Abstract
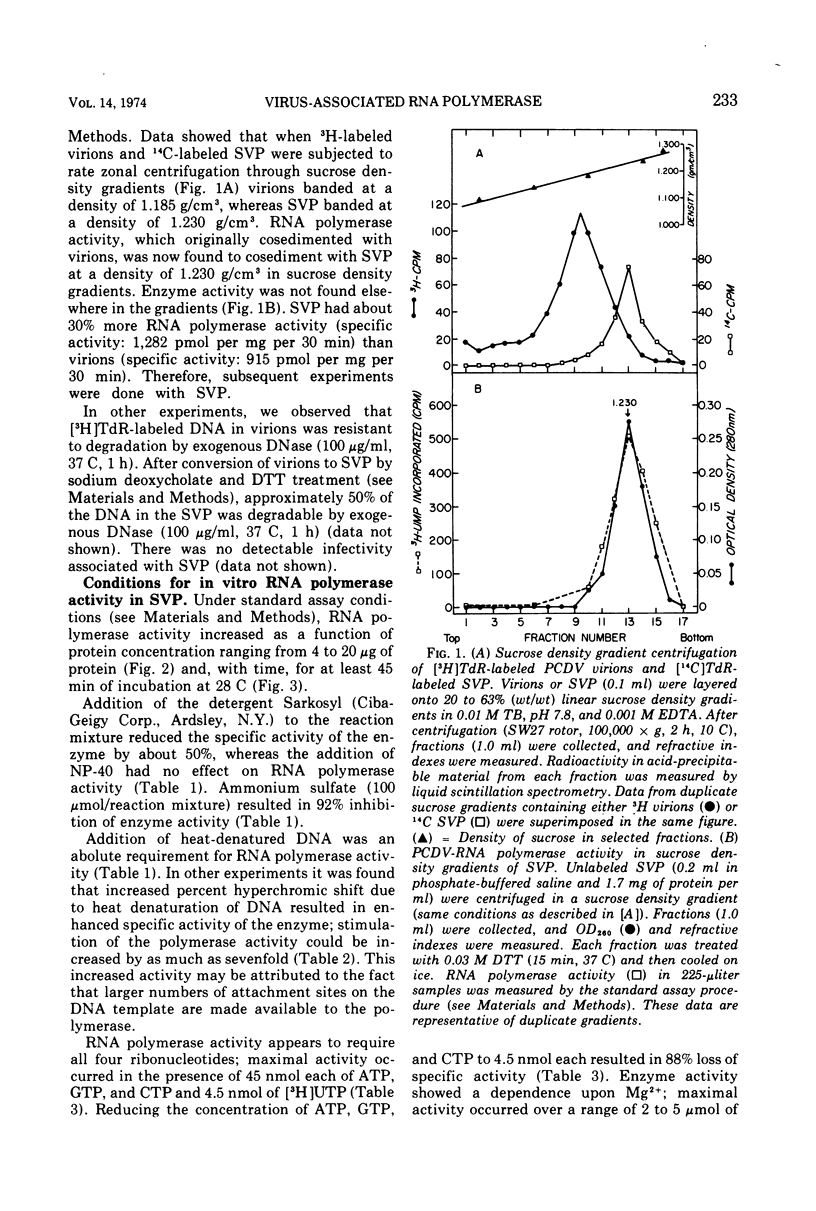
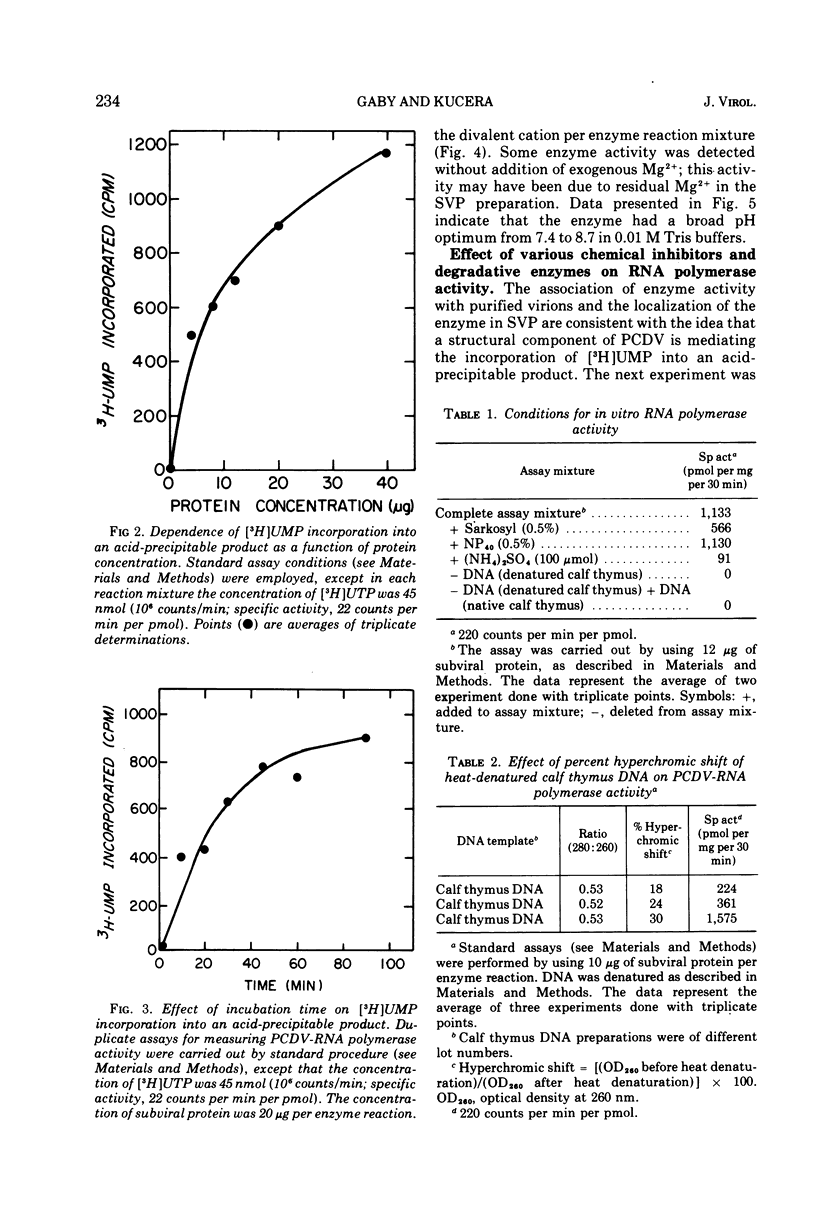
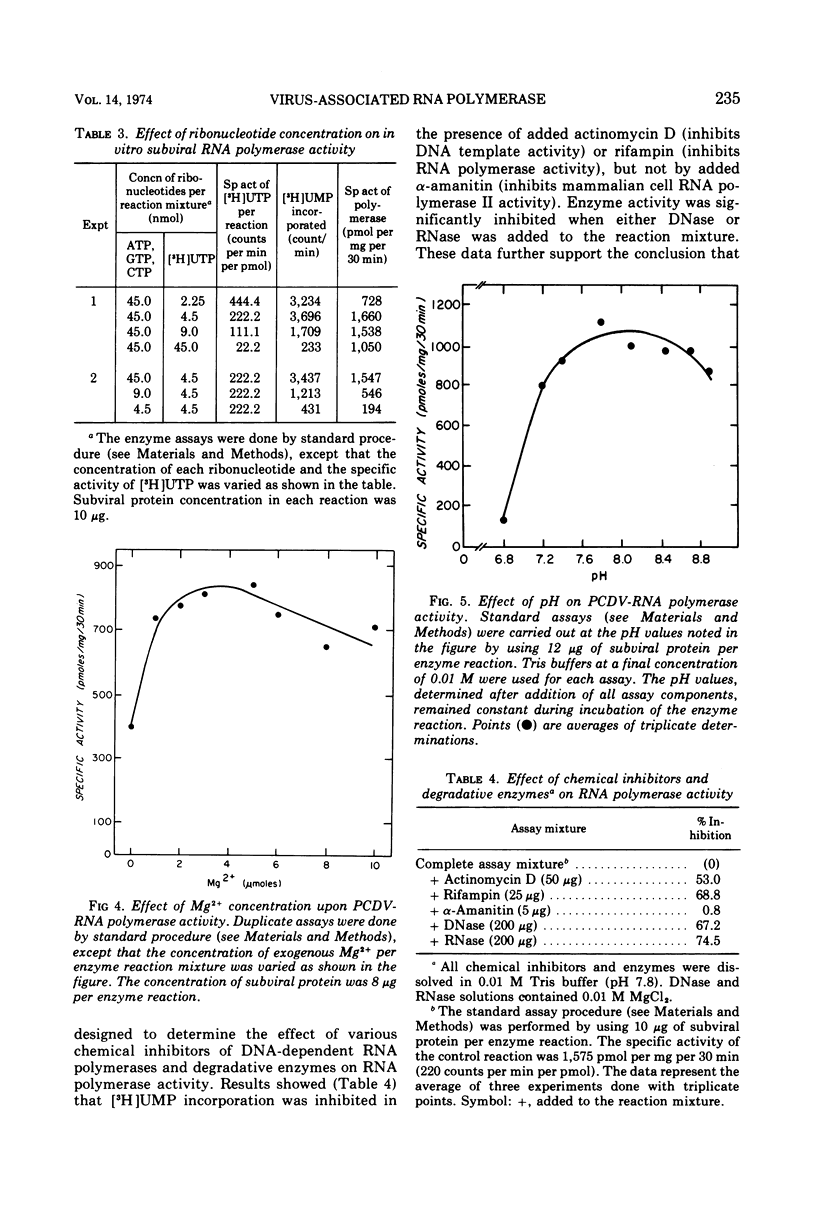
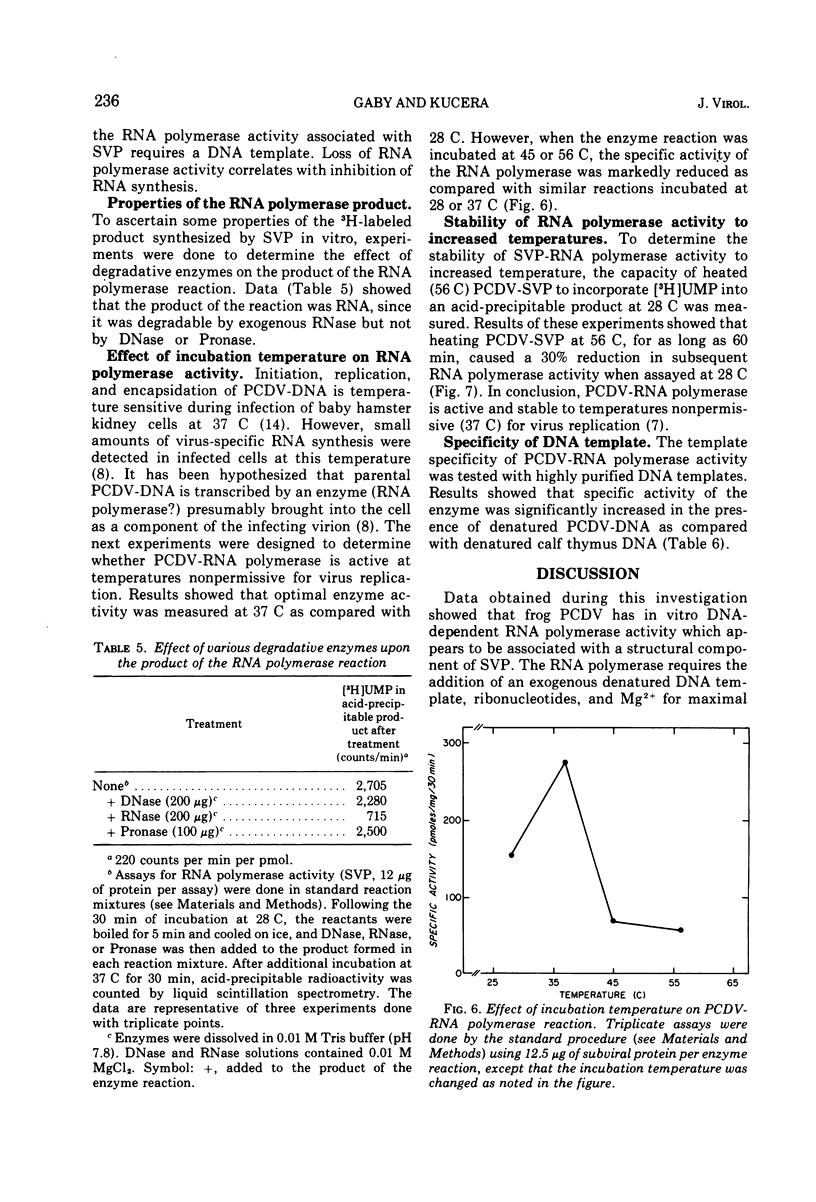
Polyhedral cytoplasmic deoxyribovirus virions contain a DNA-dependent RNA polymerase which catalyzes the incorporation of ribonucleotides into an acid-precipitable product. Treatment of virions with sodium deoxycholate and dithiothreitol resulted in the formation of subviral particles which could be separated from virions by rate zonal centrifugation in sucrose gradients. Subviral particles were RNA polymerase-positive and more active per unit mass of protein than virions. In vitro enzyme activity associated with subviral particles required addition of ribonucleotides, Mg2+, and exogenous denatured DNA template. Optimal enzyme activity occurred over a broad pH (7.2 to 8.8) and Mg2+ concentration (2 to 10 μmol) range. The specific activity of the RNA polymerase was maximal at 37 C. Addition of DNase or actinomycin D to the reaction mixture reduced the incorporation of [3H]UMP into an acid-precipitable product. The product of the reaction was sensitive to degradation by RNase but not to DNase or Pronase. These data suggest that the enzyme copies DNA into RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., Hirth C., Travo C., Nonnenmacher H., Kirn A. Preparation and properties of an inhibitory extract from frog virus 3 particles. J Virol. 1973 May;11(5):694–701. doi: 10.1128/jvi.11.5.694-701.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubertin A., Palese P., Tan K. B., Vilagines R., McAuslan B. R. Proteins of a polyhedral cytoplasmic deoxyvirus. 3. Structure of frog virus 3 and location ov virus-associated adenosine triphosphate phosphohydrolase. J Virol. 1971 Nov;8(5):643–648. doi: 10.1128/jvi.8.5.643-648.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Costanzo F., Mannini-Palenzona A., La Placa M. Impairment of host cell ribonucleic acid polymerase II after infection with frog virus 3. J Virol. 1972 Apr;9(4):698–700. doi: 10.1128/jvi.9.4.698-700.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., WOODROOFE G. M. The reactivation of poxviruses. II. The range of reactivating viruses. Virology. 1960 May;11:185–201. doi: 10.1016/0042-6822(60)90061-1. [DOI] [PubMed] [Google Scholar]
- Granoff A. Viruses of amphibia. Curr Top Microbiol Immunol. 1969;50:107–137. doi: 10.1007/978-3-642-46169-9_4. [DOI] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Mechanisms involved in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus: evidence for an RNA polymerase in the virion. Virology. 1971 Oct;46(1):39–49. doi: 10.1016/0042-6822(71)90004-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Viron-associated protein kinase and its involvement in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus. Virology. 1972 Jun;48(3):847–851. doi: 10.1016/0042-6822(72)90167-5. [DOI] [PubMed] [Google Scholar]
- Gravell M., Naegele R. F. Nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus (PCDV). Virology. 1970 Jan;40(1):170–174. doi: 10.1016/0042-6822(70)90390-9. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Bratt M. A. Ribonucleic acid polymerase in virions of Newcastle disease virus: comparison with the vesicular stomatitis virus polymerase. J Virol. 1971 Mar;7(3):389–394. doi: 10.1128/jvi.7.3.389-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. S., McAuslan B. R. Virus-associated nucleases: location and properties of deoxyribonucleases and ribonucleases in purified frog virus 3. J Virol. 1972 Aug;10(2):202–210. doi: 10.1128/jvi.10.2.202-210.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S. Effects of temperature on frog polyhedral cytoplasmic deoxyribovirus multiplication: thermosensitivity of initiation, replication, and encapsidation of viral DNA. Virology. 1970 Nov;42(3):576–589. doi: 10.1016/0042-6822(70)90304-1. [DOI] [PubMed] [Google Scholar]
- Kucera L. S. Failure of rifampin to inhibit frog polyhedral cytoplasmic deoxyribovirus multiplication. Antimicrob Agents Chemother. 1973 Sep;4(3):372–375. doi: 10.1128/aac.4.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Ribonucleic acid-dependent deoxyribonucleic acid polymerase in visna virus. J Virol. 1970 Nov;6(5):702–704. doi: 10.1128/jvi.6.5.702-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Mcauslan B. R. Rifampicin inhibition of vaccinia replication. Biochem Biophys Res Commun. 1969 Oct 8;37(2):289–295. doi: 10.1016/0006-291x(69)90733-5. [DOI] [PubMed] [Google Scholar]
- Palese P., Koch G. Degradation of single- and double-stranded RNA by frog virus 3. Proc Natl Acad Sci U S A. 1972 Mar;69(3):698–701. doi: 10.1073/pnas.69.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., McAuslan B. R. Virus-associated DNase: endonuclease in a polyhedral cytoplasmic deoxyvirus. Virology. 1972 Jul;49(1):319–321. doi: 10.1016/s0042-6822(72)80036-9. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Phosphorylation of animal virus proteins by a virion protein kinase. J Virol. 1973 Sep;12(3):511–522. doi: 10.1128/jvi.12.3.511-522.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Kingsbury D. W., Darlington R. W. Sendai virus-induced transcriptase from infected cells: polypeptides in the transcriptive complex. J Virol. 1972 Nov;10(5):1037–1043. doi: 10.1128/jvi.10.5.1037-1043.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. O., Portner A., Kingsbury D. W. Ribonucleic acid transcriptases in Sendai Virions and infected cells. J Virol. 1971 Aug;8(2):174–180. doi: 10.1128/jvi.8.2.174-180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., McAuslan B. R. Proteins of polyhedral cytoplasmic deoxyviruses. I. The structural polypeptides of FV 3 . Virology. 1971 Jul;45(1):200–207. doi: 10.1016/0042-6822(71)90127-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Vilagines R., McAuslan B. R. Proteins of polyhedal cytoplasmic deoxyvirus. II. Nucleotide phosphohydrolase activity associated with frog virus 3. J Virol. 1971 May;7(5):619–624. doi: 10.1128/jvi.7.5.619-624.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


