Abstract
AIMS
Brachial systolic blood pressure (bSBP) exceeds aortic pressure by a variable amount, and estimated central systolic blood pressure (cSBP) may be a better indicator of cardiovascular risk than bSBP. We undertook a systematic review and meta-analysis to compare the effect of single and multiple antihypertensive agents on bSBP, cSBP and augmentation index (AIx).
Methods
A random effects meta-analysis was performed on 24 randomized controlled trials of antihypertensives with measurements of bSBP, cSBP and/or AIx. Separate analyses were performed for drug comparisons with or without placebo, and drug combinations.
Results
In the placebo vs. drug meta-analysis, antihypertensive therapy reduced bSBP more than cSBP and there was no statistically significant evidence of heterogeneity by drug class, although the number of individual studies was small. In placebo-adjusted drug vs. drug comparison, treatment with β-blockers, omapatrilat and thiazide diuretics lowered cSBP significantly less than bSBP (i.e. central to brachial amplification decreased), whereas other monotherapies lowered cSBP and bSBP to similar extents. Sample sizes were too small and effect estimates insufficiently precise to allow firm conclusions to be made regarding comparisons between individual drug classes. Antihypertensive combinations that included β-blockers decreased central to brachial amplification. β-Blockers increased AIx, whereas all other antihypertensive agents reduced AIx to similar extents.
CONCLUSIONS
A reduction in central to brachial amplification by some classes of antihypertensive drug will result in lesser reductions in cSBP despite achievement of target bSBP. This effect could contribute to differences in outcomes in randomized clinical trials when β-blocker- and/or diuretic-based antihypertensive therapy are compared with other regimens.
Keywords: augmentation, central blood pressure, meta-analysis, systematic review
Introduction
Blood pressure (BP) is one of the principal modifiable risk factors for cardiovascular disease [1]. Mean and diastolic arterial pressure are relatively similar in all large elastic arteries [2], but systolic BP and pulse pressure in the brachial artery differ from the systolic BP and pulse pressure in other ‘central’ arteries, such as the aorta [2] and carotid artery [3]. This difference is thought to result from differential timing and magnitude of wave reflection attributed to differences in downstream impedance and arterial stiffness [4, 5]. Some studies have also found that measures of central systolic BP (cSBP) and central pulse pressure are better predictors of target organ damage and cardiovascular disease than brachial systolic BP (bSBP) or brachial pulse pressure [6–13], and a recent meta-analysis showed borderline superiority of central pulse pressure compared with brachial pulse pressure in the prediction of cardiovascular events [14]. There is therefore a growing interest in using central BP as the target for treatment and monitoring in hypertension [15, 16].
Comparison of central and peripheral BP waveforms
The BP waveform at any site is thought to result from the interaction of forward- and backward-travelling waves. Forward-travelling waves are predominantly due to left ventricular ejection, while backward-travelling waves usually arise from wave reflection. Wave reflection occurs at sites of impedance mismatching, for example at arterial branches [17]. Some workers have also emphasized the role of arterial, particularly aortic, compliance (termed the ‘Windkessel’ or reservoir pressure) in the generation of the pressure waveform [18, 19]. The question of whether the Windkessel/reservoir and wave models are competing or complimentary ways of understanding the BP waveform remains disputed [20].
Differences in the magnitude, type (i.e. compression or decompression) and timing of waves account for the differences in systolic pressure and waveform morphology in the different large elastic arteries [5]. Systolic BP in the brachial and radial artery is consistently higher than in the aorta and carotid artery (termed central to brachial amplification). This amplification can mostly be explained by a large early reflected compression wave arising from the distal extremity of the upper limb [21], which is superimposed on the wave resulting from left ventricular contraction.
The complex pattern of wave interaction responsible for the BP waveform means that while bSBP and cSBP are related, the nature of this relationship is neither simple nor readily predictable. Typically, bSBP exceeds cSBP by around 10 mmHg, depending on age and sex [22], but in some individuals the difference between bSBP and cSBP can be in excess of 30 mmHg [23, 24]. The relationship between bSBP and central SBP is complex and highly variable and is influenced by numerous factors, including age [22], sex [22], height [25], heart rate [26] and disease, e.g. hypertension or diabetes [24].
Measurement of cSBP
The BP waveform can be recorded non-invasively by applanation tonometry [27], cuff-based techniques applied to the brachial artery [28, 29] or a volume-clamped photoplethysmographic device on the finger [30, 31]. The arterial distension waveform can be measured by ultrasound [32, 33] and provides an acceptable estimate of the BP waveform. Tonometry and ultrasound can be used on the carotid artery to obtain a BP waveform that is similar, although not identical, to the aortic BP waveform [3, 34] without the need for mathematical transformation. However, it is technically easier and more convenient to perform tonometry on the radial artery and estimate cSBP using some form of signal processing technique. Many studies have employed Fourier analysis to derive an individualized [3] or, more commonly, a generalized [35] transfer function that can be used to synthesise an approximation of the aortic BP waveform by differentially modifying the various frequency components of the BP waveform. This approach is widely used, but has been criticised for assuming that a single transfer function can be applied to all individuals in all circumstances [36]; differences in transfer functions result in different estimates of cSBP [37]. In addition, although many studies of antihypertensive agents have used radially derived estimates of central BP, there are few specific validation data for the transfer function technique in the presence of antihypertensive drugs.
More recently, other approaches to estimate cSBP have been described, including identification of a late shoulder (SBP2) in the BP waveform that appears to correspond to cSBP [38], or use of a running-average filter [39]. All of these approaches are limited by uncertainties in methods used to calibrate the recorded waveforms and inaccuracies in the non-invasive determination of BP [38, 40].
Additional information can also be obtained from the BP waveform, in particular calculation of the augmentation index, with [41] or without [42] use of a transfer function. The central augmentation index (AIx) is often used as an index of aortic wave reflection, although it is also influenced by arterial stiffness and heart rate [43]. Nevertheless, it may provide some additional mechanistic insight into the haemodynamic response to antihypertensive agents.
The effects of antihypertensive medication on central BP
The relative efficacy of antihypertensive agents in randomized clinical trials has usually been assessed on the basis of their ability to lower brachial BP; however, because of the important influence of wave reflection, one might expect different classes of antihypertensive agents to have differential effects on aortic BP owing to their variable impact on vasodilator state and hence wave reflection. It is therefore plausible that reported differences in regression of target organ damage, such as regression of left ventricular hypertrophy [44], increased carotid intima–media thickness [45] or differences in cardiovascular event rates [46, 47] with different antihypertensive agents, despite almost equivalent lowering of brachial BP, could be attributable to differential lowering of cSBP, rather than pleiotropic mechanisms, because the left ventricle and carotid artery are exposed to central BP rather than brachial BP. In many studies and meta-analyses, however, there were small residual differences in peripheral BP [9, 47] that might also explain differences in target organ damage or cardiovascular events.
In view of the importance of cSBP and increasing evidence that antihypertensive drugs may have a differential effect on cSBP compared with brachial systolic BP, we undertook a systematic review and meta-analysis. The aim of this systematic review was to compare the effects of different classes of single and multiple antihypertensive agents on bSBP, cSBP and AIx.
Methods
We identified original randomized controlled trials by an all-language search of all articles (any year up to May 2011) in the Cochrane Controlled Trials Register (CCTR), Medline and EMBASE. We subsequently screened the references of all retrieved articles to identify additional relevant publications. The following search strings and MESH terms were used: central systolic blood pressure OR carotid systolic blood pressure OR aortic systolic blood pressure AND randomized controlled trial AND placebo.
Study selection
We included any randomized controlled clinical trial in adults with hypertension (including isolated systolic hypertension) that compared the effects of at least one antihypertensive agent or a combination of antihypertensive agents with placebo, or with another antihypertensive agent, or combinations of antihypertensive agents, on brachial and central BP and, where available, AIx.
We excluded studies that investigated the effects on central BP of dietary factors, beverages or other agents that are not generally considered antihypertensive drugs. We also excluded studies where the duration of exposure to antihypertensive agent was less than 2 weeks. Studies were also excluded where only the changes in BP from baseline with treatment were reported, with no absolute values. Vasodilating β-blockers, such as nebivolol, were analysed as a separate drug class, because there is evidence that their haemodynamic effects differ substantially from first-generation β-blockers [48]. We took care not to include any study population more than once if it featured in more than one publication. Data for men and women that were presented separately in one publication [48] were pooled prior to inclusion in the analysis.
Data items and summary measures
We extracted brachial SBP, central SBP and AIx (if reported) from all studies. The AIx was defined as the percentage ratio of augmentation pressure divided by pulse pressure, where the augmentation pressure is the pressure difference between the shoulder and peak of the pressure waveform (systolic pressure), and pulse pressure is defined as the difference between systolic and diastolic pressures. Data were extracted into a data extraction form using standard QUOROM reporting guidelines [49]. Data were verified by two independent researchers, and any discrepancies were resolved by consensus.
Data from publications comparing antihypertensive monotherapy with placebo were used to calculate weighted mean differences (WMD) between treatment and placebo by drug class (placebo vs. drug analysis) for each of bSBP and cSBP. In crossover studies where more than one drug class was compared with placebo, the number of participants in the placebo group was divided by the number of comparisons to avoid a unit of analysis error by multiple counting of placebo data [50]. In all but one of these placebo–drug trials, data for AIx were also available, and we calculated the WMD between treatment group and placebo for AIx in a similar way.
We also compared the effect of the different drug classes (drug vs. drug comparison analysis) on central to brachial amplification, i.e. the difference between cSBP and bSBP, in a further meta-analysis. As central to brachial amplification is highly variable between studies, we subtracted the central to brachial amplification on placebo from the data on drug to quantify the drug-related change in central to brachial amplification. Some studies lacked a placebo comparator and in order to include data from these trials, we imputed the placebo bSBP–cSBP difference based on the weighted mean of all placebo vs. drug trials included in the meta-analysis. We examined the effect of drugs on central to brachial amplification because in practice brachial BP is used to monitor the effect of antihypertensive treatment, and a differential effect of a drug on central to brachial amplification could result in different cSBP despite similar bSBP (e.g. [9]). Further meta-analyses were performed to compare the effect of different drug combinations (combination drug analysis) on the central to brachial amplification and also on AIx, using similar methodology.
Statistical analysis
As the underlying effect sizes can be expected to vary between studies, we performed a random-effects meta-analysis using Stata 11.2 (Stata Corp. College Station, TX, USA). Study heterogeneity was assessed using Q and I2 statistics [51]. Possible publication bias was assessed by inspection of funnel plots and use of Egger's test [52]. Weighted means or WMD of data are presented with their 95% confidence intervals in parentheses; P < 0.05 was considered statistically significant.
Results
A total of 24 individual studies involving 5071 participants were included in the meta-analysis (Figure 1 and Table 1). In eight of these studies, measurements were made at the carotid artery, while the others used the radial artery; of these, the Sphygmocor device was employed in all but one (Table 1). Data on the following drugs were available for analysis: α-blockers (AB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), β-blockers (BB), calcium channel blockers (CCB), nebivolol, omapatrilat, spironolactone and thiazide diuretics (D).
Figure 1.
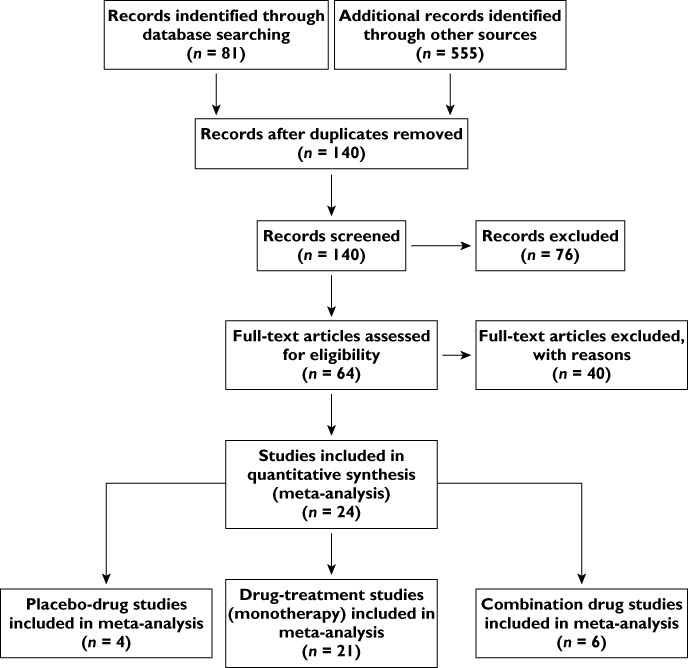
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram
Table 1.
Characteristics of included studies
| Study id | Drug class or placebo studied | Sample size | Type of study | Tonometry site | Design | Comments | Reference |
|---|---|---|---|---|---|---|---|
| Asmar 2001b | P vs. ARB | 27 | Placebo controlled | Radial | Crossover | Hypertension + Diabetes | [63] |
| Asmar 2001a | ACEI + D vs. BB | 471 | Drug comparison, combination | Carotid | Parallel | [61] | |
| Boutouyrie 2010 | ARB + C vs. ARB + BB | 393 | Combination | Radial | Parallel | [64] | |
| Chen 1995 | ACEI vs. BB* | 79 | Drug comparison | Carotid | Parallel | cSBP not reported | [65] |
| Dart 2007 | ACEI vs. D† | 479 | Drug comparison | Carotid | Parallel | [66] | |
| Deary 2002 | P vs. ACEI vs. C vs. ACEI vs. BB vs. AB vs. D | 30 | Placebo controlled, drug comparison | Radial | Crossover | Separate data for sexes | [49] |
| Dhakam 2006 | BB vs. ARB | 21 | Drug comparison | Radial | Crossover | [67] | |
| Dhakam 2008 | P vs. BB vs. nebivolol | 16 | Placebo controlled, drug comparison | Radial | Crossover | [68] | |
| Doi 2010 | C vs. D | 37 | Drug comparison | Radial | Parallel | [69] | |
| Ferdinand 2011 | Aliskiren + D vs. CCB | 53 | Drug comparison, combination | Carotid | Parallel | African-American | [70] |
| Guerin 1992 | BB vs. C | 20 | Drug comparison | Carotid | Parallel | [71] | |
| Jiang 2007 | ACEI vs. D | 101 | Drug comparison | Radial | Parallel | [72] | |
| London 1994 | C vs. ACEI | 24 | Drug comparison | Carotid | Parallel | End-stage renal disease | [73] |
| London 2004 | ACEI + D vs. BB | 181 | Drug comparison, combination | Carotid | Parallel | [74] | |
| Mackenzie 2009 | C vs. ACEI vs. BB vs. D | 59 | Drug comparison | Radial | Parallel | Isolated systolic hypertension | [75] |
| Mahmud 2000 | ARB | 18 | Drug comparison | Radial | Parallel | [76] | |
| Mahmud 2005 | D vs. spironolactone | 24 | Drug comparison | Radial | Crossover | [77] | |
| Mahmud 2008 | BB vs. nebivolol | 40 | Drug comparison | Radial | Parallel | [78] | |
| Matsui 2009 | ARB + C vs. ARB + D | 207 | Combination | Radial | Parallel | [79] | |
| Mitchell 2002 | ACEI vs. omapatrilat | 167 | Drug comparison | Carotid | Parallel | [80] | |
| Morgan 2004 | P vs. C vs. ACEI vs. BB vs. D | 32 | Placebo controlled, drug comparison | Radial | Crossover | [81] | |
| Neal 2004 | C vs. ACEI vs. BB | 24 | Drug comparison | Radial | Crossover | Liver transplantation | [82] |
| Schneider 2008 | ARB vs. BB | 156 | Drug comparison | Radial | Parallel | [83] | |
| Williams 2006 | BB + D vs ACEi + CCB | 2199 | Combination | Radial | Parallel | [9] |
Abbreviations: AB, α-blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; C, calcium channel blocker; cSBP, central systolic blood pressure; D, thiazide diuretic; P, placebo. *Some patients received hydrochlorothiazide in addition to monotherapy. †Only 65% allocated ACEI received monotherapy.
Effect of different classes of antihypertensives on bSBP and cSBP
Six drug classes (ARB, ACEI, BB, CCB, D and AB) were compared in placebo-controlled studies. Each class lowered bSBP and cSBP compared with placebo (Figures 2 and 3), although the reduction in bSBP was larger than that in cSBP [WMD −12.79 (−15.54, −10.04) vs. −9.60 (−12.61, −6.58) mmHg]. There was no evidence of publication bias (Egger's test P= 0.2 and 0.1, respectively, and Supporting Information Figure S1). For both bSBP and cSBP, differences between drug classes were relatively small and there was no statistically significant evidence of heterogeneity (I2= 0%, P= 0.8 and I2= 16.8%, P= 0.3, respectively), although the number of studies available for this analysis was limited (four studies). Different drug classes also lowered DBP to similar extents in placebo-controlled studies [overall difference between drugs and placebo =−6.4 (−8.3, −4.5) mmHg; I2= 0%; P= 0.7]. There was again no evidence of publication bias (Egger's test P= 0.3 and Supporting Information Figure S1).
Figure 3.
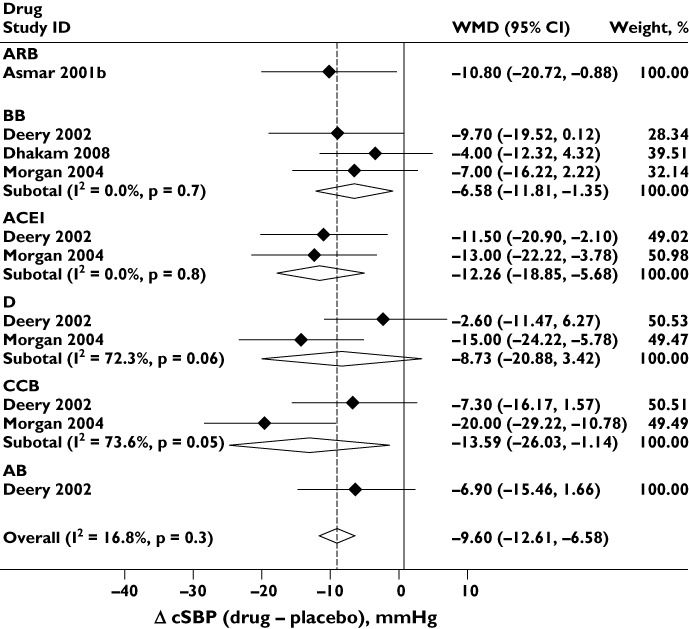
Effect of different classes of antihypertensive monotherapy vs. placebo on central systolic blood pressure (cSBP). Abbreviations are as for Figure 2
Effect of different classes of antihypertensives on central to brachial amplification
Figure 4 shows the placebo-adjusted differences between bSBP and cSBP (i.e. central to brachial amplification) for a total of nine drug classes as monotherapy; meta-analysis of the bSBP and cSBP data individually are shown in the supporting information (Figures S2 and S3). There was significant heterogeneity between drug classes (I2= 74%, P < 0.001). Treatment with BB (P < 0.001), D (P= 0.02) and omapatrilat (P < 0.001) resulted in significant changes in central to brachial amplification, whereas other drug classes had equivalent effects on bSBP and cSBP. There was no evidence of marked publication bias (Figure 4; Egger's test P= 0.1).
Figure 4.
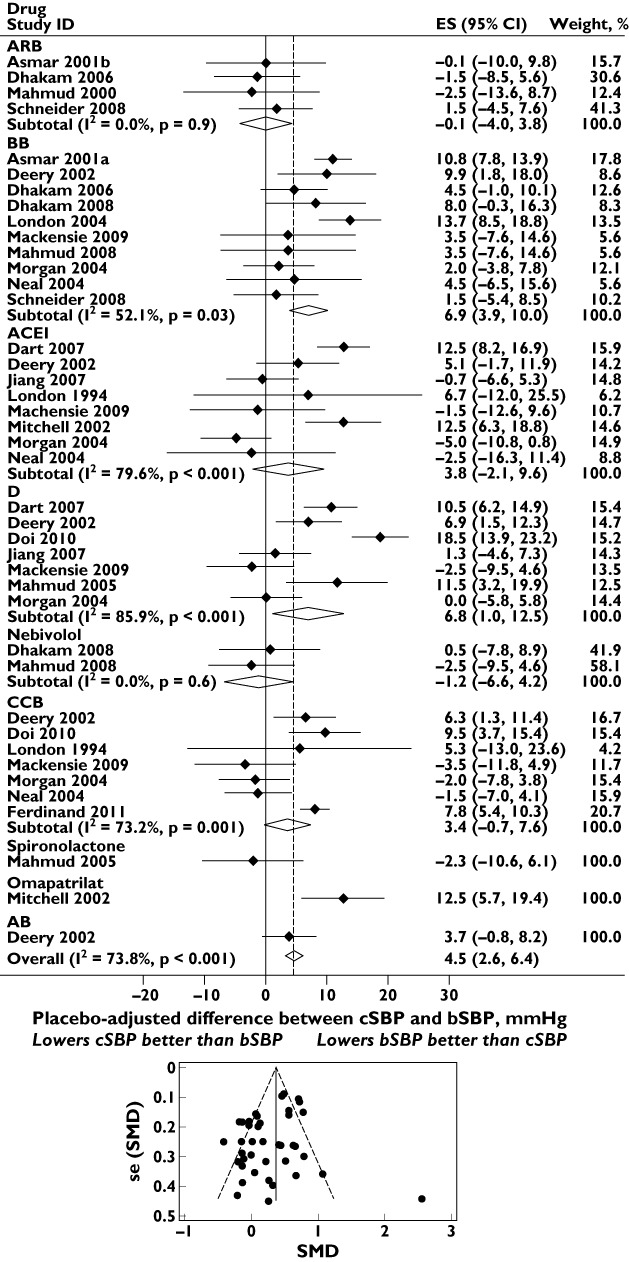
Effect of different classes of antihypertensive monotherapy on placebo-adjusted differences between central systolic blood pressure (cSBP) and brachial systolic blood pressure (bSBP). The funnel plot with pseudo 95% confidence limits is shown below. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; CCB, calcium channel blocker; CI, confidence interval; D, thiazide diuretic; ES, effect size; se (SMD), standard error of the standardized mean difference; SMD, standardized mean difference
Further analysis of these data showed marked heterogeneity by site of measurement (I2= 74%, P < 0.001). However, when the meta-analysis was restricted to studies using radial tonometry the results for BB and D were not substantially different, with placebo-adjusted weighted mean differences between bSBP and cSBP for BB and D being 4.4 (1.7, 7.1) and 6.1 (−1.0, 13.1) mmHg, respectively.
Effect of different combinations of antihypertensive drugs on central to brachial amplification
Figure 5 shows the placebo-adjusted difference between bSBP and cSBP for seven combinations of antihypertensive drug class. There was significant heterogeneity between drug classes. Combinations of ACEI + D, ARB + BB and BB + D caused a greater reduction in bSBP than cSBP; ARB + CCB, ARB + D and ACEI + CCB reduced cSBP and bSBP to similar extents; while, in a single study, aliskiren + D caused a greater reduction in placebo-adjusted cSBP than bSBP. There was no evidence of publication bias (Egger's test P= 0.8 and Supporting Information Figure S1).
Figure 5.
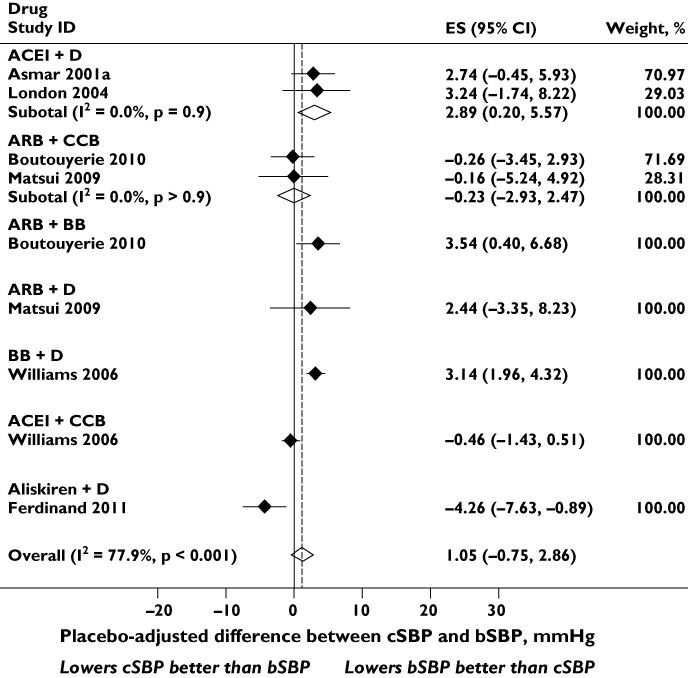
Effect of different combination of classes of antihypertensive drugs on placebo-adjusted differences between central systolic blood pressure (cSBP) and brachial systolic blood pressure (bSBP). Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; CCB, calcium channel blocker; CI, confidence interval; D, thiazide diuretic; ES, effect size
Effect of antihypertensives on AIx
Figure 6 shows a comparison of the effect of antihypertensive monotherapy (vs. placebo) on AIx. There was significant heterogeneity between drug classes; BB was associated with an increase in AIx, while the other classes reduced AIx to similar extents. There was no evidence of publication bias (Egger's test P= 0.3 and Supporting Information Figure S1).
Figure 6.
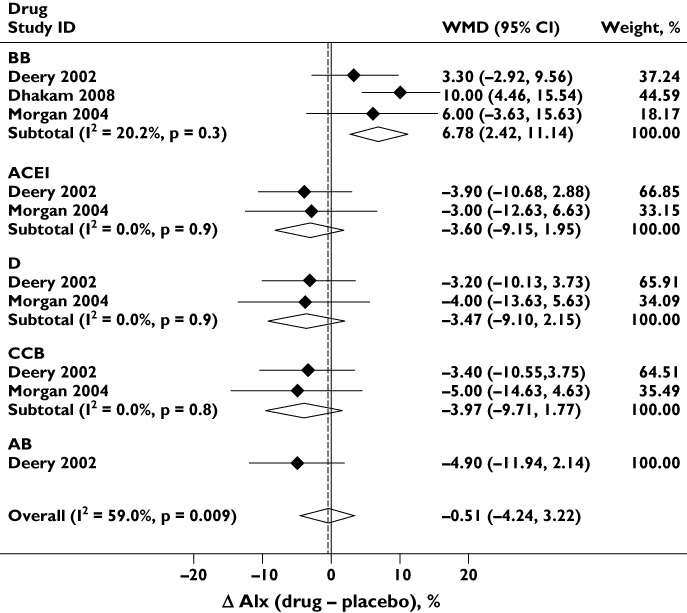
Effect of antihypertensive monotherapy compared to placebo on augmentation index (AIx). Abbreviations are as for Figure 2
Figure 7 shows the effect of six antihypertensive combinations on the placebo-adjusted difference in AIx. There was significant heterogeneity between drug classes. The combinations ACEI + D, ARB + BB and BB + D increased the placebo-adjusted difference in AIx; ARB + CCB had no overall effect on Aix, although the confidence limits of the estimate were very wide; and ACEI + D and ACEI + CCB reduced AIx. There was no evidence of publication bias (Egger's test P= 0.9 and Supporting Information Figure S1).
Figure 7.
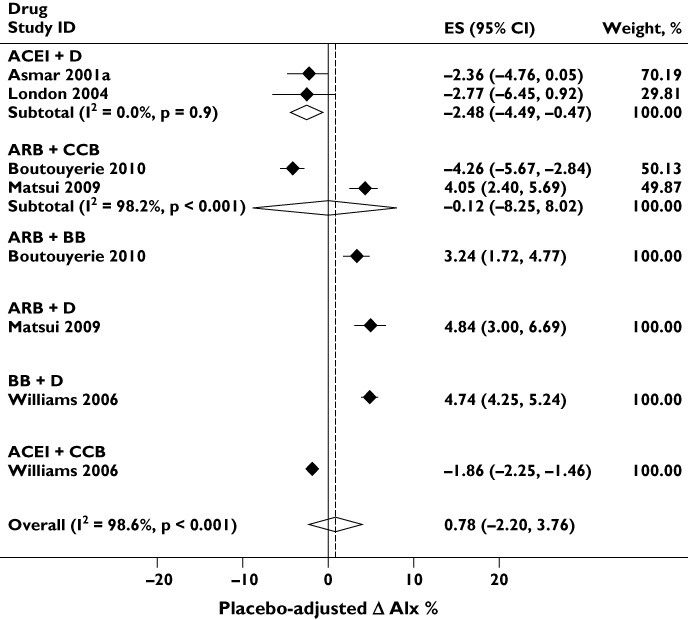
Effect of antihypertensive combinations on placebo-adjusted augmentation index (AIx). Abbreviations are as for Figure 5
Discussion
This systematic review and meta-analysis sought to compare the effects of different antihypertensive drug classes and their combinations on bSBP, cSBP and AIx. All classes of antihypertensive drugs reduced bSBP and cSBP compared with placebo, although the average reduction in bSBP was greater than the reduction in cSBP. An analysis restricted to placebo-controlled studies provided no evidence of heterogeneity between antihypertensive drugs classes on cSBP, although the number of trials included was small (four). A larger number of studies were available for a comparison of the effect of antihypertensive monotherapy on the placebo-adjusted difference between bSBP and cSBP. This analysis indicated that BB, omapatrilat and D lowered cSBP significantly less than bSBP (i.e. reduced central to brachial amplification), whereas other monotherapies lowered cSBP and bSBP to similar extents. Sample sizes and effect estimates were insufficiently precise to allow firm conclusions to be made regarding comparisons between individual drug classes with respect to differential lowering of bSBP and cSBP. Nevertheless, given the current practice of using brachial BP as a treatment target, these data imply that reductions in cSBP might be overestimated by as much as 7 mmHg when using BB or D as monotherapy. Combinations containing BB (ARB + BB and BB + D) were also less effective in lowering cSBP compared with bSBP by 3–4 mmHg.
Recently, the role of β-blockers in hypertension as has come under scrutiny [53], and evidence from meta-analyses suggests that β-blockers may be inferior to other first-line antihypertensive agents, particularly with regard to stroke [47]. If cSBP (the systolic pressure to which the brain is exposed) is more relevant to stroke, then based on the relationship between bSBP and CVD risk, a difference of 3 mmHg would be predicted to correspond to ∼10% increased risk of stroke and could therefore be a contributor to less effective stroke prevention by BB. These observations also support suggestions that measurement of central BP may offer advantages over brachial BP in CVD risk prediction and titration of therapy.
An implication of this meta-analysis is that it may be important to examine the effects of novel BP-lowering agents on central BP, as well as brachial BP. A similar consideration also applies to nonpharmacological treatments, such as weight loss, exercise, dietary change and reductions in salt and alcohol intake, and as yet there is limited information on this question [54–57]. We suggest that the effects of some vasoactive agents on cSBP might have been underestimated on the basis of conventional brachial sphygmomanometry and may be worthy of further study; organic nitrates [58] or phosphodiesterase type 5 inhibitors [59] spring to mind.
Additional insight into some of the possible mechanisms responsible for differences between antihypertensive drug classes is provided by the meta-analysis of AIx. The BB class stands out in that it increases AIx, an effect that may, at least in part, be due to the reduction in heart rate [59], but may also relate to the detrimental effects of BB-based antihypertensive therapy on the magnitude of wave reflection [60, 61]. There was no evidence that D differed from other classes in terms of its effect on AIx, and this suggests that other explanations are needed to account for the effect of D on the difference between bSBP and cSBP observed in this meta-analysis.
This meta-analysis has several limitations. The number of studies that met inclusion criteria was small, and in particular, the comparison of placebo-controlled studies using monotherapy has limited power to exclude important between-class differences in cSBP. We believe this is the most likely explanation of the different conclusions drawn from the analysis of placebo-controlled studies as compared with the analysis of placebo-adjusted differences in bSBP and cSBP (for which more studies were eligible). We were also unable to assess the differences in effects on bSBP and cSBP between individual drug classes (for example, D vs. ACEI), and further studies are required to address this question. For many drug classes, there was clear evidence of heterogeneity within studies of the same drug class. Site of measurement contributed to this heterogeneity, but other unidentified sources of heterogeneity cannot be excluded. Given the small number of studies, particularly within each drug class, a robust assessment of publication bias was not achievable, and this too may influence some of the differences seen.
Although brachial BP remains the principal tool used for the clinical diagnosis and monitoring of hypertension, there is an increasing body of evidence demonstrating that central BP measurement may be a better prognostic marker in hypertension. In addition, there are newer cuff-based measurement methods that require minimal additional training beyond conventional BP measurement, and will hopefully facilitate the transition of central BP measurement from research tool to clinical practice. This does not detract from the current importance of brachial BP, for which there are significantly more longitudinal data, and which is cheap and relatively simple to measure.
Conclusions
Brachial systolic pressure exceeds central or aortic systolic pressure owing to central to brachial amplification. β-Blockers, diuretics and combinations containing β-blockers tend to reduce central to brachial amplification, which implies that the achievement of target bSBP may be associated with lesser reductions in cSBP with these classes of agents. This could contribute to differences in outcomes in randomized clinical trials that compare β-blocker- and/or diuretic-based antihypertensive therapy with other regimens.
Acknowledgments
A.D.H. receives support from the NIHR Biomedical Research Centre scheme. C.M. is a Walport Clinical Lecturer in Cardiology.
Competing Interests
There are no competing interests to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1
Funnel plots with pseudo 95% confidence limits for: (a) Effect of different classes of antihypertensive monotherapy vs.placebo on brachial systolic blood pressure (Figure 2); (b) Effectof different classes of antihypertensive monotherapy vs.placebo on central systolic blood pressure (Figure 3); (c) Effectof different combination of classes of antihypertensive drugs onplacebo-adjusted differences between central systolic bloodpressure and brachial systolic blood pressure (Figure 5); (d)Effect of antihypertensive monotherapy compared to placebo onaugmentation index (Figure 6).
Figure 2.
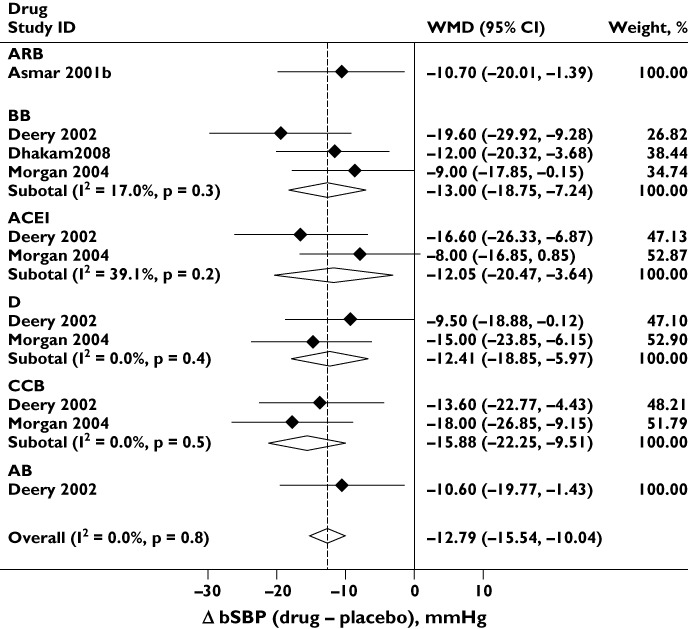
Effect of different classes of antihypertensive monotherapy vs. placebo on brachial systolic blood pressure (bSBP). Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; CCB, calcium channel blocker; CI, confidence interval; D, thiazide diuretic; WMD, weighted mean difference
Figure S2
Effect of different classes of antihypertensive monotherapy on brachial systolic blood pressure (bSBP). Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; CCB, calcium channel blocker; D,thiazide diuretic; ES, effect size.
Figure S3
Effect of different classes of antihypertensive monotherapy on central systolic blood pressure (cSBP). Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; CCB, calcium channel blocker; D,thiazide diuretic; ES, effect size.
REFERENCES
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Remington JW, Wood EH. Formation of peripheral pulse contour in man. J Appl Physiol. 1956;9:433–42. doi: 10.1152/jappl.1956.9.3.433. [DOI] [PubMed] [Google Scholar]
- 3.Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FC. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension. 1996;27:168–75. doi: 10.1161/01.hyp.27.2.168. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 5th edn. London: Hodder Arnold; 2005. [Google Scholar]
- 5.Hughes AD, Parker KH, Davies JE. Waves in arteries: a review of wave intensity analysis in the systemic and coronary circulations. Artery Res. 2008;2:51–9. [Google Scholar]
- 6.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc'h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–8. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 7.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FCP, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–7. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–55. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 9.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 10.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 11.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di BM, Devereux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–9. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–5. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 13.Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–90. doi: 10.1161/01.HYP.0000209340.33592.50. [DOI] [PubMed] [Google Scholar]
- 14.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 15.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009;54:1730–4. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agabiti-Rosei E, Mancia G, O'Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–60. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 17.Brown N. Impedance matching at arterial bifurcations. J Biomech. 1993;26:59–67. doi: 10.1016/0021-9290(93)90613-j. [DOI] [PubMed] [Google Scholar]
- 18.Wang JJ, Flewitt JA, Shrive NG, Parker KH, Tyberg JV. Systemic venous circulation. Waves propagating on a windkessel: relation of arterial and venous windkessels to systemic vascular resistance. Am J Physiol Heart Circ Physiol. 2006;290:H154–H162. doi: 10.1152/ajpheart.00494.2005. [DOI] [PubMed] [Google Scholar]
- 19.Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Foale RA, Malik IS, Tyberg JV, Parker KH, Mayet J, Hughes AD. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol. 2010;298:H580–H586. doi: 10.1152/ajpheart.00875.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyberg JV, Davies JE, Wang Z, Whitelaw WA, Flewitt JA, Shrive NG, Francis DP, Hughes AD, Parker KH, Wang JJ. Wave intensity analysis and the development of the reservoir-wave approach. Med Biol Eng Comput. 2009;47:221–32. doi: 10.1007/s11517-008-0430-z. [DOI] [PubMed] [Google Scholar]
- 21.Zambanini A, Cunningham SL, Parker KH, Khir AW, McG Thom SA, Hughes AD. Wave-energy patterns in carotid, brachial, and radial arteries: a noninvasive approach using wave-intensity analysis. Am J Physiol Heart Circ Physiol. 2005;289:H270–H276. doi: 10.1152/ajpheart.00636.2003. [DOI] [PubMed] [Google Scholar]
- 22.McEniery CM, Yasmin HIR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–60. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 23.Pauca AL, Wallenhaupt SL, Kon ND, Tucker WY. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102:1193–8. doi: 10.1378/chest.102.4.1193. [DOI] [PubMed] [Google Scholar]
- 24.McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–82. doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 25.London GM, Guerin AP, Pannier BM, Marchais SJ, Metivier F. Body height as a determinant of carotid pulse contour in humans. J Hypertens Suppl. 1992;10:S93–S95. [PubMed] [Google Scholar]
- 26.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drzewiecki GM, Melbin J, Noordergraaf A. Arterial tonometry: review and analysis. J Biomech. 1983;16:141–52. doi: 10.1016/0021-9290(83)90037-4. [DOI] [PubMed] [Google Scholar]
- 28.Horvath IG, Nemeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziraki A. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–75. doi: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 29.Lowe A, Harrison W, El-Aklouk E, Ruygrok P, Al-Jumaily AM. Non-invasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech. 2009;42:2111–5. doi: 10.1016/j.jbiomech.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Gizdulich P, Prentza A, Wesseling KH. Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc Res. 1997;33:698–705. doi: 10.1016/s0008-6363(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 31.Karamanoglu M, Feneley MP. On-line synthesis of the human ascending aortic pressure pulse from the finger pulse. Hypertension. 1997;30:1416–24. doi: 10.1161/01.hyp.30.6.1416. [DOI] [PubMed] [Google Scholar]
- 32.Haluska BA, Jeffriess L, Mottram PM, Carlier SG, Marwick TH. A new technique for assessing arterial pressure wave forms and central pressure with tissue Doppler. Cardiovasc Ultrasound. 2007;5:6. doi: 10.1186/1476-7120-5-6. doi: 10.1186/1476-7120-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kips J, Vanmolkot F, Mahieu D, Vermeersch S, Fabry I, de Hoon J, Van BL, Segers P. The use of diameter distension waveforms as an alternative for tonometric pressure to assess carotid blood pressure. Physiol Meas. 2010;31:543–53. doi: 10.1088/0967-3334/31/4/006. [DOI] [PubMed] [Google Scholar]
- 34.Karamanoglu M, Feneley MP. Derivation of the ascending aortic-carotid pressure transfer function with an arterial model. Am J Physiol. 1996;271:H2399–H2404. doi: 10.1152/ajpheart.1996.271.6.H2399. [DOI] [PubMed] [Google Scholar]
- 35.Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–7. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 36.Hope SA, Meredith IT, Cameron JD. Arterial transfer functions and the reconstruction of central aortic waveforms: myths, controversies and misconceptions. J Hypertens. 2008;26:4–7. doi: 10.1097/HJH.0b013e3282f0c9f5. [DOI] [PubMed] [Google Scholar]
- 37.Segers P, Mahieu D, Kips J, Van B. The use of a generalized transfer function: different processing, different results! J Hypertens. 2007;25:1783–7. doi: 10.1097/HJH.0b013e3282ef5c5f. [DOI] [PubMed] [Google Scholar]
- 38.Guilcher A, Brett S, Munir S, Clapp B, Chowienczyk PJ. Estimating central SBP from the peripheral pulse: influence of waveform analysis and calibration error. J Hypertens. 2011;29:1357–66. doi: 10.1097/HJH.0b013e3283479070. [DOI] [PubMed] [Google Scholar]
- 39.Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an N-point moving average method. J Am Coll Cardiol. 2011;57:951–61. doi: 10.1016/j.jacc.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 40.Vermeersch SJ, Rietzschel ER, De B, De B, De B, Van B, Gillebert TC, Verdonck PR, Segers P. Determining carotid artery pressure from scaled diameter waveforms: comparison and validation of calibration techniques in 2026 subjects. Physiol Meas. 2008;29:1267–80. doi: 10.1088/0967-3334/29/11/003. [DOI] [PubMed] [Google Scholar]
- 41.Adji A, Hirata K, O'Rourke MF. Clinical use of indices determined non-invasively from the radial and carotid pressure waveforms. Blood Press Monit. 2006;11:215–21. doi: 10.1097/01.mbp.0000218001.50333.b7. [DOI] [PubMed] [Google Scholar]
- 42.Millasseau SC, Patel SJ, Redwood SR, Ritter JM, Chowienczyk PJ. Pressure wave reflection assessed from the peripheral pulse: is a transfer function necessary? Hypertension. 2003;41:1016–20. doi: 10.1161/01.HYP.0000057574.64076.A5. [DOI] [PubMed] [Google Scholar]
- 43.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–51. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41–6. doi: 10.1016/s0002-9343(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 45.Wang JG, Staessen JA, Li Y, Van Bortel LM, Nawrot T, Fagard R, Messerli FH, Safar M. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke. 2006;37:1933–40. doi: 10.1161/01.STR.0000227223.90239.13. [DOI] [PubMed] [Google Scholar]
- 46.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 47.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–53. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 48.Weir MR. Beta-blockers in the treatment of hypertension: are there clinically relevant differences? Postgrad Med. 2009;121:90–8. doi: 10.3810/pgm.2009.05.2007. [DOI] [PubMed] [Google Scholar]
- 49.Deary AJ, Schumann AL, Murfet H, Haydock SF, Foo RSY, Brown MJ. Double-blind, placebo-controlled crossover comparison of five classes of antihypertensive drugs. J Hypertens. 2002;20:771–7. doi: 10.1097/00004872-200204000-00037. [DOI] [PubMed] [Google Scholar]
- 50.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the Quality of Reports of Meta-Analyses of Randomised Controlled Trials: the QUOROM statement. Onkologie. 2000;23:597–602. doi: 10.1159/000055014. [DOI] [PubMed] [Google Scholar]
- 51.Higgins JPT, Deeks JJ, Altman DG. Chapter 16: special topics in statistics. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (Updated September 2008) Chichester: The Cochrane Collaboration & John Wiley & Sons Ltd; 2008. pp. 481–529. The Cochrane Collaboration. Available at http://www.cochrane-handbook.org (last accessed 7 July 2011) [Google Scholar]
- 52.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothstein H, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester, England: Wiley; 2005. [Google Scholar]
- 54.National Institute for Clincial Excellence and British Hypertension Society. NICE/BHS Hypertension Guideline Review. 28-6-2006.
- 55.Starmans-Kool MJ, Stanton AV, Xu YY, McG Thom SA, Parker KH, Hughes AD. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J Appl Physiol. 2011;110:468–71. doi: 10.1152/japplphysiol.00917.2010. [DOI] [PubMed] [Google Scholar]
- 56.Van Trijp MJ, Bos WJ, van der Schouw YT, Muller M, Grobbee DE, Bots ML. Alcohol and arterial wave reflections in middle aged and elderly men. Eur J Clin Invest. 2005;35:615–21. doi: 10.1111/j.1365-2362.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- 57.Holland DJ, Sacre JW, McFarlane SJ, Coombes JS, Sharman JE. Pulse wave analysis is a reproducible technique for measuring central blood pressure during hemodynamic perturbations induced by exercise. Am J Hypertens. 2008;21:1100–6. doi: 10.1038/ajh.2008.253. [DOI] [PubMed] [Google Scholar]
- 58.Jiang XJ, O'Rourke MF, Jin WQ, Liu LS, Li CW, Tai PC, Zhang XC, Liu SZ. Quantification of glyceryl trinitrate effect through analysis of the synthesised ascending aortic pressure waveform. Heart. 2002;88:143–8. doi: 10.1136/heart.88.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliver JJ, Dear JW, Webb DJ. Clinical potential of combined organic nitrate and phosphodiesterase type 5 inhibitor in treatment-resistant hypertension. Hypertension. 2010;56:62–7. doi: 10.1161/HYPERTENSIONAHA.109.147686. [DOI] [PubMed] [Google Scholar]
- 60.Williams B, Lacy PS. Impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE-Heart Rate. J Am Coll Cardiol. 2009;54:705–13. doi: 10.1016/j.jacc.2009.02.088. [DOI] [PubMed] [Google Scholar]
- 61.Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–6. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 145.Manisty CH, Zambanini A, Parker KH, Davies JE, Francis DP, Mayet J, McG Thom SA, Hughes AD. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian cardiac outcome trial substudy. Hypertension. 2009;54:724–30. doi: 10.1161/HYPERTENSIONAHA.108.125740. [DOI] [PubMed] [Google Scholar]
- 63.Asmar R. Effect of telmisartan on arterial distensibility and central blood pressure in patients with mild to moderate hypertension and Type 2 diabetes mellitus. J Renin Angiotensin Aldosterone Syst. 2001;2(Suppl. 2):S8–11. doi: 10.3317/jraas.2001.031. [DOI] [PubMed] [Google Scholar]
- 64.Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314–22. doi: 10.1161/HYPERTENSIONAHA.109.148999. [DOI] [PubMed] [Google Scholar]
- 65.Chen CH, Ting CT, Lin SJ, Hsu TL, Yin FC, Siu CO, Chou P, Wang SP, Chang MS. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension. 1995;25:1034–41. doi: 10.1161/01.hyp.25.5.1034. [DOI] [PubMed] [Google Scholar]
- 66.Dart AM, Cameron JD, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LMH, Reid CM, Ryan P, Beilin LJ, Jennings GLR, Johnston CI, McNeil JJ, MacDonald GJ, Morgan TO, West MJ, Kingwell BA. Similar effects of treatment on central and brachial blood pressures in older hypertensive subjects in the second Australian national blood pressure trial. Hypertension. 2007;49:1242–7. doi: 10.1161/HYPERTENSIONAHA.106.085803. [DOI] [PubMed] [Google Scholar]
- 67.Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–9. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26:351–6. doi: 10.1097/HJH.0b013e3282f283c9. [DOI] [PubMed] [Google Scholar]
- 69.Doi M, Miyoshi T, Hirohata S, Kamikawa S, Usui S, Kaji Y, Sakane K, Ogawa H, Ninomiya Y, Kusachi S. Combination therapy of calcium channel blocker and angiotensin II receptor blocker reduces augmentation index in hypertensive patients. Am J Med Sci. 2010;339:433–9. doi: 10.1097/MAJ.0b013e3181d658c4. [DOI] [PubMed] [Google Scholar]
- 70.Ferdinand KC, Pool J, Weitzman R, Purkayastha D, Townsend R. Peripheral and central blood pressure responses of combination aliskiren/hydrochlorothiazide and amlodipine monotherapy in African American Patients with stage 2 hypertension: the ATLAAST trial. J Clin Hypertens (Greenwich) 2011;13:366–75. doi: 10.1111/j.1751-7176.2010.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guerin AP, Pannier BM, Marchais SJ, Metivier F, Safar M, London GM. Effects of antihypertensive agents on carotid pulse contour in humans. J Hum Hypertens. 1992;6(Suppl. 2):S37–S40. [PubMed] [Google Scholar]
- 72.Jiang XJ, O'Rourke MF, Zhang YQ, He XY, Liu LS. Superior effect of an angiotensin-converting enzyme inhibitor over a diuretic for reducing aortic systolic pressure. J Hypertens. 2007;25:1095–9. doi: 10.1097/HJH.0b013e3280ac1533. [DOI] [PubMed] [Google Scholar]
- 73.London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90:2786–96. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 74.London GM, Asmar RG, O'Rourke MF, Safar ME. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004;43:92–9. doi: 10.1016/j.jacc.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 75.Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–13. doi: 10.1161/HYPERTENSIONAHA.109.133801. [DOI] [PubMed] [Google Scholar]
- 76.Mahmud A, Feely J. Favourable effects on arterial wave reflection and pulse pressure amplification of adding angiotensin II receptor blockade in resistant hypertension. J Hum Hypertens. 2000;14:541–6. doi: 10.1038/sj.jhh.1001053. [DOI] [PubMed] [Google Scholar]
- 77.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–5. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Mahmud A, Feely J. Beta-blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21:663–7. doi: 10.1038/ajh.2008.156. [DOI] [PubMed] [Google Scholar]
- 79.Matsui Y, Eguchi K, O'Rourke MF, Ishikawa J, Miyashita H, Shimada K, Kario K. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension. 2009;54:716–23. doi: 10.1161/HYPERTENSIONAHA.109.131466. [DOI] [PubMed] [Google Scholar]
- 80.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–61. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 81.Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–23. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 82.Neal DA, Brown MJ, Wilkinson IB, Byrne CD, Alexander GJ. Hemodynamic effects of amlodipine, bisoprolol, and lisinopril in hypertensive patients after liver transplantation. Transplantation. 2004;77:748–50. doi: 10.1097/01.tp.0000116418.78963.dc. [DOI] [PubMed] [Google Scholar]
- 83.Schneider MP, Delles C, Klingbeil AU, Ludwig M, Kolloch RE, Krekler M, Stumpe KO, Schmieder RE. Effect of angiotensin receptor blockade on central haemodynamics in essential hypertension: results of a randomised trial. J Renin Angiotensin Aldosterone Syst. 2008;9:49–56. doi: 10.3317/jraas.2008.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


