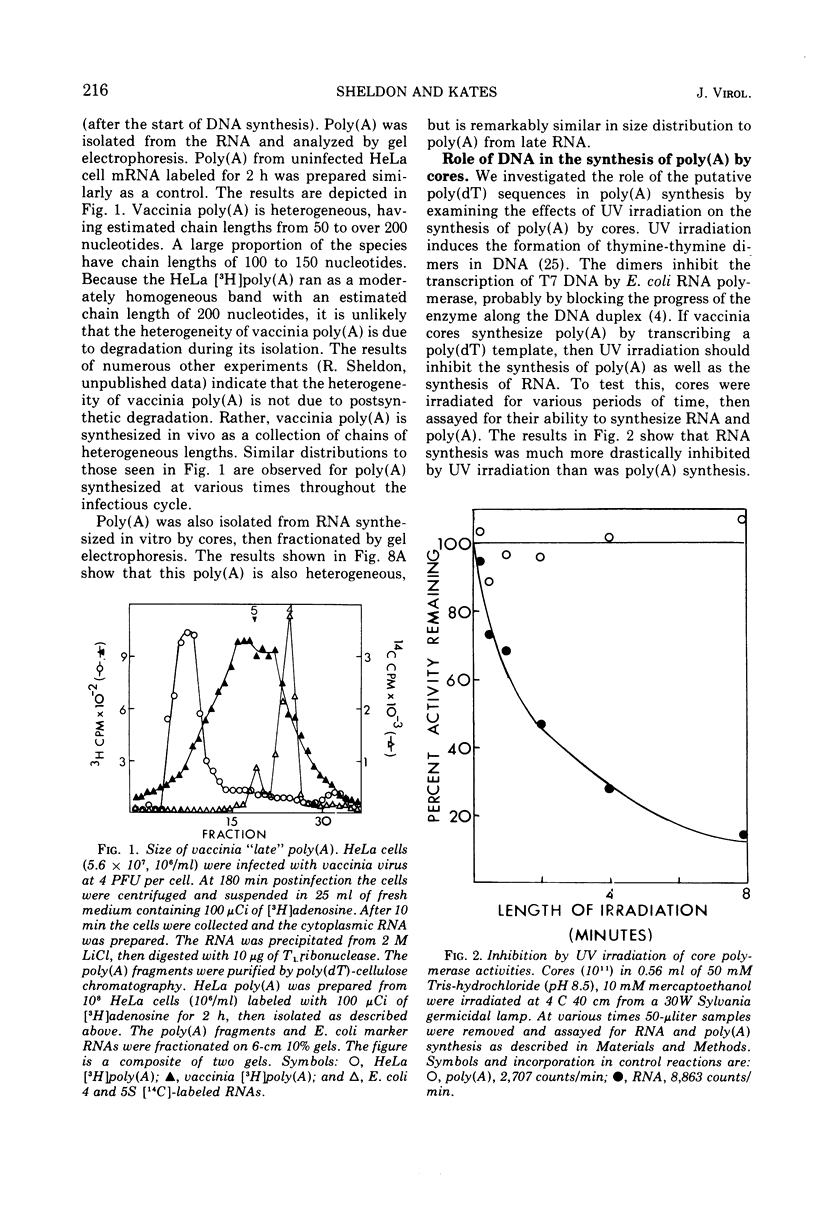
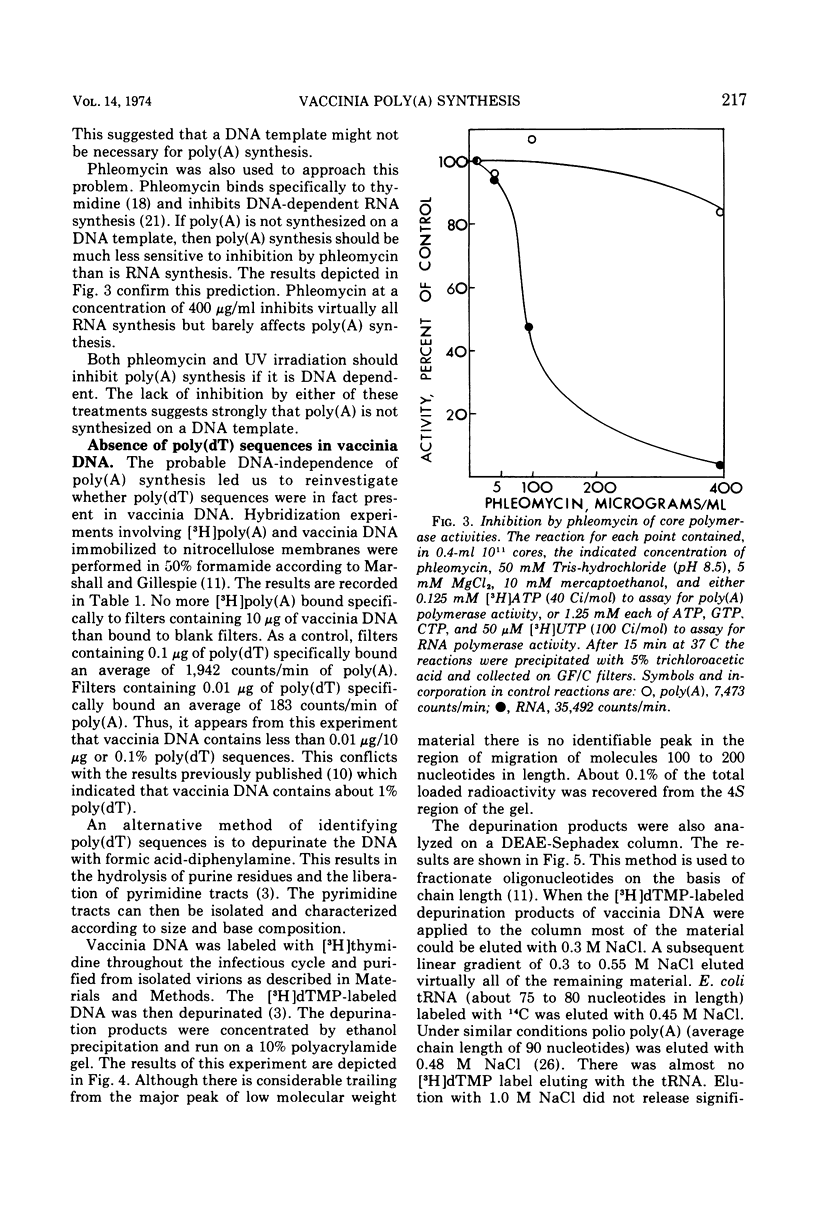
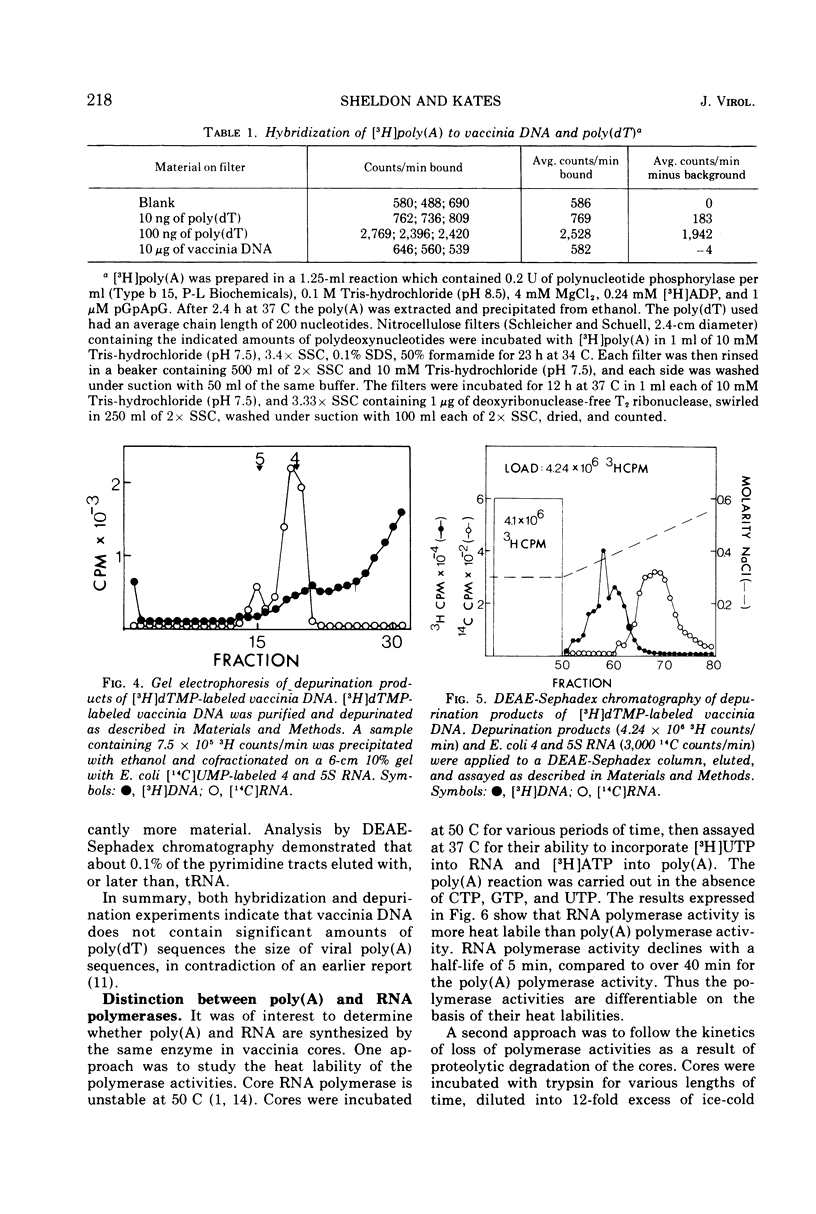
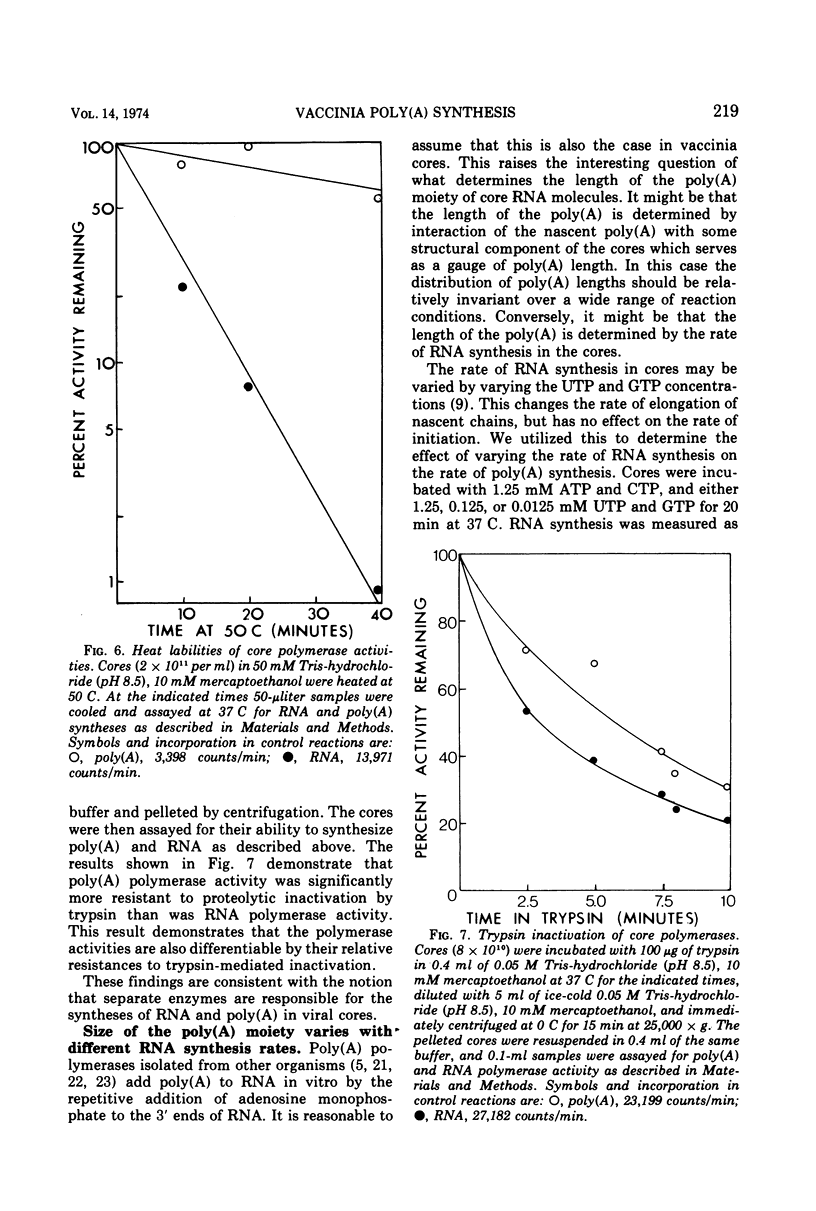
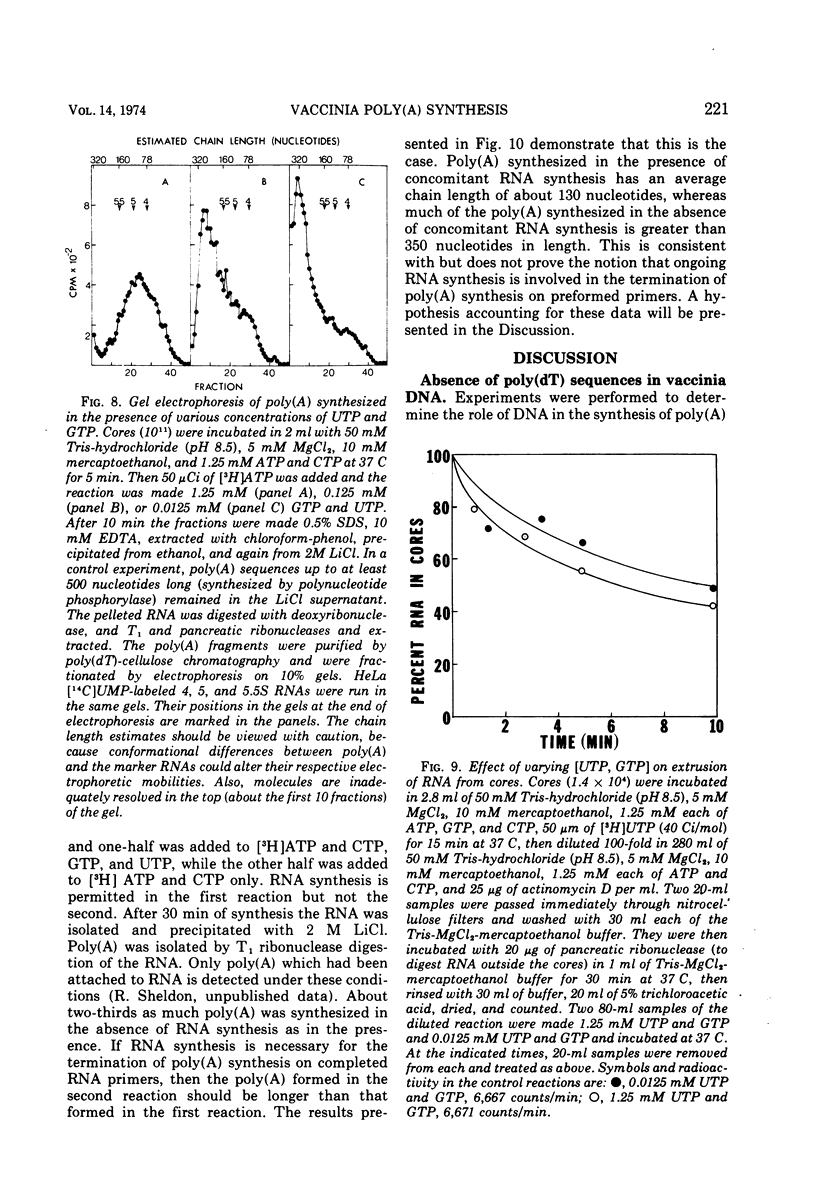
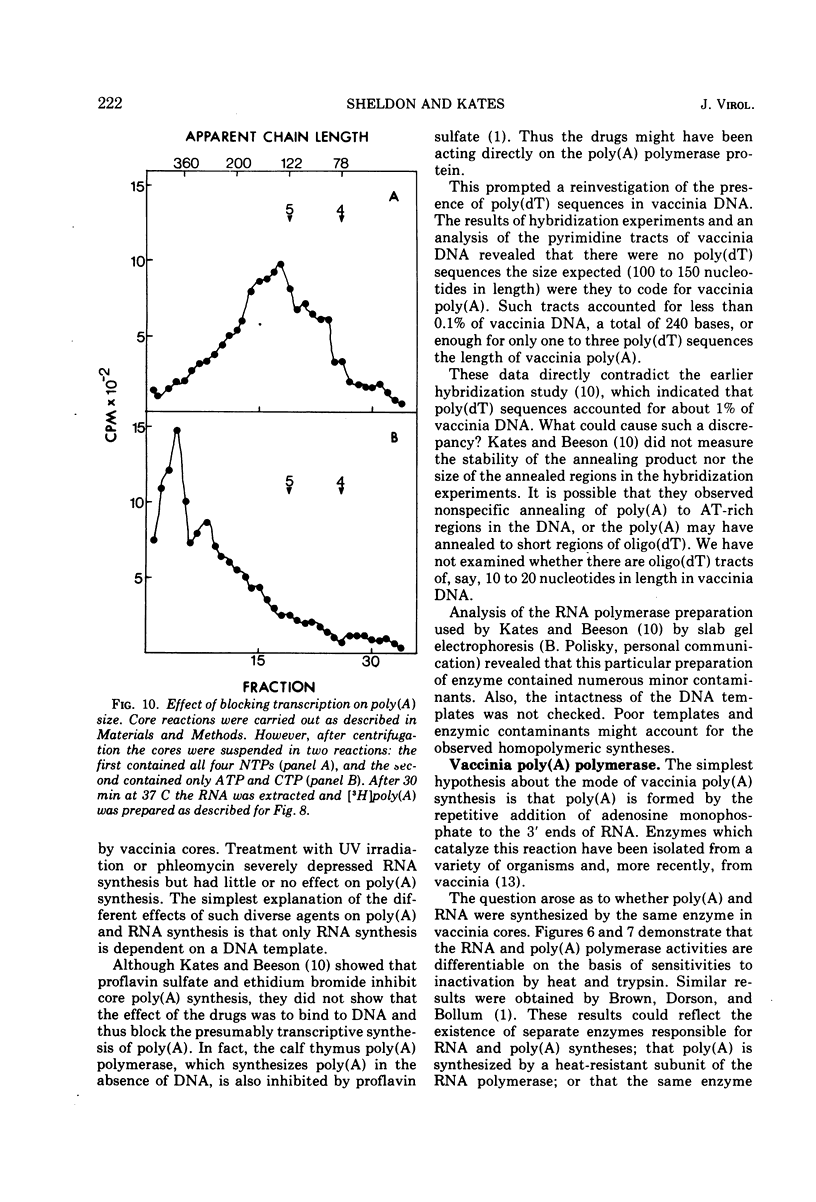
Abstract
Data are presented which indicate that vaccinia DNA does not contain poly(dT) sequences the size of poly(A) sequences (50 to 200 nucleotides in length) found in vaccinia RNA. A hybridization experiment and polyacrylamide gel electrophoresis and DEAE-Sephadex chromatography of pyrimidine tracts show that poly(dT) sequences can account for no more than 0.1% of vaccinia DNA. Ultraviolet irradiation (which causes thymine dimer formation) and phleomycin (which binds to thymidine) both inhibit RNA synthesis but not poly(A) synthesis by vaccinia cores. These data are consistent with a nontranscriptive mechanism for vaccinia poly(A) synthesis. Both trypsin and 50 C heat treatment inhibit RNA synthesis more than poly(A) synthesis by cores, suggesting that separate enzymes may be involved in these syntheses. When the rate of core RNA synthesis is reduced by lowering the UTP and GTP concentrations, the size of the poly(A) sequences increase. These and other data suggest that transcription is involved in the termination of poly(A) synthesis in cores. This might be due to the displacement of growing poly(A) chains by recently completed RNA 3′ termini which have not yet acquired poly(A) sequences.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M., Dorson J. W., Bollum F. J. Terminal riboadenylate transferase: a poly A polymerase in purified vaccinia virus. J Virol. 1973 Aug;12(2):203–208. doi: 10.1128/jvi.12.2.203-208.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Chessin H., Summers W. C. Initiation by RNA polymerase on UV or x-ray damaged T7 DNA. Biochem Biophys Res Commun. 1970 Jan 6;38(1):40–45. doi: 10.1016/0006-291x(70)91080-6. [DOI] [PubMed] [Google Scholar]
- EDMONDS M., ABRAMS R. Polynucleotide biosynthesis: formation of a sequence of adenylate units from adenosine triphosphate by an enzyme from thymus nuclei. J Biol Chem. 1960 Apr;235:1142–1149. [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Marshall S., Gillespie D. Poly U tracts absent from viral RNA. Nat New Biol. 1972 Nov 8;240(97):43–45. doi: 10.1038/newbio240043a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Paoletti E. Polyadenylate polymerase from vaccinia virions. Nat New Biol. 1973 Sep 12;245(141):59–63. doi: 10.1038/newbio245059a0. [DOI] [PubMed] [Google Scholar]
- Munyon W., Mann J., Grace J. T., Jr Protection of vaccinia from heat inactivation by nucleotide triphosphates. J Virol. 1970 Jan;5(1):32–38. doi: 10.1128/jvi.5.1.32-38.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Pietsch P., Corbett C. Competitive effects of phleomycin and mercuric chloride in vivo. Nature. 1968 Aug 31;219(5157):933–934. doi: 10.1038/219933a0. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R., Kates J., Kelley D. E., Perry R. P. Polyadenylic acid sequences on 3' termini of vaccinia messenger ribonucleic acid and mammalian nuclear and messenger ribonucleic acid. Biochemistry. 1972 Sep 26;11(20):3829–3834. doi: 10.1021/bi00770a023. [DOI] [PubMed] [Google Scholar]
- Shishido K., Ikeda Y. Preferential binding of E. coli RNA polymerase to the AT-rich regions of bacteriophage f1 DNA. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1420–1428. doi: 10.1016/s0006-291x(71)80244-9. [DOI] [PubMed] [Google Scholar]
- Tsiapalis C. M., Dorson J. W., De Sante D. M., Bollum F. J. Terminal riboadenylate transferase: a polyadenylate polymerase from calf thymus gland. Biochem Biophys Res Commun. 1973 Feb 5;50(3):737–743. doi: 10.1016/0006-291x(73)91306-5. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Bretthauer R. K. Properties of a polyriboadenylate polymerase isolated from yeast ribosomes. Biochemistry. 1971 Apr 27;10(9):1576–1582. doi: 10.1021/bi00785a011. [DOI] [PubMed] [Google Scholar]
- VENKATARAMAN P. R., MAHLER H. R. Incorporation of adenylic acid into ribonucleic acid and synthetic polynucleotides. J Biol Chem. 1963 Mar;238:1058–1067. [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]


