Abstract
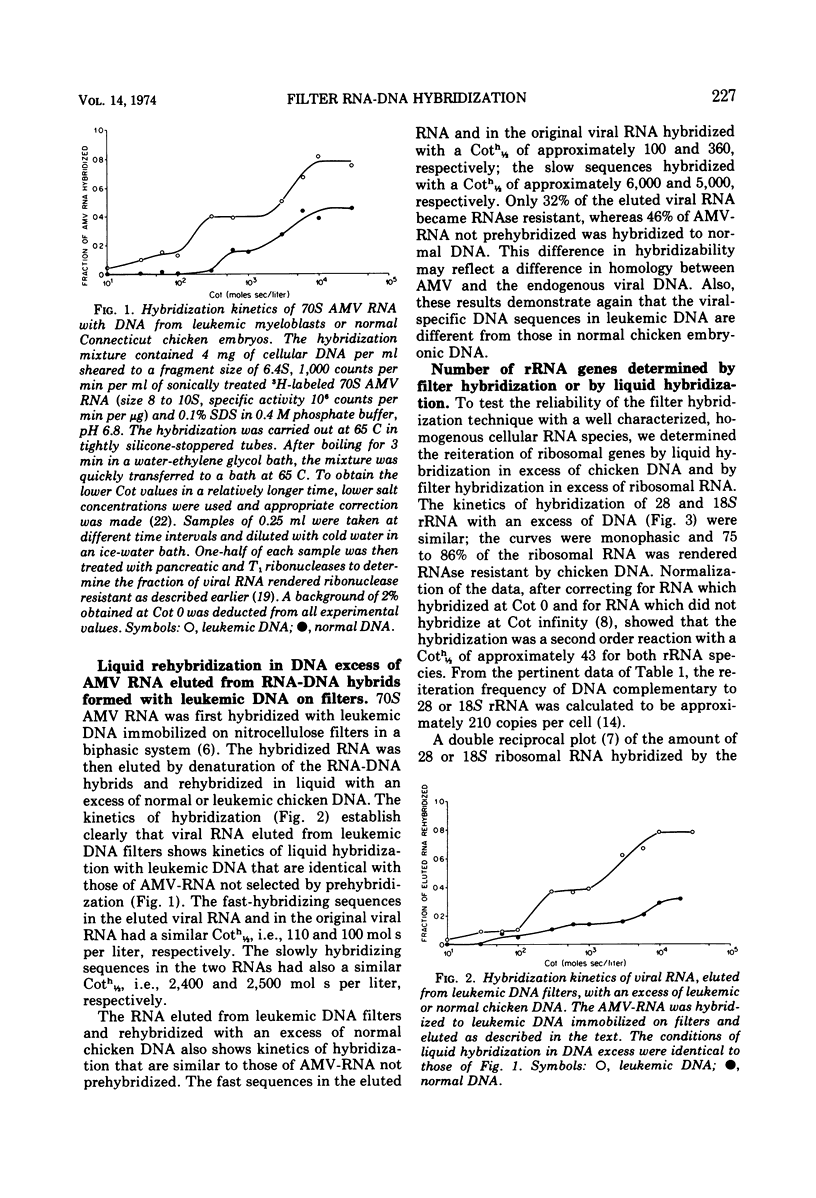
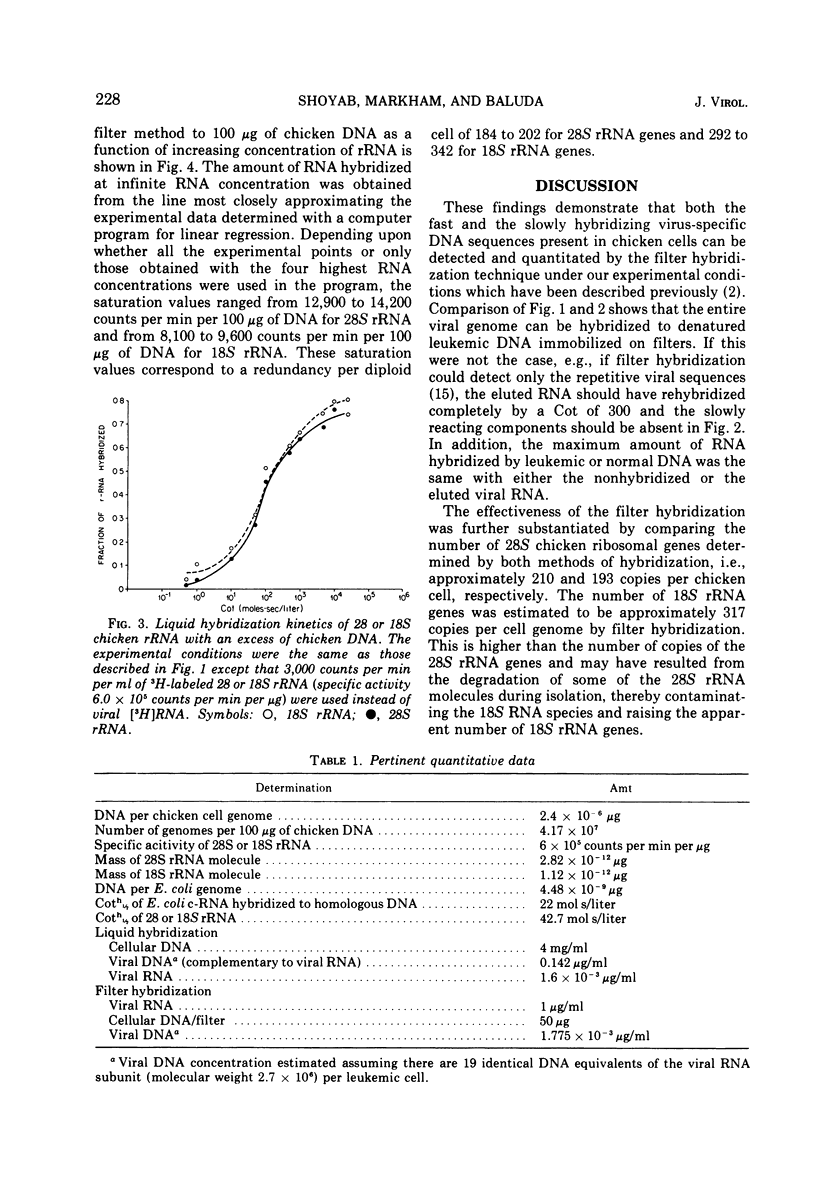
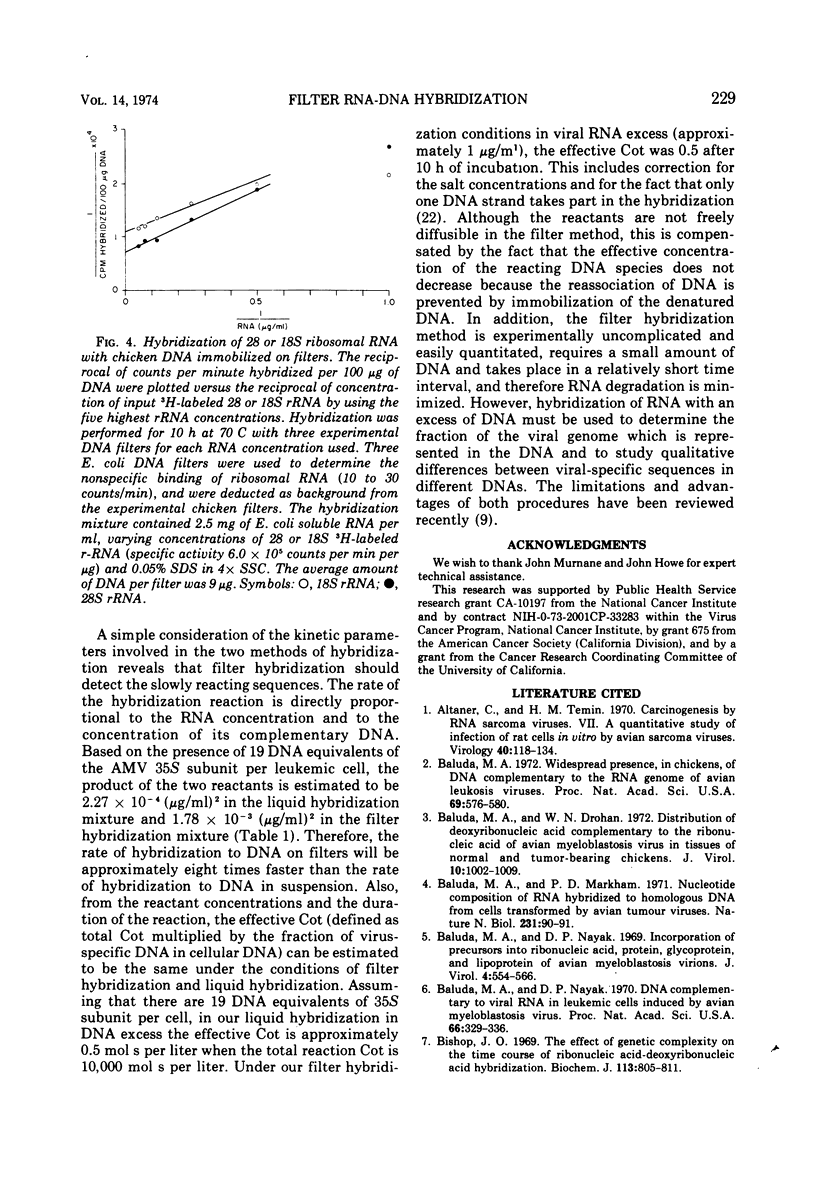
Denatured DNA from leukemic myeloblasts or uninfected chicken embryos, immobilized on nitrocellulose filters, was hybridized to a vast excess of [3H]70S RNA from purified avian myeloblastosis virus. The viral RNA was eluted from the RNA-DNA hybrids, purified, and then rehybridized in solution to an excess of either leukemic or normal chicken embryonic DNA. This study revealed that all the slow and the fast hybridizing viral RNA sequences detectable by liquid hybridization in DNA excess had hybridized to the filter bound DNA. Both techniques also gave similar values for the number of 28S ribosomal RNA genes contained in a chicken cell genome: 210 by the liquid hybridization procedure and 218 by the filter hybridization technique. Therefore, filter hybridization can accurately detect DNA sequences present in relatively few numbers in the genome of higher organisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Drohan W. N. Distribution of deoxyribonucleic acid complementary to the ribonucleic acid of avian myeloblastosis virus in tissues of normal and tumor-bearing chickens. J Virol. 1972 Nov;10(5):1002–1009. doi: 10.1128/jvi.10.5.1002-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Markham P. D. Nucleotide composition of RNA hybridized to homologous DNA from cells transformed by avian tumour viruses. Nat New Biol. 1971 May 19;231(20):90–91. doi: 10.1038/newbio231090a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O. DNA-RNA hybridization. Acta Endocrinol Suppl (Copenh) 1972;168:247–276. doi: 10.1530/acta.0.071s247. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. Molecular hybridization of ribonucleic acid with a large excess of deoxyribonucleic acid. Biochem J. 1972 Jan;126(1):171–185. doi: 10.1042/bj1260171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O. The effect of genetic complexity on the time-course of ribonucleic acid-deoxyribonucleic acid hybridization. Biochem J. 1969 Aug;113(5):805–811. doi: 10.1042/bj1130805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. INTERACTION OF COMPLEMENTARY RNA AND DNA. J Mol Biol. 1964 Feb;8:184–200. doi: 10.1016/s0022-2836(64)80128-5. [DOI] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. The formation of hybrid DNA molecules and their use in studies of DNA homologies. J Mol Biol. 1961 Oct;3:595–617. doi: 10.1016/s0022-2836(61)80024-7. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Separation of DNA sequences complementary to the RNA of avian myeloblastosis virus from chicken DNA by alkaline cesium chloride density sedimentation. J Virol. 1973 Sep;12(3):534–537. doi: 10.1128/jvi.12.3.534-537.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. F., Bugianesi R. L., Shen T. Y. Preparation of sepharose-bound poly (rI:rC). Biochem Biophys Res Commun. 1971 Oct 1;45(1):184–189. doi: 10.1016/0006-291x(71)90067-2. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


