IL-6, IL-8 and IL-12p40 also elevated) | (Ashwood, Krakowiak et al. 2011) |
| Elevated plasma levels in high functioning children with ASD. (IL-1RA, IL-5, IL-8, IL-12p70, IL-13, IL-17 and GRO-α also elevated) | (Suzuki, Matsuzaki et al. 2011) | |
| Elevated plasma levels in adults with severe ASD. (IL-6 and endotoxin levels also elevated) | (Emanuele, Orsi et al. 2010) | |
| Peripheral blood cells from ASD subjects produce higher baseline levels. (Similar trends for IL-6 and TNF-α) | (Jyonouchi, Sun et al. 2001) | |
| Peripheral blood cells from ASD subjects produce higher levels with TLR2 or TLR4 stimulation, and lower levels with TLR-9 stimulation. (Similar trends for IL-6 and TNFα) | (Enstrom, Onore et al. 2010) | |
| IL-6 | Elevated plasma levels in children with ASD, correlated with regressive onset. (IL-1Β, IL-8, and IL-12p40 also elevated) | (Ashwood, Krakowiak et al. 2011) |
| Elevated plasma levels in adults with severe autism. (IL-Β and endotoxin levels also elevated) | (Emanuele, Orsi et al. 2010) | |
| Peripheral blood cells from ASD subjects produce higher baseline levels. (Similar trends for IL-1Β and TNF-α) | (Jyonouchi, Sun et al. 2001) | |
| Peripheral blood cells from children with ASD produce higher levels with TLR2 or TLR4 stimulation, and lower levels with TLR-9 stimulation. (Similar trends for IL-6 and TNFα | (Enstrom, Onore et al. 2010) | |
| Lymphoblasts from ASD subjects produce more IL-6. (Also TNF-α) | (Malik, Sheikh et al. 2011) | |
| Increased IL-6 staining in postmortem cerebellar sections from ASD subjects | (Wei, Zou et al. 2011) | |
| Increased IL-6 in postmortem brain specimens (various regions) from ASD subjects. (Also increased TGF-Β and inflammatory chemokines). | (Vargas, Nascimbene et al. 2005) | |
| Increased IL-6 in postmortem brain tissue from ASD subjects. (Also increased TNF-α, IFN-γ, GM-CSF, and IL-8) | (Li, Chauhan et al. 2009) | |
| IL-4 | Increased IL-4 in mid-gestational serum samples from mothers giving birth to a child with ASD. (Also IL-5 and IFN-γ) | (Goines, Croen et al. 2011) |
| Increased IL-4 in amniotic fluid samples from mothers giving birth to a child with ASD (Also IL-10, TNF-α and TNF-Β) | (Abdallah, Larsen et al. 2011) | |
| Peripheral blood cells from ASD subjects stimulated with PMA-ionomycin were more likely to be IL-4+ (And less likely to be IFN-γ+) | (Gupta, Aggarwal et al. 1998) | |
| IFN-γ | Increased IFN-γ in mid-gestational serum samples from mothers giving birth to a child with ASD. (Also IL-4 and IL-5) | (Goines, Croen et al. 2011) |
| Increased plasma levels in individuals with ASD. (Also IL-12) | (Singh 1996) | |
| Peripheral blood cells stimulated with PMA-ionomycin are less likely to be IFN-γ+ (And more likely to be IL-4+) | (Gupta, Aggarwal et al. 1998) | |
| Unstimulated whole blood from ASD subjects produced significantly more IFN-γ compared to controls. (Also increased IL-1RA, IL-6, and TNF-α) | (Croonenberghs, Bosmans et al. 2002) | |
| NK cells from children with ASD produced higher IFN-γ under resting conditions, and lower levels after stimulation. (Also observed with perforin and granzyme B) | (Enstrom, Lit et al. 2009) | |
| Increased IFN-γ in post mortem brain specimens from ASD subjects. (Also increased TNF-α, IL-6, GM-CSF, and IL-8) | (Li, Chauhan et al. 2009) | |
| TGF-Β | Decreased plasma TGF-Β in children with ASD. Lower levels correlated with more severe behavioral scores. | (Ashwood, Enstrom et al. 2008) |
| Decreased serum TGF-Β in adults with ASD. | (Okada, Hashimoto et al. 2007) | |
| Increased TGF-Β levels in postmortem brain specimens (various regions) from ASD subjects. (Also IL-6 and inflammatory chemokines) | (Vargas, Nascimbene et al. 2005) |
Interleukin (IL)-1B
IL-1Β is an inflammatory cytokine expressed very early in immune responses (Jiang, Tian et al. 1997). In tissue, IL-1Β propagates inflammation by activating local immune cells and the vascular endothelium. Systemically, IL-1Β stimulates IL-6 production and eventually an acute phase response in the liver. Systemic IL-1Β can cross the blood brain barrier (Banks, Ortiz et al. 1991) and stimulate its own expression in the hypothalamus, which leads to neuroendocrine changes associated with fever and sickness behavior (Dantzer 2001; Skurlova, Stofkova et al. 2006). IL-1Β receptors are structurally related to toll-like receptors (TLRs), and signaling is achieved through NF-κB and MAP kinase (MAPK) signaling cascades (O'Neill 2000). IL-1Β belongs to an evolutionarily conserved family of proteins that function beyond immunity (Barksby, Lea et al. 2007). It shares structural homology with fibroblast growth factors (Zhang, Cousens et al. 1991), which are critical in embryonic neurodevelopment, and are implicated in autism and schizophrenia (Tabares-Seisdedos and Rubenstein 2009; Stevens, Smith et al. 2010).
Genes for IL-1Β, its receptor, and its receptor-associated proteins are associated with intellectual disability, schizophrenia, and autism (Katila, Hanninen et al. 1999; Piton, Michaud et al. 2008; Handley, Lian et al. 2010). Children and adults with autism have increased plasma IL-1Β and skewed cellular IL-1Β responses following stimulation (Ashwood, Krakowiak et al. 2011; Suzuki, Matsuzaki et al. 2011). Compared to controls, monocytes from children with ASD produce excessive IL-1Β following LPS exposure (Jyonouchi, Sun et al. 2001; Enstrom, Onore et al. 2010), and lower levels following exposure to TLR 9 agonists (Enstrom, Onore et al. 2010). The IL-1 antagonist, IL-1ra, is also increased among ASD subjects (Suzuki, Matsuzaki et al. 2011). IL-1ra reduces inflammation by competing for the IL-1Β receptor, and increased levels may represent an attempt to counteract inflammation in ASD. Postmortem brains from ASD subjects had normal IL-1Β levels (Li, Chauhan et al. 2009), but given that peripheral IL-1Β can enter the brain (Banks, Ortiz et al. 1991), increased systemic levels could directly impact neurological processes.
IL-1Β disruption can have a variety of neurological consequences relevant to autism. The cytokine and its receptors are found throughout the nervous system during critical developmental periods (Giulian, Young et al. 1988). IL-1Β induces neural progenitor cell proliferation in some CNS regions, while inhibiting it in others (de la Mano, Gato et al. 2007). This could contribute to the region-specific overgrowth and undergrowth observed in the ASD brain. Excitatory synapse formation is partially mediated by the IL-1 receptor and receptor-associated proteins (Yoshida, Yasumura et al. 2011).
Altering these proteins can tip the balance between excitatory and inhibitory signaling, which might underlie neurological features of autism (Rubenstein and Merzenich 2003). Increased IL-1ra in autism suggests an attempt to counterbalance IL-1Β and may or may not be beneficial. Following brain injury, IL-1ra upregulation serves a neuroprotective role by dampening excessive inflammation (Loddick and Rothwell 1996). However, if administered during critical windows of neurodevelopment, IL-1ra can negatively impact neurogenesis, brain morphology, memory consolidation, and behavior (Spulber, Oprica et al. 2008; Spulber, Bartfai et al. 2010; Spulber, Bartfai et al. 2011). This shows that some level of IL-1B signaling is essential during development. In adulthood, IL-1Β is implicated in CNS disorders like Alzheimer’s disease and the advancement of amyloid-containing plaques (Griffin, Sheng et al. 1995). While excessive IL-1B contributes to pathology in some cases, it may have a protective role in others. For example, IL-1Β limits neuronal damage following excitotoxic exposures (Strijbos and Rothwell 1995), and mice lacking IL-1Β fail to undergo remyelination following experimental autoimmune encephalitis (EAE) induction (Mason, Suzuki et al. 2001). IL-1Β is involved in higher order brain processes and is induced in the hippocampus during learning processes, and is critical for maintenance of long-term potentiation (LTP) (Ross, Allan et al. 2003). Both over expression (Barrientos, Frank et al. 2009) and under expression of IL-1 beta (Goshen, Kreisel et al. 2007; Labrousse, Costes et al. 2009)are associated with impairments in memory and learning.
In summary, IL-1Β participates in neurological processes, and appears to have a role in both CNS pathology and healing. Normal, homeostatic levels of IL-1Β and its antagonist IL-1ra are necessary for proper brain development and function. This “Goldilocks” state is typical of many cytokines, where too much or too little is not desirable. Alterations in IL-1Β systems due to genetic mechanisms or environmental exposures may contribute to autism.
Interleukin (IL)-6
IL-6 is an inflammatory cytokine that shares functional properties with IL-1Β. Like IL-1Β, IL-6 is produced early in immune reactions, although it appears later and persists longer (Jiang, Tian et al. 1997). IL-6 is best known for stimulating the acute phase response in the liver, generating fever, and activating lymphocytes. Despite the functional similarities with IL-1Β, IL-6 differs drastically in terms of structure and signaling properties. It is a member of the neuropoietic cytokine family, which includes leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), and IL-11. These cytokines signal through a gp130 receptor complex (Ward, Howlett et al. 1994), and activate JAK-STAT (specifically STAT 3) and MAPK signaling pathways (Heinrich, Behrmann et al. 2003). In addition to their inflammatory properties, neuropoietic cytokines have a number of well-described roles in the nervous system, and are intricately involved in neurodevelopment and function (Bauer, Kerr et al. 2007; McAfoose and Baune 2009). IL-6 and its receptors are expressed at low levels in the healthy brain (Gadient and Otten 1994; Gadient and Otten 1994) and at higher levels in a variety of disease states (Huell, Strauss et al. 1995; Hang, Shi et al. 2004). Peripheral IL-6 can cross the blood brain barrier and influence a variety of processes in the adult brain (Banks, Kastin et al. 1994).
Prenatal cytokine imbalances may contribute to neurodevelopmental disorders like autism and schizophrenia through “fetal programming”. Fetal programming is the concept that maternal factors like inflammation and chronic stress can alter the gestational environment, skew development, and lead to long term physiological and behavioral consequences (Patterson 2009; Bilbo 2010). IL-6 readily crosses the placenta and enters fetal tissues, which is unique among cytokines, and can induce changes in placental physiology and gene expression (Zaretsky, Alexander et al. 2004; Aaltonen, Heikkinen et al. 2005; Dahlgren, Samuelsson et al. 2006; Hsiao and Patterson 2011). Animal models show that IL-6 is necessary and sufficient to alter neurodevelopmental outcomes, leading to changes in behavior, cognition, neuropathology, GABA dysregulation, and skewed immune function among offspring (Samuelsson, Jennische et al. 2006; Smith, Li et al. 2007). Similar effects are seen with prenatal exposure to infection or the infectious mimic poly I:C (Smith, Li et al. 2007; Malkova, Yu et al. 2012). IL-6 can impact a variety of processes in the developing brain. IL-6 and its family members regulate self-renewal among neuronal precursors (Escary, Perreau et al. 1993; Yoshimatsu, Kawaguchi et al. 2006), direct neuronal migration (Wei, Zou et al. 2011), promote cell survival (Kushima, Hama et al. 1992), and regulate neurite outgrowth (Ihara, Nakajima et al. 1997). IL-6 exposure during critical windows can also alter synaptic networks. Chronic IL-6 overexpression reduces expression of glutamate receptors and L-type calcium channels in culture and in vivo (Vereyken, Bajova et al. 2007), and increases the ratio of excitatory to inhibitory synapses in cultures of cerebellar granular cell cultures (Wei, Zou et al. 2011). This is of particular interest in autism, given that skewed excitatory and inhibitory ratios may be an underlying factor in its pathogenesis.
Despite intriguing evidence from animal models, two recent human studies question whether gestational IL-6 alone contributes to autism. A retrospective examination of IL-6 in archived mid-pregnancy maternal serum and amniotic fluid showed that increased levels associated with developmental disorders, but not autism (Abdallah, Larsen et al. 2011; Goines, Croen et al. 2011). This suggests that gestational IL-6 might be a marker for neurodevelopmental diseases, but is insufficient on its own to cause ASD. IL-6 can affect many sequential phases of neurodevelopment, so the timing of the exposure will largely dictate the neurological outcome. Therefore, excessive IL-6 during one phase of neurodevelopment could have one set of consequences, while similar expression during another phase has an entirely different effect. A longitudinal examination of IL-6 throughout gestation is therefore needed to obtain a more complete picture of its relevance in neurodevelopmental disorders.
IL-6 can also impact processes in the adult brain, and physiological levels are critical for homeostasis, cognition, learning, and memory. Physiological levels of IL-6 are critical for normal CNS function, and both over and under expression leads to neurological problems. Mice overexpressing IL-6 in the CNS have overt symptoms including tremor, ataxia, and seizure (Campbell, Abraham et al. 1993), and more subtle alterations in cognition and avoidance behaviors (Heyser, Masliah et al. 1997). IL-6 is transcribed in the hippocampus during LTP (Balschun, Wetzel et al. 2004). Overexpression of IL-6 reduces LTP (Bellinger, Madamba et al. 1995; Li, Katafuchi et al. 1997), whilst under expression increases it and improves learning and memory (Balschun, Wetzel et al. 2004; Braida, Sacerdote et al. 2004). With regard to social behaviors, mice overexpressing IL-6 are more social than mice that lack the cytokine, while mice lacking IL-6 demonstrate higher aggression and emotionality (Alleva, Cirulli et al. 1998; Armario, Hernandez et al. 1998).
Many independent studies show IL-6 dysregulation in individuals with autism. Children and adults with the disorder have higher circulating IL-6 levels compared to typical controls (Emanuele, Orsi et al. 2010; Ashwood, Krakowiak et al. 2011). Further, cellular IL-6 production is increased with and without stimulation (Jyonouchi, Sun et al. 2001; Enstrom, Onore et al. 2010; Malik, Sheikh et al. 2011). Increased IL-6 is also found in postmortem brain specimens from ASD subjects. Specifically, immunohistochemical analysis of cerebellar sections showed significantly more IL-6 staining in autism postmortem brain specimens (Wei, Zou et al. 2011). Two additional analyses of homogenates of the frontal cortex and anterior cingulate gyrus also showed higher IL-6 levels (Vargas, Nascimbene et al. 2005; Li, Chauhan et al. 2009). Given the ability of IL-6 to impact processes in the adult brain, it is conceivable that increased IL-6 in autism could contribute to ongoing aspects of the disorder. Alternatively, it might be an epiphenomenon, and represent a biomarker of infectious or toxic environmental exposures and altered biological homeostasis.
In summary, there is extensive evidence that IL-6 can alter neurodevelopment and function. While it is unclear whether gestational IL-6 in humans is related to autism, a dysregulation of IL-6 is observed later in life in individuals with autism. The significance of these findings is unclear, and may be the result of other genetic and environmental factors in autism. These possibilities warrant further investigation.
Interleukin (IL)-4
IL-4 is a class I cytokine that activates Jak/Stat (STAT 6), MAPK, and PI3 kinase signaling cascades (Nelms, Keegan et al. 1999). Immunologically, IL-4 has a variety of interesting roles, and can 1) induce “alternatively activated” macrophages that promote tissue repair over inflammation 2) activate basophils and mast cells, 3) promote B-cell isotype switching towards IgG1 and IgE, 4) participate in immune responses against helminthes by inducing epithelial cell turnover in the gut, and 5) participate in allergy and asthma-related immune responses (Kuperman and Schleimer 2008; Byers and Holtzman 2011; Oliphant, Barlow et al. 2011).
The receptors for IL-4 are expressed in the brain under normal conditions throughout life (Nolan, Maher et al. 2005). During development, IL-4 promotes oligodendrogenesis among neuronal progenitor cells, (Butovsky, Ziv et al. 2006), and improves survival in embryonic hippocampal cultures (Araujo and Cotman 1993). IL-4 influences retinal circuitry by regulating progenitor cell proliferation and differentiation (da Silva, Campello-Costa et al. 2008). During later phases of neurodevelopment, IL-4 can alter synapse formation; increasing the proportion of GABAergic synapses in cell culture models (Sholl-Franco, Marques et al. 2002).
Two recent studies have linked developmental IL-4 exposures to autism, though its role in pathogenicity versus protection is unclear. Mothers giving birth to a child with autism show higher levels of IL-4 in mid-pregnancy serum samples (Goines, Croen et al. 2011) and amniotic fluid (Abdallah, Larsen et al. 2011) compared to controls. IL-4 is not thought to cross the placenta, and maternal serum and amniotic fluid IL-4 may or may not relate to IL-4 in fetal tissues. Other cytokines were also upregulated in these archived samples, including IFN-γ, TNF-α, and the anti-inflammatory cytokine IL-10. This raises the question of whether IL-4 acts alone, or in concert with other cytokines. Increased IL-4 may represent a regulatory reflex to inflammation along with IL-10. IL-4’s role in pregnancy and fetal health is unclear. Increased levels during pregnancy have been associated with poor outcomes such as preterm labor (Dudley, Hunter et al. 1996) but also healthy outcomes such as protection from preeclampsia (Kronborg, Gjedsted et al. 2011; Rajakumar, Chu et al. 2011). More subtle neurodevelopmental outcomes have not thoroughly been explored with respect to gestational IL-4.
In the adult brain, IL-4 largely serves a neuroprotective role, and is associated with higher order cognitive processes. It is upregulated during CNS inflammation, inducing alternative activation of glial cells and protecting from apoptosis (Garg, Kipnis et al. 2009; Sholl-Franco, da Silva et al. 2009; You, Luo et al. 2011). In a mouse model for Alzheimer’s disease, IL-4 can attenuate disease progression (Kiyota, Okuyama et al. 2010). Following LPS exposure, IL-4 reduces inflammation and improves memory and LTP in the aged hippocampus (Nolan, Maher et al. 2005). An elegant study by Derecki et al showed that IL-4-producing T cells accumulate in the meningeal spaces during cognitive tasks. Depletion of IL-4 led to an inflammatory phenotype among meningeal myeloid cells, and a dramatic decline in cognitive capacity. Remarkably, cognitive deficits in IL-4 deficient mice could be reversed by reintroducing the cytokine in adulthood (Derecki, Cardani et al. 2010).
Among individuals diagnosed with autism, plasma and CNS IL-4 levels appear to be normal (Vargas, Nascimbene et al. 2005; Li, Chauhan et al. 2009; Ashwood, Krakowiak et al. 2011). However, IL-4 producing T cells are proportionately higher in children with autism compared to controls (Gupta, Aggarwal et al. 1998). Given the evidence that meningeal IL-4 producing T cells are critical for normal cognitive function in adulthood, it is possible that dysregulation in this cell population could contribute to altered behavior throughout life (Derecki, Cardani et al. 2010).
Collectively, IL-4 serves a variety of neurological roles, and is increased in autism. Its role during gestation is unclear due to a dearth of in-vivo studies of pregnancy and neurobehavioral outcomes following developmental IL-4 exposures. The significance of increased IL-4 producing T cells in subjects with autism is also unclear. Extensive evidence suggests that IL-4 is neurologically beneficial, so it may be that increased IL-4 in autism represents an immunological attempt to regulate other detrimental processes, and does not contribute to the disease itself. Future studies should explore this possibility.
Interferon-gamma (IFN-γ)
Interferon gamma (IFN-γ) is the sole type II interferon. It shares some functional similarities with type I interferons like IFN-α and IFN-Β but has unique structural features, receptors, and signaling pathways. IFN-γ is produced primarily by T cells and Natural Killer (NK) cells during cell-mediated immune responses, and functions largely to activate macrophages and combat viral infections (Boehm, Klamp et al. 1997; Schroder, Hertzog et al. 2004). It signals mainly through the JAK/STAT (STAT1), and MAPK cascades (Hu, Roy et al. 2001; Platanias 2005). IFN-γ and IL-4 counterbalance one another’s activity via TH1/TH2 interactions, so dysregulation in one cytokine often impacts the other. It is therefore not surprising that both cytokines are implicated in ASD.
Developmental exposure to IFN-γ has been linked to autism. Mothers of children with autism have higher serum IFN-γ during the second trimester compared to controls (Goines, Croen et al. 2011). Like IL-4, IFN-γ does not cross the placenta, and the relationship between maternal serum levels and fetal exposure to the cytokine is unclear. If the cytokine is present in fetal tissues, it could interfere with normal neural development and synapse formation. IFN-γ promotes neuronal differentiation among neural progenitor cells (Barish, Mansdorf et al. 1991; Jonakait, Wei et al. 1994; Wong, Goldshmit et al. 2004; Butovsky, Ziv et al. 2006; Zahir, Chen et al. 2009; Leipzig, Xu et al. 2010; Li, Walker et al. 2010), however, these cells appear to be abnormal and exhibit compromised function and strange patterns of neuronal marker expression (Walter, Honsek et al. 2011). IFN-γ also impacts dendritic morphology and synapse formation, leading to long-term changes in cellular connectivity and communication. Depending on cell culture conditions, IFN-γ either promotes or inhibits dendrite outgrowth through STAT 1 and MAPK signaling pathways (Barish, Mansdorf et al. 1991; Kim, Beck et al. 2002; Wong, Goldshmit et al. 2004; Song, Wang et al. 2005; Andres, Shi et al. 2008). In culture, excessive IFN-γ alters patterns of excitatory signaling and receptor expression (Vikman, Owe-Larsson et al. 2001), and animals lacking the cytokine have fewer pre-synaptic terminals (Victorio, Havton et al. 2010). Interestingly, mice overexpressing IFN-γ show increased MHC I in the brain (Corbin, Kelly et al. 1996). MHC I is critical for T cell and NK cell recognition of self and foreign entities, and was historically thought to be absent in the CNS. However, recent studies have demonstrated that it is expressed in the CNS, and has an essential role in synapse formation and plasticity (Shatz 2009). IFN-γ may therefore induce abnormalities in synaptic organization by altering MHC I expression. Collectively, these studies show that direct exposure to IFN-γ can cause abnormal neurodevelopment, which may explain features of autism.
If excess IFN-γ is not present in fetal tissues, excessive maternal levels could have an indirect impact on fetal development. Interestingly, IFN-γ has a variety of critical roles in pregnancy, and directs aspects of placental development, health, and maintenance (Murphy, Tayade et al. 2009). Increased gestational IFN-γ is associated with adverse pregnancy outcomes including recurrent miscarriage (Jenkins, Roberts et al. 2000). Therefore, IFN-γ might be an indicator of compromised health in pregnancy, which could lead to neurodevelopmental abnormalities.
Peripheral IFN-γ is up-regulated in a number of neurological disorders including multiple sclerosis (Martins, Rose et al. 2011) and Down’s syndrome (Torre, Broggini et al. 1995). Individuals with autism also have increased plasma levels of IFN-γ (Singh 1996), which correlates with other peripheral inflammatory mediators such as nitric oxide (Sweeten, Posey et al. 2004). Peripheral immune cells from ASD subjects produce higher basal levels of IFN-γ but fail to respond further following immunological stimulation (Gupta, Aggarwal et al. 1998; Croonenberghs, Bosmans et al. 2002; Enstrom, Lit et al. 2009). In addition to peripheral IFN-γ dysregulation, postmortem brain specimens showed increased levels of IFN-γ (Li, Chauhan et al. 2009), suggesting IFN-γ may directly impact CNS processes in autism. In the developed nervous system, IFN-γ is historically associated with neurodegeneration, although some evidence suggests it may have a beneficial role. IFN-γ can cross the blood brain barrier at low levels (Pan, Banks et al. 1997), but is barely detectable in the healthy nervous system (Traugott and Lebon 1988; De Simone, Levi et al. 1998). In the CNS, IFN-γ is up-regulated following infectious exposures (De Simone, Levi et al. 1998), and in diseases including cerebral palsy (Folkerth, Keefe et al. 2004), multiple sclerosis (Traugott and Lebon 1988), HIV dementia (Nolting, Lindecke et al. 2009), and Parkinson’s (Barcia, Ros et al. 2011; Mangano, Litteljohn et al. 2011). High levels are harmful in cell culture, and cause enhanced glutamate induced neurotoxicity (Mizuno, Zhang et al. 2008). However, in cell culture and in vivo, low IFN-γ levels reduce oxidative-stress induced apoptosis through activation of astrocytes (Garg, Kipnis et al. 2009; Victorio, Havton et al. 2010) and may be neuroprotective in some cases. In a mouse model of Alzheimer’s disease, overexpressing IFN-γ actually attenuated plaque formation (Chakrabarty, Ceballos-Diaz et al. 2010), whilst protective roles for IFN-γ have been suggested during some phases of demyelaniting autoimmune disorders (Kumar and Sercarz 1998). It is therefore difficult to determine whether IFN-γ has a pathogenic role in ASD, or if it represents a potentially beneficial immune response to damage associated with genetic and/or environmental influences.
Transforming Growth Factor-beta (TGF-Β)
TGF-Β is a highly pleiotropic cytokine that maintains immune homeostasis, directs lymphocyte differentiation, and orchestrates aspects of embryonic development. TGF-Β is largely immunosuppressive; limiting excessive T cell activity and inflammation (Mantel and Schmidt-Weber 2011). It exists in three isoforms, each with distinct and overlapping roles. TGF-Β1 is best characterized, and is the founding member of the TGF-Β superfamily of proteins, which includes growth differentiation factors, bone morphogenic proteins (BMPs), activins, and inhibins (Kingsley 1994). TGF-Β superfamily signaling occurs largely via SMAD pathways, though MAPK cascades are also triggered (Yu, Hebert et al. 2002; Shi and Massague 2003).
TGF-Β superfamily proteins are critical for proper neurodevelopment. For example, BMPs have an important role in early neural induction and differentiation (Reissmann, Ernsberger et al. 1996; Bachiller, Klingensmith et al. 2000; Tropepe, Hitoshi et al. 2001). TGF-Β1 is involved in neuronal migration, survival, and synapse formation. Mice lacking the cytokine demonstrate improper CNS development, including a disorganized extracellular matrix, widespread neuronal degeneration, microgliosis, reduced expression of synaptophysin, and deficits in both glutamatergic and GABAergic synapses (Brionne, Tesseur et al. 2003; Heupel, Sargsyan et al. 2008; Vashlishan, Madison et al. 2008). Overexpressing TGF-Β in vivo also disrupts the extracellular matrix, and leads to seizures, motor incoordination, hydrocephalus, and behavioral abnormalities (Wyss-Coray, Feng et al. 1995; Depino, Lucchina et al. 2011). Changes in CNS expression levels of IL-6, Neuroligin 3 and reelin are also observed with TGF- Β overexpression (Depino, Lucchina et al. 2011), which is intriguing because many of these proteins are altered in autism (Persico, D'Agruma et al. 2001; Laumonnier, Bonnet-Brilhault et al. 2004; Fatemi, Snow et al. 2005). Interestingly, CNS overexpression of TGF-Β during development vs. adulthood leads to opposite behavioral consequences (Depino, Lucchina et al. 2011). Early in life, overexpression led to decreased social behavior and heightened anxiety/depression behaviors, while overexpression later in life had the exact opposite effect. This highlights the importance of timing when considering the neurological consequences of cytokine imbalances.
There is no evidence for TGF-Β dysregulation during gestational development in autism. This doesn’t negate the possibility that it is involved, as these endpoints are extremely difficult to measure in vivo. However, there is evidence for TGF-Β dysregulation in individuals diagnosed with the disorder themselves. Plasma TGF-Β is decreased in children and adults with ASD (Okada, Hashimoto et al. 2007; Ashwood, Enstrom et al. 2008), and lower levels of the cytokine correlate with more severe autism behaviors (Ashwood, Enstrom et al. 2008). The connection between low TGF-Β and behavioral phenotype is unclear, although this finding lends promise to the goal of developing a simple ASD testing regime based on biological markers in addition to behavioral symptoms. In contrast to peripheral TGF-Β in ASD, postmortem brain specimens show increased levels compared to controls (Vargas, Nascimbene et al. 2005). The reason for this periphery/brain disparity is unclear. Although, TGF-Β does not cross the blood brain barrier (Kastin, Akerstrom et al. 2003), the cytokine and its receptors are expressed normally throughout the nervous system (Gomes, Sousa Vde et al. 2005) and it is conceivable that peripheral and brain levels are independent. In the adult brain, TGF-Β is generally thought to be neuroprotective. CNS levels spike following injury and infection, and increase with age, HIV dementia, and Alzheimer’s disease, which leads to protection against disease-related neural degeneration and apoptosis (Henrich-Noack, Prehn et al. 1994; Krupinski, Kumar et al. 1996; Ruocco, Nicole et al. 1999; Buckwalter and Wyss-Coray 2004; Doyle, Cekanaviciute et al. 2010). However, TGF-Β can also contribute to pathogenicity. For example, high levels of the cytokine cause glial scarring and fibrosis (Moon and Fawcett 2001), and increase disease susceptibility and severity in a murine model of multiple sclerosis (Wyss-Coray, Borrow et al. 1997).
Overall, the role of TGF-Β in the nervous system is complex, and varies based on the timing and context of the interaction. Increased TGF-Β in the brain of ASD subjects might represent a protective reflex to a disease state, or perhaps contribute to the pathology of the disease itself. In the periphery, ASD subjects have decreased TGF-Β as well as increased inflammatory markers (Jyonouchi, Sun et al. 2001; Croonenberghs, Bosmans et al. 2002; Emanuele, Orsi et al. 2010; Ashwood, Krakowiak et al. 2011; Ashwood, Krakowiak et al. 2011). This suggests a global immune dysregulation in ASD, with an improper balance between regulation and activation, which could have wide reaching consequences for many systems in the body.
Environmental factors in autism and immune dysfunction
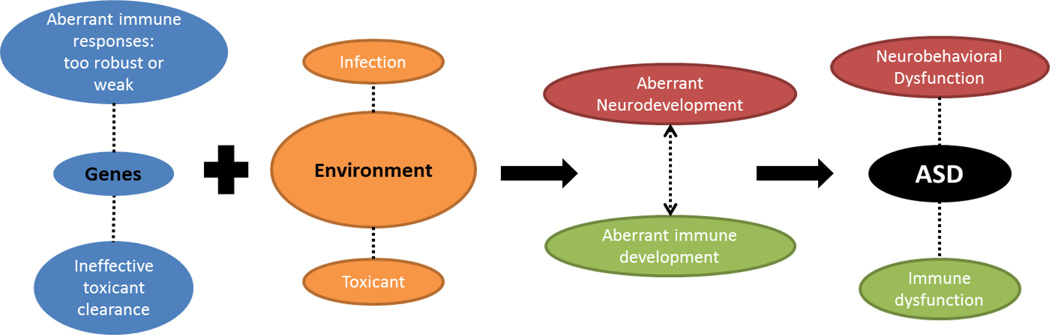
There is now general consensus that autism has an environmental component (Pessah 2008; Hallmayer, Cleveland et al. 2011). For our purposes, the “environment” is a broad term used to define non-genetic, toxic, infectious, and/or immune factors that may contribute to the disorder. The hypothesis follows that an ill-timed exposure could cause autism in genetically susceptible individuals (Figure 1). For example, some genes associated with autism may cause inappropriate immune responses (Heuer 2011; Onore, Careaga et al. 2011), while others reduce the capacity to deal appropriately with toxins (D'Amelio, Ricci et al. 2005). These individuals are more susceptible to environmental influences, which could lead to ASD.
Figure 1. Multifactorial model for ASD etiology involving genes, the environment, and immunity.
This model begins with a genetic background that predisposes an individual to respond inappropriately to environmental influences, like an infection or toxic exposure. An environmental exposure during a critical period could disrupt development of the nervous and immune system. This disruption could lead to ASD, as well as the neurological, behavioral, and immune dysfunctions observed in the disorder.
Environmental toxicants can cause both neural and immune dysfunction. This is likely mediated through disrupted cell signaling and homeostasis. Many toxicants alter calcium homeostasis, which can have a variety of consequences for immune development and function (Limke, Otero-Montanez et al. 2003; Toscano and Guilarte 2005; Savignac, Mellstrom et al. 2007; Vig and Kinet 2009; Pessah, Cherednichenko et al. 2010; Bhatti, Bhatti et al. 2011). Toxicants can also disrupt endocrine function, which can have a variety of immunological consequences (Rivest 2010; De Vito, Incerpi et al. 2011; Schug, Janesick et al. 2011). The following section considers environmental factors that may be related to autism, and focuses on their role in the immune system.
Heavy Metals
Heavy metals like lead and mercury are widespread environmental toxins. Developmental exposure to these compounds is associated with lower IQ, endocrine disruptions, and behavioral disturbances (Bellinger and Dietrich 1994; Steuerwald, Weihe et al. 2000; Cordier, Garel et al. 2002; Selevan, Rice et al. 2003; Winneke 2011). Heavy metals also have immunotoxic properties, leading in some instances to autoantibody production (Waterman, el-Fawal et al. 1994; Bagenstose, Salgame et al. 1999; Rowley and Monestier 2005) and skewed cytokine profiles (discussed below). Both of these immunological phenomena are observed in ASD (Enstrom, Van de Water et al. 2009; Onore, Careaga et al. 2011). These features have made them possible, though controversial, candidates in autism.
Heavy Metals: Lead
Lead has a variety of toxic mechanisms, and shares structural features with calcium which allows it to compete for binding sites (Toscano and Guilarte 2005). In addition to its neurotoxic activity, lead is highly immunotoxic (Toscano and Guilarte 2005; Mishra 2009). At high levels, it is immunosuppressive, leading to increased production of regulatory cytokines and enhanced susceptibility to infection (Valentino, Rapisarda et al. 2007). At lower levels, lead appears to be immunostimulatory (Flohe, Bruggemann et al. 2002). One study showed that lead can tip the balance between inflammation and regulation by increasing expression of IFN-γ and reducing TGF-Β (Goebel, Flohe et al. 2000): a cytokine profile which has been observed in autism (Singh 1996; Gupta, Aggarwal et al. 1998; Croonenberghs, Bosmans et al. 2002; Ashwood, Enstrom et al. 2008; Enstrom, Lit et al. 2009; Li, Chauhan et al. 2009). Lead and pro-inflammatory cytokines can function in concert to alter the nervous system. When coadministered to glial cells, lead and cytokines such as IL-1Β changed matrix metalloproteinase expression in a manner that was not observed when either was administered alone (Lahat, Shapiro et al. 2002) and suggests that immune and environmental factors could act synergistically on tissue remodeling.
There are conflicting reports regarding lead in autism. Higher serum lead levels have been documented in a few studies (Cohen, Johnson et al. 1976; Shannon and Graef 1996; Filipek, Accardo et al. 1999), although more recent studies show no difference between autism and control populations (Tian, Green et al. 2011; Albizzati, More et al. 2012). Polymorphisms in ALAD, a gene associated with heavy metal toxicity, have been described in ASD (Rose, Melnyk et al. 2008) leading, some authors to speculate that children with ASD have higher lead levels due to a decreased capacity to eliminate lead from the body (Kern, Grannemann et al. 2007). Others suggest increased frequency of pica (hand-to-mouth behaviors) in ASD may also alter exposures to lead (Cohen, Johnson et al. 1976; Shannon and Graef 1996). Despite lead’s well-established neurotoxic and immunotoxic mechanisms, it is currently debatable whether it contributes to autism.
Heavy Metals: Mercury
Mercury exerts its toxicity broadly throughout the body. It binds a wide range of molecular groups including thiols, hydroxyls, and carboxyls (Bridges and Zalups 2010), and can dramatically increase intracellular calcium levels (Sakamoto, Ikegami et al. 1996; Limke, Otero-Montanez et al. 2003). In the immune system, mercury’s effects depend heavily on genetic background. For example, certain strains of mice reliably develop anti-nuclear antibodies after mercury exposure, while others do not (Bagenstose, Salgame et al. 1999; Rowley and Monestier 2005). Mercury also impacts cytokine profiles; and some strains of mice strongly up-regulate the TH2 cytokine IL-4 in response to mercury, while other strains up-regulate TH1 cytokines like IFN-γ (Wu, Turner et al. 2001; Hemdan, Lehmann et al. 2007). In two human mast cell lines, mercury exposure was shown to increase IL-6 production (Kempuraj, Asadi et al. 2010). Further, mercury interferes with cytokine-related signaling cascades including NF-κB and p38 MAPK (Dieguez-Acuna, Ellis et al. 2001; Kim, Johnson et al. 2002). Altered cytokine repertoires in response to mercury exposure may contribute to the development of autoimmunity. Interestingly, IL-4 deficient mice produce autoantibodies in response to mercury exposure (Bagenstose, Salgame et al. 1998; Kono, Balomenos et al. 1998), while IFN-γ deficient mice do not (Kono, Balomenos et al. 1998), suggesting that autoantibody production is IFN-γ dependent.
The evidence implicating mercury in autism is somewhat contradictory. Several recent studies have shown no link between mercury body burdens and autism (Ip, Wong et al. 2004; Hertz-Picciotto, Green et al. 2010; Albizzati, More et al. 2012; Wright, Pearce et al. 2012). However, a reanalysis of data generated by Ip et al showed there was in fact an association between mercury levels and autism that had been overlooked due to a statistical error (Desoto and Hitlan 2007). Ethyl mercury is a component in the vaccine preservative thimerosal, which has received attention in recent years. It has neurotoxic capacities, and can alter calcium signaling and cytokine production (specifically IL-6) (Goth, Chu et al. 2006). While in vitro studies suggest toxic potential for thimerosal, a large number of independent epidemiological studies show no link to autism (DeStefano 2007; Miller and Reynolds 2009; Price, Thompson et al. 2010).
Two recent studies examined the correlation between gene transcription profiles (35,000+ genes) and heavy metal body burdens in children with autism and controls (Stamova, Green et al. 2011; Tian, Green et al. 2011). Mercury loads correlated with the expression of several immunologically relevant genes across all study participants. Further, there were some unique correlations in the autism group for genes involved in antigen presentation and recognition of self. This suggests that individuals with ASD may have a unique immunological susceptibility to heavy metals, but the significance of these findings is not clear. Although genomic expression profiles may suggest correlative changes in autism, analysis of common polymorphisms associated with mercury transport and excretion, namely MT1a, DMT1, LAT1, and MTF1, were unable to detect any differences in autism (Owens, Summar et al. 2011). However, in contrast, a different study showed that polymorphisms in MTF1 and the heavy metal transport gene (SLC11A3) are associated with the ASD(Serajee, Nabi et al. 2004). Additional genetic analyses are needed to rectify these disparate findings.
Pesticides
Pesticides are fairly non-persistent toxic compounds that are deliberately spread throughout the environment in mass quantities. While minimizing off-target toxicity is a primary goal in pesticide development, several products have been banned once their toxic potential in humans was recognized. Developmental exposure to several types of pesticides, including organophosphates (OPs), organochlorines (OCs), and pyrethroids, is associated with neurological dysfunction and an increased risk for ASD (Garry, Harkins et al. 2002; Kamel and Hoppin 2004; Eskenazi, Marks et al. 2007; Roberts, English et al. 2007; Eskenazi, Huen et al. 2010; Bouchard, Chevrier et al. 2011). Genetic analyses also suggest that individuals with ASD may be less capable of excreting pesticides, due to expression of a less-active variant of the OP-metabolizing enzyme paroxonase (D'Amelio, Ricci et al. 2005; Pasca, Nemes et al. 2006). In addition to their neurotoxicity, many pesticides impact the immune system and cytokine production, which may be relevant for ASD (Banerjee, Koner et al. 1996; Li 2007).
Pesticides: Organochlorines
Organochlorine (OC) pesticides are structurally and functionally variable toxic compounds that include members like hexachlorobenzene, dicofol, and DDT; many of which have been banned (Crinnion 2009). OCs interferes with calcium signaling, voltage sensitive sodium channels, and GABA receptors, leading to neuro- and immunotoxicity (Casida 2009; Crinnion 2009; Heusinkveld and Westerink 2012). Immunologically, these compounds impact both humoral and cell-mediated processes, and reduce the host response to infectious challenges (Banerjee, Koner et al. 1996; Reed, Dzon et al. 2004; Nagayama, Tsuji et al. 2007). A handful of studies have explored the impact of OCs on cytokine profiles. DDT reduced IL-2 production in cell culture by interfering with the transcription factor NF-κB (Ndebele, Tchounwou et al. 2004). Given the central role of NF-κB in cytokine production and function, this is likely to have a wide-ranging immunological impact. In contrast, cases of DDT or lindane poisoning in humans is associated with increased serum levels of IL-2, IL-4, and TNF-alpha, as well as decreased levels of IFN-γ (Daniel, Huber et al. 2002; Seth, Ahmad et al. 2005). It is not clear why IL-2 production in response to OCs differs in cell culture versus in vivo. However, the findings of increased IL-4 and decreased IFN-γ in humans suggest a TH2 immune bias following OC exposure, which could have downstream consequences for allergic and asthmatic disorders. Similar immune profiles have been reported in some studies of autism (Gupta, Aggarwal et al. 1998).
Pesticides: Organophosphates
Organophosphates (OP) are esters of phosphoric acid that were introduced as replacements for various banned OC pesticides. They act primarily through acetylcholinesterase (AChE) inhibition, leading to altered cholinergic signaling, parasympathetic and sympathetic perturbations, seizures, and/or respiratory arrest. OPs can also be toxic in the absence of AChE inhibition, and may induce higher order neural and cognitive dysfunction (Duysen, Li et al. 2001; Costa 2006; Pancetti, Olmos et al. 2007). Some OP developmental effects are more severe in males than females (Levin, Addy et al. 2001; Levin, Timofeeva et al. 2010), which is intriguing given that autism also has a heavy male bias (Baron-Cohen, Knickmeyer et al. 2005).
OPs induce a variety of immunological phenomena relevant to autism (Galloway and Handy 2003; Li 2007). In general, OPs appear to induce a prolonged inflammatory state that may evolve into an adaptive response characterized by up-regulation of both TH1 and TH2 cytokines. Acute OP intoxication is related to system-wide production of inflammatory mediators (Hamaguchi, Namera et al. 2006; Roeyen, Chapelle et al. 2008; Anand, Singh et al. 2009). Within the CNS, acute and chronic exposure to OPs results in increased inflammatory cytokines including IL-1Β and IL-6 in multiple brain regions (Svensson, Waara et al. 2001; Henderson, Barr et al. 2002; Williams, Berti et al. 2003; Dhote, Peinnequin et al. 2007; Dillman, Phillips et al. 2009; Johnson and Kan 2010); similar to findings in the autism brain (Vargas, Nascimbene et al. 2005; Li, Chauhan et al. 2009). OP induced inflammation can be long term, re-emerging and persisting long after the initial exposure (Chapman, Kadar et al. 2006). In primary cultures of human peripheral blood cells or astrocytes, the OP pesticide chlorpyrifos up-regulates IL-6 and IFN-γ production and the expression of related genes (Mense, Sengupta et al. 2006). Children born to mothers working in agriculture had higher production of TH2 cells at 12 and 24 months of age. (Duramad, Harley et al. 2006; Duramad, Tager et al. 2006). An immunosuppressive response can also be induced following exposure to various OPs, perhaps as a reflex to the toxin’s initial inflammatory properties (Williams, Berti et al. 2003; Damodaran, Greenfield et al. 2006; Dhote, Peinnequin et al. 2007). Collectively, these data suggest that OPs impact cytokine profiles in the short and long term, increasing inflammatory cytokines, TH1 and TH2 profiles and compensatory regulatory activity.
Pesticides: Pyrethroids
Pyrethroids are a group of insecticides and repellants derived from natural compounds in the Chrysanthemum genus of plants. They mediate their toxicity by disrupting calcium signaling, interfering with voltage sensitive sodium channels, and inducing oxidative stress (Shafer, Meyer et al. 2005; Soderlund 2012). Exposure to these compounds is associated with a wide range of neurodevelopmental problems in mammalian models (Shafer, Meyer et al. 2005; Wolansky and Harrill 2008). Immunological abnormalities are also linked to pyrethroids. In human peripheral blood mononuclear cells, exposure to several different pyrethroid compounds suppressed both IFN-γ and IL-4 expression in a time and concentration dependent manner (Diel, Horr et al. 2003). In a monocytic cell line, various synthetic pyrethroids and their metabolites reduced expression of immunoregulatory IL-10, and increased production of more inflammatory cytokines IL-12 and TNF-α (Zhang, Zhao et al. 2010). In a Xenopus laevis model, application of environmentally relevant concentrations of various pyrethroids increased IL-1Β expression (Martini, Fernandez et al. 2010). In primary human fetal astrocytes, the pyrethroid pesticide cyfluthrin was found to have an activating effect, and increased the expression of genes involved in IFN-γ and IL-6 production and signaling (Mense, Sengupta et al. 2006)
Halogenated Aromatic Hydrocarbons
Halogenated aromatic hydrocarbons are toxic compounds that are highly resistant to degradation. Two examples are polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs). PCBs and PBDEs consist of two aromatic rings with various chlorine (PCBs) or bromine (PBDEs) substitutions. There are over two hundred different congeners each of PCBs and PBDEs, which differ based on the number and orientation of halogen substitutions. Toxicity in this class of molecules is congener-specific, and involves varying degrees of 1) interaction with the Aryl hydrocarbon receptor (Ahr) (Mitchell and Elferink 2009; Gu, Goodarzi et al. 2012), 2) disruption of endocrine systems (Morse, Groen et al. 1993; Van Birgelen, Smit et al. 1995; Stoker, Cooper et al. 2005; Kuriyama, Wanner et al. 2007; Lema, Dickey et al. 2008), and/or 3) interference with calcium homeostasis through interactions with the ryanodine receptor (Coburn, Curras-Collazo et al. 2008; Pessah, Lehmler et al. 2009; Pessah, Cherednichenko et al. 2010; Kim, Bose et al. 2011; Langeveld, Meijer et al. 2012). These toxic mechanisms have both neurological and immunological significance.
Children with ASD may be uniquely susceptible to halogenated aromatic hydrocarbons. Postmortem analysis showed altered ryanodine receptor expression in the brain of autism subjects compared to controls, which could alter their sensitivity to Ryr-reactive compounds (Voineagu, Wang et al. 2011). Further, in a mouse model of Rett syndrome, a genetic disorder that shares features with autism, developmental exposure to PBDE-47 caused epigenetic, cognitive, and social differences that were not observed in wild type mice (Amir, Van den Veyver et al. 1999; Woods, Vallero et al. 2012). Finally, children with ASD have unique immune responses to PBDEs, which is discussed in detail below (Ashwood, Schauer et al. 2009).
Halogenated Aromatic Hydrocarbons: Polychlorinated Biphenyls (PCBs)
Polychlorinated biphenyls (PCBs) are ubiquitous in the environment and in animal and human tissues. They were widely used as additives to industrial oils and lubricants until the late 1970’s when their adverse health effects became apparent. PCB exposure is linked to adverse pregnancy outcomes and neurobehavioral deficits (Kuratsune, Yoshimura et al. 1971; Rogan, Gladen et al. 1988; Chen and Hsu 1994; Eriksson and Fredriksson 1998; Howard, Fitzpatrick et al. 2003; Kenet, Froemke et al. 2007; Lein, Yang et al. 2007; Tsukimori, Tokunaga et al. 2008; Kim, Inan et al. 2009; Boix, Cauli et al. 2010; Kim and Pessah 2011), as well as immune dysfunction. In studies involving marine animals, murine models, and humans, PCBs lead to generalized immune suppression, characterized by diminished cellular immunity and atrophied lymphoid organs (Davis and Safe 1990; Narayanan, Carter et al. 1998; Fournier, Degas et al. 2000; Shin, Bae et al. 2000; Tan, Li et al. 2003; Beineke, Siebert et al. 2005; Leijs, Koppe et al. 2009). Cytokine profiles are also impacted by PCBs. In a murine model, perinatal exposure to a PCB mixture induced inflammatory cytokine expression (primarily IL-6) in the brain of adult offspring (Hayley, Mangano et al. 2011). In human blood cells, PCB 52 and PCB 133 induced transcriptional changes in several cytokine signaling and regulation pathways (Wens, De Boever et al. 2011). This effect was only observed in cells from male donors, suggesting a male-bias for some PCB effects. Another study showed that PCB 118 enhanced IL-4 producing T-cell development (Gaspar-Ramirez, Perez-Vazquez et al. 2012), which correlates with findings in children with ASD and their mothers (Gupta, Aggarwal et al. 1998; Abdallah, Larsen et al. 2011; Goines, Croen et al. 2011).
PCBs likely exert their immunotoxicity by interfering with immunologically-relevant signaling pathways. For example, dioxin-like PCBs interfere with Ahr signaling, which is critical for maintaining a healthy immune balance in the skin and gut (Li, Innocentin et al. 2011; Monteleone, Rizzo et al. 2011). PCBs also disrupt the key cytokine signaling pathways JAK/STAT and MAPK. Immune cells exposed to PCB 47 and 153 have compromised function and altered STAT 5 and ERK phosphorylation (Canesi, Ciacci et al. 2003). STAT 5 activation is central for regulatory T cell development (Burchill, Goetz et al. 2003; Adamson, Collins et al. 2009), and activation of this molecule by PCBs might mediate some of their immunosuppressive effects. Finally, some PCB congeners interfere with ryanodine receptors, which are expressed widely in the immune system. These receptors are regulated by cytokines including TGF-Β (Hosoi, Nishizaki et al. 2001; Pessah, Cherednichenko et al. 2010), and can induce IL-6 production after engangement (Treves, Vukcevic et al. 2011). In summary, PCBs alter immune function through a variety of mechanisms that may be relevant to immune profiles observed in autism.
Halogenated Aromatic Hydrocarbons: Polybrominated Diphenyl Ethers (PBDEs)
PBDEs are a group of flame retardants that are the subject of growing concern due to their structural and functional similarities to PCBs. They are widely dispersed in the environment and bioaccumulate up the food chain (Johnson-Restrepo and Kannan 2009). Environmental PBDE levels have jumped dramatically in the last several decades, and are increasingly found in human tissues and breast milk (Noren and Meironyte 2000; Darnerud, Eriksen et al. 2001). Of note, this heightened prevalence is concurrent with the apparent rise in ASD diagnoses (Hertz-Picciotto and Delwiche 2009; Messer 2010). While not linked specifically to ASD, PBDE exposure is associated with improper neurodevelopment, hormonal disruptions, and a variety of behavioral, motor, and cognitive issues (Alm, Kultima et al. 2008; He, He et al. 2008; Roze, Meijer et al. 2009; Herbstman, Sjodin et al. 2010; Kodavanti, Coburn et al. 2010; Schreiber, Gassmann et al. 2010; Dingemans, van den Berg et al. 2011).
Some evidence shows that PBDEs can impact immune activity. Studies involving marine and murine models link PBDEs to changes in the immune system, including thymic and splenic atrophy, increased production of IL-10, lymphocyte depletion, reduced antibody recall responses, and decreased responses to pathogens (Fowles, Fairbrother et al. 1994; Thuvander 1999; Beineke, Siebert et al. 2005; Zhou, Chen et al. 2006; Beineke, Siebert et al. 2007; Beineke, Siebert et al. 2007; Lundgren, Darnerud et al. 2009; Watanabe, Shimizu et al. 2010; Bondy, Lefebvre et al. 2011; Fair, Stavros et al. 2012). There is little data regarding the impact of PBDEs on cytokine production and signaling, and future studies should examine these endpoints in more detail. One recent study considered the effect of BDE-47 on cytokine responses in children with autism and controls. Peripheral blood mononuclear cells obtained from ASD and typically developing controls were pretreated with PBDE-47 and stimulated with LPS. Analysis of supernatant cytokines showed that BDE-47 had a divergent impact on cells from ASD versus typical controls. Among controls, BDE-47 caused a significant decrease in inflammatory cytokines production (IL-6, IL-12, GM-CSF, TNF-α), indicating broad immune suppression. In contrast, children with ASD only down-regulated IL-6 in the presence of BDE-47, and had significantly higher IL-1Β responses (Ashwood, Schauer et al. 2009). This suggests that BDE-47 can enhance some aspects of inflammation in ASD. This diagnosis-specific immunotoxic effect suggests that children with ASD respond differently to PBDEs than typical children. Robust inflammation in response to such exposures during critical developmental windows could have long term neurological effects, and may be a possible mechanism in ASD. Future studies should continue to examine a potential role for PBDEs in immune dysfunction and neurodevelopmental disorders like autism.
Pathogenic exposures in autism
Early-life infections can skew fetal development, leading to aberrant neural and immune activity. This is a widely suggested etiological mechanism in schizophrenia, and is increasingly implicated in autism as well (Patterson 2009). Several infections, including measles, cytomegalovirus, and rubella during preand perinatal periods have been associated with autism (Chess 1977; Markowitz 1983; Ivarsson, Bjerre et al. 1990; Sweeten, Posey et al. 2004; Libbey, Sweeten et al. 2005; Meyer, Feldon et al. 2011). A recent large-scale epidemiological study showed that infection-related hospitalizations during pregnancy significantly increased the risk of ASD (Atladottir, Thorsen et al. 2010). Interestingly, the risk was not associated with any specific type of infection, suggesting that the general immune mechanism controlling the response to the pathogen rather than the pathogen itself were involved. This response is likely / guaranteed to include cytokines. Indeed, there is clinical evidence for altered gestational cytokine milieus in autism (Abdallah, Larsen et al. 2011; Goines, Croen et al. 2011), which could be related to infectious exposures, and may mediate aspects of the disorder.
As discussed in previous sections, several studies have linked early immune challenges to long-term changes in behavioral and immune parameters. For example, mice prenatally exposed to influenza (Fatemi, Earle et al. 2002; Shi, Fatemi et al. 2003), the viral mimic Poly I:C (Vuillermot, Weber et al. 2010), or the bacterial component LPS (Romero, Guaza et al. 2010) demonstrate long term neurological and behavioral abnormalities. This effect is largely mediated by cytokines like IL-6 and IL-1Β (Samuelsson, Jennische et al. 2006; Smith, Li et al. 2007; Bilbo, Barrientos et al. 2008). Prenatal infectious exposures can also impact the developing immune system, and lead to long term immune dysregulation. This was demonstrated by increased levels of IL-6, IL-2, and TNF-α in adult animals that had been prenatally exposed to LPS (Romero, Guaza et al. 2010). Another interesting set of studies showed that an early life infection led to neurological deficits in adulthood that only became apparent after an immunological stimulation (Bilbo, Biedenkapp et al. 2005; Bilbo, Levkoff et al. 2005; Bilbo and Schwarz 2009). This suggests that early life infection can change immune responses later in life, and that this has neurological consequences. The concept that prenatal infections can impact both brain and immune development is intriguing, and should be explored further in autism.
Overall, when considering environmental exposures, it is important to take time to consider that the prevalence rates for ASD have increased dramatically over the last 10–20 years. These rates continue to increase year-on-year. Arguably concentrations for some compounds such as lead and chlorinated pesticides have fallen in the population since their removal or reduction from the environment in the 1970’s. In contrast, levels of PBDEs and Ops have increased. Although it may be tempting to link compounds as risk factors for ASD based solely on similar time trends there is a need for more extensive research to begin to understand whether such temporal relationships are associated with risk for ASD. Future research should focus on the relationships between environmental exposures and risk for ASD diagnosis and whether environmental exposures to such compounds as PBDE induce cytokine responses that could modulate neuronal function in the pediatric population. Moreover, future research should discern whether children who develop a ASD are more sensitive to specific environmental exposures using cytokine production as readouts. More research focused on environmental exposures and ASD is warranted.
Conclusions
Cytokine imbalances are well documented in autism and have many interesting implications. Cytokines are intricately involved in neurodevelopment and neuronal function, and an ill-timed cytokine disruption can have long term neurological consequences. Further, cytokine expression is largely dependent on genetic and environmental influences. Therefore, they may represent a biomarker for genetic or environmental factors at play in autism. To illustrate the connection between immunity, genes, the environment, and neurodevelopmental outcome, consider two scenarios: First, an individual may be genetically poised to mount an inappropriate immune response to an infectious or toxic exposure. This individual might respond either too robustly or too weakly to resolve the threat without collateral damage to the brain and other body systems (including the immune system). Second, an individual may lack appropriate genetic machinery to excrete toxins; leading to their accumulation in tissue. This could lead to an amplification of the toxin’s effects on a variety of body systems, including the brain and immune system. For each child, an environmental challenge during a critical window of development could have especially severe consequences, causing abnormal CNS function, altered immune phenotypes, and perhaps autism. These scenarios represent an emerging global view of autism that considers a broad contribution of several factors, including genes, the environment, and the immune system. Cross-disciplinary investigations that consider diverse biological contributions will be essential to untangle ASD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors report no conflict of interest.
References
- Aaltonen R, Heikkinen T, et al. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106(4):802–807. doi: 10.1097/01.AOG.0000178750.84837.ed. [DOI] [PubMed] [Google Scholar]
- Abdallah MW, Larsen N, et al. Amniotic fluid inflammatory cytokines: Potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- Adamson AS, Collins K, et al. The Current STATus of lymphocyte signaling: new roles for old players. Curr Opin Immunol. 2009;21(2):161–166. doi: 10.1016/j.coi.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizzati A, More L, et al. Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr. 2012;64(1):27–31. [PubMed] [Google Scholar]
- Alleva E, Cirulli F, et al. Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur J Neurosci. 1998;10(12):3664–3672. doi: 10.1046/j.1460-9568.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- Alm H, Kultima K, et al. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology. 2008;29(4):628–637. doi: 10.1016/j.neuro.2008.04.021. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Amir RE, Van den Veyver IB, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Anand S, Singh S, et al. Cardiac abnormalities in acute organophosphate poisoning. Clin Toxicol (Phila) 2009;47(3):230–235. doi: 10.1080/15563650902724813. [DOI] [PubMed] [Google Scholar]
- Andres DA, Shi GX, et al. Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation. J Neurochem. 2008;107(5):1436–1447. doi: 10.1111/j.1471-4159.2008.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600(1):49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- Armario A, Hernandez J, et al. IL-6 deficiency leads to increased emotionality in mice: evidence in transgenic mice carrying a null mutation for IL-6. Journal of Neuroimmunology. 1998;92(1–2):160–169. doi: 10.1016/s0165-5728(98)00199-4. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Anthony A, et al. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24(6):664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, et al. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204(1–2):149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, et al. Altered T cell responses in children with autism. Brain Behav Immun. 2011;25(5):840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, et al. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol. 2009;208(1–2):130–135. doi: 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403(6770):658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Bagenstose LM, Salgame P, et al. Mercury-induced autoimmunity in the absence of IL-4. Clin Exp Immunol. 1998;114(1):9–12. doi: 10.1046/j.1365-2249.1998.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagenstose LM, Salgame P, et al. Murine mercury-induced autoimmunity: a model of chemically related autoimmunity in humans. Immunol Res. 1999;20(1):67–78. doi: 10.1007/BF02786508. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, et al. Interleukin-6: a cytokine to forget. FASEB J. 2004;18(14):1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Banerjee BD, Koner BC, et al. Immunotoxicity of pesticides: perspectives and trends. Indian J Exp Biol. 1996;34(8):723–733. [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, et al. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179(1–2):53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Ortiz L, et al. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991;259(3):988–996. [PubMed] [Google Scholar]
- Barcia C, Ros CM, et al. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis. 2011;2:e142. doi: 10.1038/cddis.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish ME, Mansdorf NB, et al. Gamma-interferon promotes differentiation of cultured cortical and hippocampal neurons. Dev Biol. 1991;144(2):412–423. doi: 10.1016/0012-1606(91)90433-4. [DOI] [PubMed] [Google Scholar]
- Barksby HE, Lea SR, et al. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149(2):217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, et al. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, et al. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, et al. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8(3):221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Beineke A, Siebert U, et al. Investigations of the potential influence of environmental contaminants on the thymus and spleen of harbor porpoises (Phocoena phocoena) Environ Sci Technol. 2005;39(11):3933–3938. doi: 10.1021/es048709j. [DOI] [PubMed] [Google Scholar]
- Beineke A, Siebert U, et al. Increased blood interleukin-10 mRNA levels in diseased free-ranging harbor porpoises (Phocoena phocoena) Vet Immunol Immunopathol. 2007;115(1–2):100–106. doi: 10.1016/j.vetimm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Beineke A, Siebert U, et al. Phenotypical characterization of changes in thymus and spleen associated with lymphoid depletion in free-ranging harbor porpoises (Phocoena phocoena) Vet Immunol Immunopathol. 2007;117(3–4):254–265. doi: 10.1016/j.vetimm.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Dietrich KN. Low-level lead exposure and cognitive function in children. Pediatr Ann. 1994;23(11):600–605. doi: 10.3928/0090-4481-19941101-08. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba SG, et al. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neurosci Lett. 1995;198(2):95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- Bhatti GK, Bhatti JS, et al. Alterations in Ca(2) homeostasis and oxidative damage induced by ethion in erythrocytes of Wistar rats: ameliorative effect of vitamin E. Environ Toxicol Pharmacol. 2011;31(3):378–386. doi: 10.1016/j.etap.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94(1):57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, et al. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22(4):451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, et al. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25(35):8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, et al. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119(1):293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U, Klamp T, et al. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Boix J, Cauli O, et al. Developmental exposure to polychlorinated biphenyls 52, 138 or 180 affects differentially learning or motor coordination in adult rats. Mechanisms involved. Neuroscience. 2010;167(4):994–1003. doi: 10.1016/j.neuroscience.2010.02.068. [DOI] [PubMed] [Google Scholar]
- Bondy GS, Lefebvre DE, et al. Toxicologic and immunologic effects of perinatal exposure to the brominated diphenyl ether (BDE) mixture DE-71 in the Sprague-Dawley rat. Environ Toxicol. 2011 doi: 10.1002/tox.20713. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Sacerdote P, et al. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res. 2004;153(2):423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13(5):385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, et al. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40(6):1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Wyss-Coray T. Modelling neuroinflammatory phenotypes in vivo. J Neuroinflammation. 2004;1(1):10. doi: 10.1186/1742-2094-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Goetz CA, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171(11):5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest. 2011;140(3):768–774. doi: 10.1378/chest.10-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90(21):10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, et al. Effects of PCB congeners on the immune function of Mytilus hemocytes: alterations of tyrosine kinase-mediated cell signaling. Aquat Toxicol. 2003;63(3):293–306. doi: 10.1016/s0166-445x(02)00186-8. [DOI] [PubMed] [Google Scholar]
- Casida JE. Pest toxicology: the primary mechanisms of pesticide action. Chem Res Toxicol. 2009;22(4):609–619. doi: 10.1021/tx8004949. [DOI] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Chakrabarty P, Ceballos-Diaz C, et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol. 2010;184(9):5333–5343. doi: 10.4049/jimmunol.0903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Kadar T, et al. Seizure duration following sarin exposure affects neuroinflammatory markers in the rat brain. Neurotoxicology. 2006;27(2):277–283. doi: 10.1016/j.neuro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Hsu CC. Effects of prenatal exposure to PCBs on the neurological function of children: a neuropsychological and neurophysiological study. Dev Med Child Neurol. 1994;36(4):312–320. doi: 10.1111/j.1469-8749.1994.tb11851.x. [DOI] [PubMed] [Google Scholar]
- Chess S. Follow-up report on autism in congenital rubella. J Autism Child Schizophr. 1977;7(1):69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, et al. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem Res. 2008;33(2):355–364. doi: 10.1007/s11064-007-9430-x. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Johnson WT, et al. Pica and elevated blood lead level in autistic and atypical children. Am J Dis Child. 1976;130(1):47–48. doi: 10.1001/archpedi.1976.02120020049007. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Kelly D, et al. Targeted CNS expression of interferon-gamma in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development. Mol Cell Neurosci. 1996;7(5):354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- Cordier S, Garel M, et al. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Res. 2002;89(1):1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366(1–2):1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. Chlorinated pesticides: threats to health and importance of detection. Altern Med Rev. 2009;14(4):347–359. [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, et al. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45(1):1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- D'Amelio M, Ricci I, et al. Paraoxonase gene variants are associated with autism in North America, but not in Italy: possible regional specificity in gene-environment interactions. Mol Psychiatry. 2005;10(11):1006–1016. doi: 10.1038/sj.mp.4001714. [DOI] [PubMed] [Google Scholar]
- da Silva AG, Campello-Costa P, et al. Interleukin-4 blocks proliferation of retinal progenitor cells and increases rod photoreceptor differentiation through distinct signaling pathways. J Neuroimmunol. 2008;196(1–2):82–93. doi: 10.1016/j.jneuroim.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, et al. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60(2):147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Damodaran TV, Greenfield ST, et al. Toxicogenomic studies of the rat brain at an early time point following acute sarin exposure. Neurochem Res. 2006;31(3):367–381. doi: 10.1007/s11064-005-9023-5. [DOI] [PubMed] [Google Scholar]
- Daniel V, Huber W, et al. Associations of dichlorodiphenyltrichloroethane (DDT) 4.4 and dichlorodiphenyldichloroethylene (DDE) 4.4 blood levels with plasma IL-4. Arch Environ Health. 2002;57(6):541–547. doi: 10.1080/00039890209602086. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15(1):7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, et al. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Safe S. Immunosuppressive activities of polychlorinated biphenyls in C57BL/6N mice: structure-activity relationships as Ah receptor agonists and partial antagonists. Toxicology. 1990;63(1):97–111. doi: 10.1016/0300-483x(90)90072-o. [DOI] [PubMed] [Google Scholar]
- de la Mano A, Gato A, et al. Role of interleukin-1beta in the control of neuroepithelial proliferation and differentiation of the spinal cord during development. Cytokine. 2007;37(2):128–137. doi: 10.1016/j.cyto.2007.03.004. [DOI] [PubMed] [Google Scholar]
- De Simone R, Levi G, et al. Interferon gamma gene expression in rat central nervous system glial cells. Cytokine. 1998;10(6):418–422. doi: 10.1006/cyto.1997.0314. [DOI] [PubMed] [Google Scholar]
- De Vito P, Incerpi S, et al. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21(8):879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- DeFelice ML, Ruchelli ED, et al. Intestinal cytokines in children with pervasive developmental disorders. Am J Gastroenterol. 2003;98(8):1777–1782. doi: 10.1111/j.1572-0241.2003.07593.x. [DOI] [PubMed] [Google Scholar]
- Depino AM, Lucchina L, et al. Early and adult hippocampal TGF-beta1 overexpression have opposite effects on behavior. Brain Behav Immun. 2011;25(8):1582–1591. doi: 10.1016/j.bbi.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoto MC, Hitlan RT. Blood levels of mercury are related to diagnosis of autism: a reanalysis of an important data set. J Child Neurol. 2007;22(11):1308–1311. doi: 10.1177/0883073807307111. [DOI] [PubMed] [Google Scholar]
- DeStefano F. Vaccines and autism: evidence does not support a causal association. Clin Pharmacol Ther. 2007;82(6):756–759. doi: 10.1038/sj.clpt.6100407. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dhote F, Peinnequin A, et al. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology. 2007;238(2–3):166–176. doi: 10.1016/j.tox.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Dieguez-Acuna FJ, Ellis ME, et al. Mercuric ion attenuates nuclear factor-kappaB activation and DNA binding in normal rat kidney epithelial cells: implications for mercury-induced nephrotoxicity. Toxicol Appl Pharmacol. 2001;173(3):176–187. doi: 10.1006/taap.2001.9195. [DOI] [PubMed] [Google Scholar]
- Diel F, Horr B, et al. Pyrethroid insecticides influence the signal transduction in T helper lymphocytes from atopic and nonatopic subjects. Inflamm Res. 2003;52(4):154–163. doi: 10.1007/s000110300066. [DOI] [PubMed] [Google Scholar]
- Dillman FJ, 3rd, Phillips CS, et al. Gene expression profiling of rat hippocampus following exposure to the acetylcholinesterase inhibitor soman. Chem Res Toxicol. 2009;22(4):633–638. doi: 10.1021/tx800466v. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, et al. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119(7):900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, et al. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DJ, Hunter C, et al. Elevation of amniotic fluid interleukin-4 concentrations in women with preterm labor and chorioamnionitis. Am J Perinatol. 1996;13(7):443–447. doi: 10.1055/s-2007-994385. [DOI] [PubMed] [Google Scholar]
- Duramad P, Harley K, et al. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114(12):1916–1922. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duramad P, Tager IB, et al. Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1. J Appl Toxicol. 2006;26(5):458–465. doi: 10.1002/jat.1162. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, et al. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther. 2001;299(2):528–535. [PubMed] [Google Scholar]
- Emanuele E, Orsi P, et al. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471(3):162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Enstrom AM, Lit L, et al. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009;23(1):124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, et al. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24(1):64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Van de Water JA, et al. Autoimmunity in autism. Curr Opin Investig Drugs. 2009;10(5):463–473. [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Fredriksson A. Neurotoxic effects in adult mice neonatally exposed to 3,3'4,4'5-pentachlorobiphenyl or 2,3,3'4,4'-pentachlorobiphenyl. Changes in brain nicotinic receptors and behaviour. Environ Toxicol Pharmacol. 1998;5(1):17–27. doi: 10.1016/s1382-6689(97)10002-3. [DOI] [PubMed] [Google Scholar]
- Escary JL, Perreau J, et al. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993;363(6427):361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, et al. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ Health Perspect. 2010;118(12):1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair PA, Stavros HC, et al. Immune function in female B(6)C(3)F(1) mice is modulated by DE-71, a commercial polybrominated diphenyl ether mixture. J Immunotoxicol. 2012;9(1):96–107. doi: 10.3109/1547691X.2011.643418. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22(1):25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, et al. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40(6):743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Snow AV, et al. Reelin signaling is impaired in autism. Biol Psychiatry. 2005;57(7):777–787. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29(6):439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- Flohe SB, Bruggemann J, et al. Enhanced proinflammatory response to endotoxin after priming of macrophages with lead ions. J Leukoc Biol. 2002;71(3):417–424. [PubMed] [Google Scholar]
- Folkerth RD, Keefe RJ, et al. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 2004;14(3):265–274. doi: 10.1111/j.1750-3639.2004.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Degas V, et al. Immunosuppression in mice fed on diets containing beluga whale blubber from the St Lawrence estuary and the Arctic populations. Toxicol Lett. 2000;112–113:311–317. doi: 10.1016/s0378-4274(99)00241-6. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Fairbrother A, et al. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology. 1994;86(1–2):49–61. doi: 10.1016/0300-483x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Res. 1994;637(1–2):10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Identification of interleukin-6 (IL-6)-expressing neurons in the cerebellum and hippocampus of normal adult rats. Neurosci Lett. 1994;182(2):243–246. doi: 10.1016/0304-3940(94)90807-9. [DOI] [PubMed] [Google Scholar]
- Galloway T, Handy R. Immunotoxicity of organophosphorous pesticides. Ecotoxicology. 2003;12(1–4):345–363. doi: 10.1023/a:1022579416322. [DOI] [PubMed] [Google Scholar]
- Garg SK, Kipnis J, et al. IFN-gamma and IL-4 differentially shape metabolic responses and neuroprotective phenotype of astrocytes. J Neurochem. 2009;108(5):1155–1166. doi: 10.1111/j.1471-4159.2009.05872.x. [DOI] [PubMed] [Google Scholar]
- Garry VF, Harkins ME, et al. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Perspect. 2002;110(Suppl 3):441–449. doi: 10.1289/ehp.02110s3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Ramirez O, Perez-Vazquez FJ, et al. Effect of polychlorinated biphenyls 118 and 153 on Th1/Th2 cells differentiation. Immunopharmacol Immunotoxicol. 2012 doi: 10.3109/08923973.2011.648265. [DOI] [PubMed] [Google Scholar]
- Giulian D, Young DG, et al. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8(2):709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel C, Flohe SB, et al. Orally administered lead chloride induces bias of mucosal immunity. Cytokine. 2000;12(9):1414–1418. doi: 10.1006/cyto.2000.0708. [DOI] [PubMed] [Google Scholar]
- Goines P, Haapanen L, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25(3):514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Croen LA, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FC, Sousa Vde O, et al. Emerging roles for TGF-beta1 in nervous system development. Int J Dev Neurosci. 2005;23(5):413–424. doi: 10.1016/j.ijdevneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8–10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Goth SR, Chu RA, et al. Uncoupling of ATP-mediated calcium signaling and dysregulated interleukin-6 secretion in dendritic cells by nanomolar thimerosal. Environ Health Perspect. 2006;114(7):1083–1091. doi: 10.1289/ehp.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, et al. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54(2):276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Han SS, et al. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122(2):e438–e445. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Goodarzi M, et al. Predictive insight into the relationship between AhR binding property and toxicity of polybrominated diphenyl ethers by PLS-derived QSAR. Toxicol Lett. 2012;208(3):269–274. doi: 10.1016/j.toxlet.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, et al. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85(1):106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Namera A, et al. Severe acute pancreatitis caused by organophosphate poisoning. Chudoku Kenkyu. 2006;19(4):395–399. [PubMed] [Google Scholar]
- Handley MT, Lian LY, et al. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS One. 2010;5(5):e10534. doi: 10.1371/journal.pone.0010534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang CH, Shi JX, et al. Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res. 2004;1026(1):23–32. doi: 10.1016/j.brainres.2004.07.090. [DOI] [PubMed] [Google Scholar]
- Hayley S, Mangano E, et al. An in vivo animal study assessing long-term changes in hypothalamic cytokines following perinatal exposure to a chemical mixture based on Arctic maternal body burden. Environ Health. 2011;10:65. doi: 10.1186/1476-069X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, He W, et al. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008;29(1):124–129. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemdan NY, Lehmann I, et al. Immunomodulation by mercuric chloride in vitro: application of different cell activation pathways. Clin Exp Immunol. 2007;148(2):325–337. doi: 10.1111/j.1365-2249.2007.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RF, Barr EB, et al. Response of rats to low levels of sarin. Toxicol Appl Pharmacol. 2002;184(2):67–76. [PubMed] [Google Scholar]
- Henrich-Noack P, Prehn JH, et al. Neuroprotective effects of TGF-beta 1. J Neural Transm Suppl. 1994;43:33–45. [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Green PG, et al. Blood mercury concentrations in CHARGE Study children with and without autism. Environ Health Perspect. 2010;118(1):161–166. doi: 10.1289/ehp.0900736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer LBD, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Translational Psychiatry. 2011;1 doi: 10.1038/tp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heupel K, Sargsyan V, et al. Loss of transforming growth factor-beta 2 leads to impairment of central synapse function. Neural Dev. 2008;3:25. doi: 10.1186/1749-8104-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinkveld HJ, Westerink RH. Organochlorine insecticides lindane and dieldrin and their binary mixture disturb calcium homeostasis in dopaminergic PC12 cells. Environ Sci Technol. 2012;46(3):1842–1848. doi: 10.1021/es203303r. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, et al. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94(4):1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi E, Nishizaki C, et al. Expression of the ryanodine receptor isoforms in immune cells. J Immunol. 2001;167(9):4887–4894. doi: 10.4049/jimmunol.167.9.4887. [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, et al. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190(1):72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25(4):604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Roy SK, et al. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J Biol Chem. 2001;276(1):287–297. doi: 10.1074/jbc.M004885200. [DOI] [PubMed] [Google Scholar]
- Huell M, Strauss S, et al. Interleukin-6 is present in early stages of plaque formation and is restricted to the brains of Alzheimer's disease patients. Acta Neuropathol. 1995;89(6):544–551. doi: 10.1007/BF00571510. [DOI] [PubMed] [Google Scholar]
- Ihara S, Nakajima K, et al. Dual control of neurite outgrowth by STAT3 and MAP kinase in PC12 cells stimulated with interleukin-6. EMBO J. 1997;16(17):5345–5352. doi: 10.1093/emboj/16.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip P, Wong V, et al. Mercury exposure in children with autistic spectrum disorder: case-control study. J Child Neurol. 2004;19(6):431–434. doi: 10.1177/088307380401900606. [DOI] [PubMed] [Google Scholar]
- Ivarsson SA, Bjerre I, et al. Autism as one of several disabilities in two children with congenital cytomegalovirus infection. Neuropediatrics. 1990;21(2):102–103. doi: 10.1055/s-2008-1071471. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Roberts J, et al. Evidence of a T(H) 1 type response associated with recurrent miscarriage. Fertil Steril. 2000;73(6):1206–1208. doi: 10.1016/s0015-0282(00)00517-3. [DOI] [PubMed] [Google Scholar]
- Jiang J, Tian K, et al. Expression of TNF alpha, IL-1 beta, IL-6 mRNA, release of TNF alpha in vital organs and their relationship with endotoxin translocation following hemorrhagic shock. Chin Med Sci J. 1997;12(1):41–46. [PubMed] [Google Scholar]
- Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009 doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Kan RK. The acute phase response and soman-induced status epilepticus: temporal, regional and cellular changes in rat brain cytokine concentrations. J Neuroinflammation. 2010;7:40. doi: 10.1186/1742-2094-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonakait GM, Wei R, et al. Interferon-gamma promotes cholinergic differentiation of embryonic septal nuclei and adjacent basal forebrain. Neuron. 1994;12(5):1149–1159. doi: 10.1016/0896-6273(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, et al. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120(1–2):170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112(9):950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, et al. Circulating TGF-beta1 does not cross the intact blood-brain barrier. J Mol Neurosci. 2003;21(1):43–48. doi: 10.1385/JMN:21:1:43. [DOI] [PubMed] [Google Scholar]
- Katila H, Hanninen K, et al. Polymorphisms of the interleukin-1 gene complex in schizophrenia. Mol Psychiatry. 1999;4(2):179–181. doi: 10.1038/sj.mp.4000483. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Asadi S, et al. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, et al. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci U S A. 2007;104(18):7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Grannemann BD, et al. Sulfhydryl-reactive metals in autism. J Toxicol Environ Health A. 2007;70(8):715–721. doi: 10.1080/15287390601188060. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Beck HN, et al. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J Neurosci. 2002;22(11):4530–4539. doi: 10.1523/JNEUROSCI.22-11-04530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bose DD, et al. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect. 2011;119(4):519–526. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Inan SY, et al. Excitatory and inhibitory synaptic transmission is differentially influenced by two ortho-substituted polychlorinated biphenyls in the hippocampal slice preparation. Toxicol Appl Pharmacol. 2009;237(2):168–177. doi: 10.1016/j.taap.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Pessah IN. Perinatal exposure to environmental polychlorinated biphenyls sensitizes hippocampus to excitotoxicity ex vivo. Neurotoxicology. 2011;32(6):981–985. doi: 10.1016/j.neuro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Johnson VJ, et al. Mercury inhibits nitric oxide production but activates proinflammatory cytokine expression in murine macrophage: differential modulation of NF-kappaB and p38 MAPK signaling pathways. Nitric Oxide. 2002;7(1):67–74. doi: 10.1016/s1089-8603(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Okuyama S, et al. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24(8):3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Coburn CG, et al. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116(1):297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- Kono DH, Balomenos D, et al. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol. 1998;161(1):234–240. [PubMed] [Google Scholar]
- Kronborg CS, Gjedsted J, et al. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 2011;90(7):791–796. doi: 10.1111/j.1600-0412.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, et al. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27(5):852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sercarz E. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J Immunol. 1998;161(12):6585–6591. [PubMed] [Google Scholar]
- Kuperman DA, Schleimer RP. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr Mol Med. 2008;8(5):384–392. doi: 10.2174/156652408785161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratsune M, Yoshimura T, et al. Yusho, a poisoning caused by rice oil contaminated with polychlorinated biphenyls. HSMHA Health Rep. 1971;86(12):1083–1091. [PMC free article] [PubMed] [Google Scholar]
- Kuriyama SN, Wanner A, et al. Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology. 2007;242(1–3):80–90. doi: 10.1016/j.tox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kushima Y, Hama T, et al. Interleukin-6 as a neurotrophic factor for promoting the survival of cultured catecholaminergic neurons in a chemically defined medium from fetal and postnatal rat midbrains. Neurosci Res. 1992;13(4):267–280. doi: 10.1016/0168-0102(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Costes L, et al. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS One. 2009;4(6):e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat N, Shapiro S, et al. Inorganic lead enhances cytokine-induced elevation of matrix metalloproteinase MMP-9 expression in glial cells. J Neuroimmunol. 2002;132(1–2):123–128. doi: 10.1016/s0165-5728(02)00323-5. [DOI] [PubMed] [Google Scholar]
- Langeveld WT, Meijer M, et al. Differential effects of 20 non-dioxin-like PCBs on basal and depolarisation-evoked intracellular calcium levels in PC12 cells. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfr346. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74(3):552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Zachary AA, et al. HLA-DR4 in families with autism. Pediatr Neurol. 2006;35(5):303–307. doi: 10.1016/j.pediatrneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Leijs MM, Koppe JG, et al. Effects of dioxins, PCBs, and PBDEs on immunology and hematology in adolescents. Environ Sci Technol. 2009;43(20):7946–7951. doi: 10.1021/es901480f. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Yang D, et al. Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect. 2007;115(4):556–563. doi: 10.1289/ehp.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig ND, Xu C, et al. Functional immobilization of interferon-gamma induces neuronal differentiation of neural stem cells. J Biomed Mater Res A. 2010;93(2):625–633. doi: 10.1002/jbm.a.32573. [DOI] [PubMed] [Google Scholar]
- Lema SC, Dickey JT, et al. Dietary exposure to 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ Health Perspect. 2008;116(12):1694–1699. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, et al. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res Dev Brain Res. 2001;130(1):83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, et al. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res. 2010;208(2):319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Katafuchi T, et al. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997;748(1–2):30–38. doi: 10.1016/s0006-8993(96)01283-8. [DOI] [PubMed] [Google Scholar]
- Li L, Walker TL, et al. Endogenous interferon gamma directly regulates neural precursors in the non-inflammatory brain. J Neurosci. 2010;30(27):9038–9050. doi: 10.1523/JNEUROSCI.5691-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. New mechanism of organophosphorus pesticide-induced immunotoxicity. J Nihon Med Sch. 2007;74(2):92–105. doi: 10.1272/jnms.74.92. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207(1–2):111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Innocentin S, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Sweeten TL, et al. Autistic disorder and viral infections. J Neurovirol. 2005;11(1):1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- Limke TL, Otero-Montanez JK, et al. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J Pharmacol Exp Ther. 2003;304(3):949–958. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16(5):932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, et al. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lundgren M, Darnerud PO, et al. Polybrominated diphenyl ether exposure suppresses cytokines important in the defence to coxsackievirus B3 infection in mice. Toxicol Lett. 2009;184(2):107–113. doi: 10.1016/j.toxlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Malik M, Sheikh AM, et al. Expression of inflammatory cytokines, Bcl2 and cathepsin D are altered in lymphoblasts of autistic subjects. Immunobiology. 2011;216(1–2):80–85. doi: 10.1016/j.imbio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, et al. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano EN, Litteljohn D, et al. Interferon-gamma plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Mantel PY, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol. 2011;677:303–338. doi: 10.1007/978-1-60761-869-0_21. [DOI] [PubMed] [Google Scholar]
- Markowitz PI. Autism in a child with congenital cytomegalovirus infection. J Autism Dev Disord. 1983;13(3):249–253. doi: 10.1007/BF01531564. [DOI] [PubMed] [Google Scholar]
- Martini F, Fernandez C, et al. Assessment of potential immunotoxic effects caused by cypermethrin, fluoxetine, and thiabendazole using heat shock protein 70 and interleukin-1beta mRNA expression in the anuran Xenopus laevis. Environ Toxicol Chem. 2010;29(11):2536–2543. doi: 10.1002/etc.313. [DOI] [PubMed] [Google Scholar]
- Martins TB, Rose JW, et al. Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassay. Am J Clin Pathol. 2011;136(5):696–704. doi: 10.1309/AJCP7UBK8IBVMVNR. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, et al. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21(18):7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, et al. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol Sci. 2006;93(1):125–135. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- Messer A. Mini-review: polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol Behav. 2010;100(3):245–249. doi: 10.1016/j.physbeh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, et al. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69(5 Pt 2):26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Reynolds J. Autism and vaccination-the current evidence. J Spec Pediatr Nurs. 2009;14(3):166–172. doi: 10.1111/j.1744-6155.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- Mishra KP. Lead exposure and its impact on immune system: a review. Toxicol In Vitro. 2009;23(6):969–972. doi: 10.1016/j.tiv.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Mitchell KA, Elferink CJ. Timing is everything: consequences of transient and sustained AhR activity. Biochem Pharmacol. 2009;77(6):947–956. doi: 10.1016/j.bcp.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Zhang G, et al. Interferon-gamma directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-gamma receptor and AMPA GluR1 receptor. FASEB J. 2008;22(6):1797–1806. doi: 10.1096/fj.07-099499. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172(1–2):198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Rizzo A, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–248. doi: 10.1053/j.gastro.2011.04.007. 248 e231. [DOI] [PubMed] [Google Scholar]
- Moon LD, Fawcett JW. Reduction in CNS scar formation without concomitant increase in axon regeneration following treatment of adult rat brain with a combination of antibodies to TGFbeta1 and beta2. Eur J Neurosci. 2001;14(10):1667–1677. doi: 10.1046/j.0953-816x.2001.01795.x. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68(4):368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Morse DC, Groen D, et al. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol Appl Pharmacol. 1993;122(1):27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Tayade C, et al. Interferon gamma in successful pregnancies. Biol Reprod. 2009;80(5):848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama J, Tsuji H, et al. Immunologic effects of perinatal exposure to dioxins, PCBs and organochlorine pesticides in Japanese infants. Chemosphere. 2007;67(9):S393–S398. doi: 10.1016/j.chemosphere.2006.05.134. [DOI] [PubMed] [Google Scholar]
- Narayanan PK, Carter WO, et al. Impairment of human neutrophil oxidative burst by polychlorinated biphenyls: inhibition of superoxide dismutase activity. J Leukoc Biol. 1998;63(2):216–224. doi: 10.1002/jlb.63.2.216. [DOI] [PubMed] [Google Scholar]
- Ndebele K, Tchounwou PB, et al. Coumestrol, bisphenol-A, DDT, and TCDD modulation of interleukin-2 expression in activated CD+4 Jurkat T cells. Int J Environ Res Public Health. 2004;1(1):3–11. doi: 10.3390/ijerph2004010003. [DOI] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, et al. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, et al. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280(10):9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Nolting T, Lindecke A, et al. Measurement of soluble inflammatory mediators in cerebrospinal fluid of human immunodeficiency virus-positive patients at distinct stages of infection by solid-phase protein array. J Neurovirol. 2009;15(5–6):390–400. doi: 10.3109/13550280903350192. [DOI] [PubMed] [Google Scholar]
- Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40(9–11):1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2000;2000(44):re1. doi: 10.1126/stke.442000re1. [DOI] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, et al. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Oliphant CJ, Barlow JL, et al. Insights into the initiation of type 2 immune responses. Immunology. 2011;134(4):378–385. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, et al. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens SE, Summar ML, et al. Lack of association between autism and four heavy metal regulatory genes. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Banks WA, et al. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76(12):105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Pancetti F, Olmos C, et al. Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase. J Toxicol Environ Health B Crit Rev. 2007;10(8):623–630. doi: 10.1080/10937400701436445. [DOI] [PubMed] [Google Scholar]
- Pasca SP, Nemes B, et al. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78(19):2244–2248. doi: 10.1016/j.lfs.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Persico AM, D'Agruma L, et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry. 2001;6(2):150–159. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- Pessah ILP. Autism: Current Theories and Evidence. Z. AW, Humana Press; 2008. Chapter 19: Evidence for Environmental Susceptivility in Autism. What we know what we need to know about gene × environment interactions. [Google Scholar]
- Pessah IN, Cherednichenko G, et al. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125(2):260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lehmler HJ, et al. Enantiomeric specificity of (−)-2,2',3,3',6,6'-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol. 2009;22(1):201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piton A, Michaud JL, et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum Mol Genet. 2008;17(24):3965–3974. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Prevention, C. f. D. C. a. Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2009. MMWR Surveill Summ. 2006;58(10):1–20. [PubMed] [Google Scholar]
- Price CS, Thompson WW, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126(4):656–664. doi: 10.1542/peds.2010-0309. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Chu T, et al. Maternal gene expression profiling during pregnancy and preeclampsia in human peripheral blood mononuclear cells. Placenta. 2011;32(1):70–78. doi: 10.1016/j.placenta.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Dzon L, et al. Immunomodulation of human natural killer cell cytotoxic function by organochlorine pesticides. Hum Exp Toxicol. 2004;23(10):463–471. doi: 10.1191/0960327104ht477oa. [DOI] [PubMed] [Google Scholar]
- Reissmann E, Ernsberger U, et al. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122(7):2079–2088. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- Rivest S. Interactions between the immune and neuroendocrine systems. Prog Brain Res. 2010;181:43–53. doi: 10.1016/S0079-6123(08)81004-7. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB, et al. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeyen G, Chapelle T, et al. Necrotizing pancreatitis due to poisoning with organophosphate pesticides. Acta Gastroenterol Belg. 2008;71(1):27–29. [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, et al. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science. 1988;241(4863):334–336. doi: 10.1126/science.3133768. [DOI] [PubMed] [Google Scholar]
- Romero E, Guaza C, et al. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15(4):372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011 doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Rose S, Melnyk S, et al. The Frequency of Polymorphisms affecting Lead and Mercury Toxicity among Children with Autism. American Journal of Biochemistry and Biotechnology. 2008;4(2):84–95. [Google Scholar]
- Ross FM, Allan SM, et al. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144(1–2):61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Rowley B, Monestier M. Mechanisms of heavy metal-induced autoimmunity. Mol Immunol. 2005;42(7):833–838. doi: 10.1016/j.molimm.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, et al. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117(12):1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco A, Nicole O, et al. A transforming growth factor-beta antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischemic brain injury. J Cereb Blood Flow Metab. 1999;19(12):1345–1353. doi: 10.1097/00004647-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Ikegami N, et al. Protective effects of Ca2+ channel blockers against methyl mercury toxicity. Pharmacol Toxicol. 1996;78(3):193–199. doi: 10.1111/j.1600-0773.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, et al. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Savignac M, Mellstrom B, et al. Calcium-dependent transcription of cytokine genes in T lymphocytes. Pflugers Arch. 2007;454(4):523–533. doi: 10.1007/s00424-007-0238-y. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118(4):572–578. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Schug TT, Janesick A, et al. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127(3–5):204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26(29):7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Rice DC, et al. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348(16):1527–1536. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Nabi R, et al. Polymorphisms in xenobiotic metabolism genes and autism. J Child Neurol. 2004;19(6):413–417. doi: 10.1177/088307380401900603. [DOI] [PubMed] [Google Scholar]
- Seth V, Ahmad RS, et al. Lindane-induced immunological alterations in human poisoning cases. Clin Biochem. 2005;38(7):678–680. doi: 10.1016/j.clinbiochem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, et al. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113(2):123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M, Graef JW. Lead intoxication in children with pervasive developmental disorders. J Toxicol Clin Toxicol. 1996;34(2):177–181. doi: 10.3109/15563659609013767. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64(1):40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, et al. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shin KJ, Bae SS, et al. 2,2',4,6,6'-pentachlorobiphenyl induces apoptosis in human monocytic cells. Toxicol Appl Pharmacol. 2000;169(1):1–7. doi: 10.1006/taap.2000.9034. [DOI] [PubMed] [Google Scholar]
- Sholl-Franco A, da Silva AG, et al. Interleukin-4 as a neuromodulatory cytokine: roles and signaling in the nervous system. Ann N Y Acad Sci. 2009;1153:65–75. doi: 10.1111/j.1749-6632.2008.03962.x. [DOI] [PubMed] [Google Scholar]
- Sholl-Franco A, Marques PM, et al. IL-4 increases GABAergic phenotype in rat retinal cell cultures: involvement of muscarinic receptors and protein kinase C. J Neuroimmunol. 2002;133(1–2):20–29. doi: 10.1016/s0165-5728(02)00327-2. [DOI] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. Journal of Neuroimmunology. 1996;66(1–2):143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Skurlova M, Stofkova A, et al. Exogenous IL-1beta induces its own expression, but not that of IL-6 in the hypothalamus and activates HPA axis and prolactin release. Endocr Regul. 2006;40(4):125–128. [PubMed] [Google Scholar]
- Smith SE, Li J, et al. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol. 2012;86(2):165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Wang CX, et al. Interferon gamma induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of ERK1/2 pathway. J Biol Chem. 2005;280(13):12896–12901. doi: 10.1074/jbc.M412139200. [DOI] [PubMed] [Google Scholar]
- Spulber S, Bartfai T, et al. Morphological and behavioral changes induced by transgenic overexpression of interleukin-1ra in the brain. J Neurosci Res. 2010 doi: 10.1002/jnr.22534. [DOI] [PubMed] [Google Scholar]
- Spulber S, Bartfai T, et al. Morphological and behavioral changes induced by transgenic overexpression of interleukin-1ra in the brain. J Neurosci Res. 2011;89(2):142–152. doi: 10.1002/jnr.22534. [DOI] [PubMed] [Google Scholar]
- Spulber S, Oprica M, et al. Blunted neurogenesis and gliosis due to transgenic overexpression of human soluble IL-1ra in the mouse. Eur J Neurosci. 2008;27(3):549–558. doi: 10.1111/j.1460-9568.2008.06050.x. [DOI] [PubMed] [Google Scholar]
- Stamova B, Green PG, et al. Correlations between gene expression and mercury levels in blood of boys with and without autism. Neurotox Res. 2011;19(1):31–48. doi: 10.1007/s12640-009-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci. 2011 doi: 10.1038/nn.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J Pediatr. 2000;136(5):599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Smith KM, et al. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, et al. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol. 2005;207(1):78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Strijbos PJ, Rothwell NJ. Interleukin-1 beta attenuates excitatory amino acid-induced neurodegeneration in vitro: involvement of nerve growth factor. J Neurosci. 1995;15(5 Pt 1):3468–3474. doi: 10.1523/JNEUROSCI.15-05-03468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6(5):e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson I, Waara L, et al. Soman-induced interleukin-1 beta mRNA and protein in rat brain. Neurotoxicology. 2001;22(3):355–362. doi: 10.1016/s0161-813x(01)00022-5. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, et al. Brief report: autistic disorder in three children with cytomegalovirus infection. J Autism Dev Disord. 2004;34(5):583–586. doi: 10.1007/s10803-004-2552-y. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, et al. High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol Psychiatry. 2004;55(4):434–437. doi: 10.1016/j.biopsych.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Tabares-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14(6):563–589. doi: 10.1038/mp.2009.2. [DOI] [PubMed] [Google Scholar]
- Tan Y, Li D, et al. Ortho-substituted PCBs kill thymocytes. Toxicol Sci. 2003;76(2):328–337. doi: 10.1093/toxsci/kfg233. [DOI] [PubMed] [Google Scholar]
- Thuvander AaDPO. Effects of polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) on some immunological parameters after oral exposure in rats and mice. Toxicological and Environmental Chemistry. 1999;70:229–242. [Google Scholar]
- Tian Y, Green PG, et al. Correlations of gene expression with blood lead levels in children with autism compared to typically developing controls. Neurotox Res. 2011;19(1):1–13. doi: 10.1007/s12640-009-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre D, Broggini M, et al. Serum levels of gamma interferon in patients with Down's syndrome. Infection. 1995;23(1):66–67. doi: 10.1007/BF01710065. [DOI] [PubMed] [Google Scholar]
- Torres AR, Maciulis A, et al. The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum Immunol. 2002;63(4):311–316. doi: 10.1016/s0198-8859(02)00374-9. [DOI] [PubMed] [Google Scholar]
- Torres AR, Sweeten TL, et al. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67(45):346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49(3):529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Traugott U, Lebon P. Interferon-gamma and Ia antigen are present on astrocytes in active chronic multiple sclerosis lesions. J Neurol Sci. 1988;84(23):257–264. doi: 10.1016/0022-510x(88)90130-x. [DOI] [PubMed] [Google Scholar]
- Treves S, Vukcevic M, et al. Enhanced excitation-coupled Ca(2+) entry induces nuclear translocation of NFAT and contributes to IL-6 release from myotubes from patients with central core disease. Hum Mol Genet. 2011;20(3):589–600. doi: 10.1093/hmg/ddq506. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, et al. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30(1):65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Tsukimori K, Tokunaga S, et al. Long-term effects of polychlorinated biphenyls and dioxins on pregnancy outcomes in women affected by the Yusho incident. Environ Health Perspect. 2008;116(5):626–630. doi: 10.1289/ehp.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino M, Rapisarda V, et al. Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum Exp Toxicol. 2007;26(7):551–556. doi: 10.1177/0960327107073817. [DOI] [PubMed] [Google Scholar]
- Van Birgelen AP, Smit EA, et al. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur J Pharmacol. 1995;293(1):77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Varga EA, Pastore M, et al. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009;11(2):111–117. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vashlishan AB, Madison JM, et al. An RNAi screen identifies genes that regulate GABA synapses. Neuron. 2008;58(3):346–361. doi: 10.1016/j.neuron.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Vereyken EJ, Bajova H, et al. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci. 2007;25(12):3605–3616. doi: 10.1111/j.1460-9568.2007.05615.x. [DOI] [PubMed] [Google Scholar]
- Victorio SC, Havton LA, et al. Absence of IFNgamma expression induces neuronal degeneration in the spinal cord of adult mice. J Neuroinflammation. 2010;7:77. doi: 10.1186/1742-2094-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10(1):21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman KS, Owe-Larsson B, et al. Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Res. 2001;896(1–2):18–29. doi: 10.1016/s0006-8993(00)03238-8. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, et al. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30(4):1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Honsek SD, et al. A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol Neurodegener. 2011;6:18. doi: 10.1186/1750-1326-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LD, Howlett GJ, et al. High affinity interleukin-6 receptor is a hexameric complex consisting of two molecules each of interleukin-6, interleukin-6 receptor, and gp-130. J Biol Chem. 1994;269(37):23286–23289. [PubMed] [Google Scholar]
- Watanabe W, Shimizu T, et al. Functional disorder of primary immunity responding to respiratory syncytial virus infection in offspring mice exposed to a flame retardant, decabrominated diphenyl ether, perinatally. J Med Virol. 2010;82(6):1075–1082. doi: 10.1002/jmv.21770. [DOI] [PubMed] [Google Scholar]
- Waterman SJ, el-Fawal HA, et al. Lead alters the immunogenicity of two neural proteins: a potential mechanism for the progression of lead-induced neurotoxicity. Environ Health Perspect. 1994;102(12):1052–1056. doi: 10.1289/ehp.941021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Zou H, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wens B, De Boever P, et al. Transcriptomics identifies differences between ultrapure non-dioxin-like polychlorinated biphenyls (PCBs) and dioxin-like PCB126 in cultured peripheral blood mononuclear cells. Toxicology. 2011;287(1–3):113–123. doi: 10.1016/j.tox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Berti R, et al. Central neuro-inflammatory gene response following soman exposure in the rat. Neurosci Lett. 2003;349(3):147–150. doi: 10.1016/s0304-3940(03)00818-8. [DOI] [PubMed] [Google Scholar]
- Winneke G. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J Neurol Sci. 2011;308(1–2):9–15. doi: 10.1016/j.jns.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30(2):55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Wong G, Goldshmit Y, et al. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187(1):171–177. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Woods R, Vallero RO, et al. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Pearce H, et al. A Comparison of Urinary Mercury between Children with Autism Spectrum Disorders and Control Children. PLoS One. 2012;7(2):e29547. doi: 10.1371/journal.pone.0029547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Turner DR, et al. IL-4 gene expression up-regulated by mercury in rat mast cells: a role of oxidant stress in IL-4 transcription. Int Immunol. 2001;13(3):297–304. doi: 10.1093/intimm/13.3.297. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Borrow P, et al. Astroglial overproduction of TGF-beta 1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol. 1997;77(1):45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Feng L, et al. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol. 1995;147(1):53–67. [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yasumura M, et al. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase delta. J Neurosci. 2011;31(38):13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu T, Kawaguchi D, et al. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex. Development. 2006;133(13):2553–2563. doi: 10.1242/dev.02419. [DOI] [PubMed] [Google Scholar]
- You Z, Luo C, et al. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225(1):135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Yu L, Hebert MC, et al. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21(14):3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir T, Chen YF, et al. Neural stem/progenitor cells differentiate in vitro to neurons by the combined action of dibutyryl cAMP and interferon-gamma. Stem Cells Dev. 2009;18(10):1423–1432. doi: 10.1089/scd.2008.0412. [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, et al. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103(3):546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- Zhang JD, Cousens LS, et al. Three-dimensional structure of human basic fibroblast growth factor, a structural homolog of interleukin 1 beta. Proc Natl Acad Sci U S A. 1991;88(8):3446–3450. doi: 10.1073/pnas.88.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao M, et al. Immunotoxicity of pyrethroid metabolites in an in vitro model. Environ Toxicol Chem. 2010;29(11):2505–2510. doi: 10.1002/etc.298. [DOI] [PubMed] [Google Scholar]
- Zhou J, Chen DJ, et al. Impact of PBDE-209 exposure during pregnancy and lactation on immune function of offspring rats. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26(6):738–741. [PubMed] [Google Scholar]
- Ziats MN, Rennert OM. Expression profiling of autism candidate genes during human brain development implicates central immune signaling pathways. PLoS One. 2011;6(9):e24691. doi: 10.1371/journal.pone.0024691. [DOI] [PMC free article] [PubMed] [Google Scholar]